Abstract
Purpose
To summarise our experience treating patients with spinal angiolipomas (SAs) and to evaluate factors relating to its prognosis.
Methods
We retrospectively reviewed the records of patients diagnosed with SAs who received surgical treatment from January 2001 to February 2013.
Results
Twenty-one patients were described. We divide SAs into two types: “intraspinal” and “dumbbell-shaped”. The former were further subclassified as “with lipomatosis” and “without lipomatosis”. Overweight people are more likely to get the “with lipomatosis” type which needs different surgical strategy and/or a diet therapy to get better outcomes.
Conclusion
Diagnosis of SAs should be made with reference to clinical, radiological, and pathological findings. Application of different methods is needed to treat SAs.
Keywords: Diagnosis, Spinal angiolipomas, Prognosis, Spinal tumour, Treatment
Introduction
Spinal angiolipomas (SAs) are benign tumours composed of both mature fatty tissue and abnormal vascular elements [1] that represent a distinct clinical and pathological entity. These lesions are most commonly found in the subcutaneous tissue of the trunk and extremities [2], but other sites have been reported as well. They account for 0.04–1.2 % of all spinal axis tumours [3], and are predominantly found in the epidural space, where they represent 2–3 % of spinal tumours. SAs have rarely been reported and indeed to our knowledge there has been no systemic study of SAs involving a relatively large number of patients. Reports in the literature comprise either individual case reports or literature reviews. Because of its rare occurrence and the misdiagnosis frequently occurred in clinical practices, we believe that sharing information regarding the diagnosis, treatment, and prognosis of patients with SAs is valuable.
In the present report, we summarise our experience treating a relatively large number (n = 21) of patients with SAs over approximately 10 years. More specifically, we describe the correlations between Body Mass Index (BMI) and SAs, the imaging features of SA, including the new classification of SA based on Magnetic Resonance Imaging (MRI) findings, the surgical treatment strategies, and the factors influencing prognosis.
Materials and methods
Patients
We retrospectively reviewed the records of all patients who were diagnosed with SA at the Third Hospital of Peking University from January 2001 to February 2013. The study inclusion criteria were as follows: a diagnosis of SA as confirmed by two pathologists after review of pathological sections, surgical treatment for SA.
The following details were obtained from each patient’s medical records: demographic details, BMI, history of disease, clinical signs, radiological manifestations, tumour pathology, surgical approach used and operative details, pre- and post-operative JOA scores. After surgery, patients were followed up at 3, 6, and 12 months and then once every 6 months, during which clinical follow-up, computed tomography (CT) and/or MRI scans were performed.
The study was approved by the ethics committee of the Third Hospital of Peking University.
Clinical evaluation
Clinical investigations of back or leg symptoms and neurological status were performed before and after surgery using the modified JOA scales for thoracic disease (Table 1; full normal score 11 points) [4, 5]. The JOA recovery rate was calculated as proposed by Hirabayashi et al. [6] as follows: 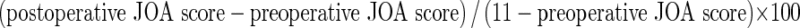 .
.
Table 1.
Modified JOA scores system to evaluate treatment of thoracic spinal disorders
| Physiological test criteria | Score |
|---|---|
| Lower limb function | |
| Unable to walk | 0 |
| Need assistance when walking on flat ground | 1 |
| Need assistance only when going up and down stairs | 2 |
| Can walk clumsily | 3 |
| Normal | 4 |
| Sensory function of the trunk | |
| Severe disturbance | 0 |
| Slight disturbance | 1 |
| Normal | 2 |
| Sensory function of the legs | |
| Severe disturbance | 0 |
| Slight disturbance | 1 |
| Normal | 2 |
| Bladder function | |
| Fully urinary retention | 0 |
| Severe dysuria | 1 |
| Mild dysuria | 2 |
| Normal | 3 |
| Total | 11 |
Radiological manifestations of SAs and tumour classification
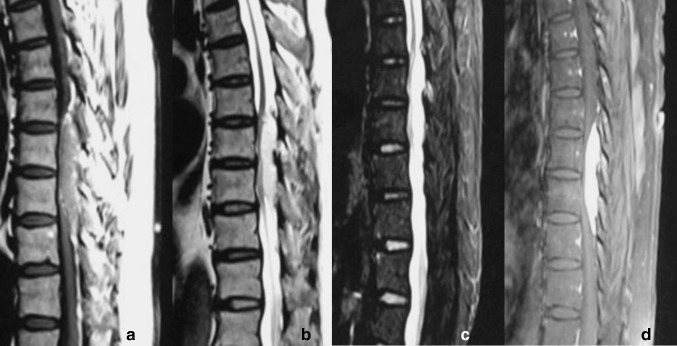
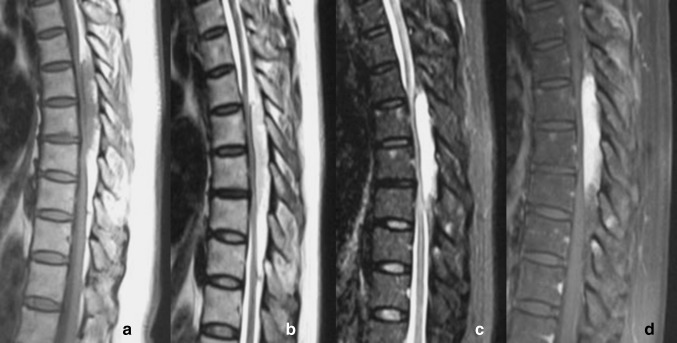
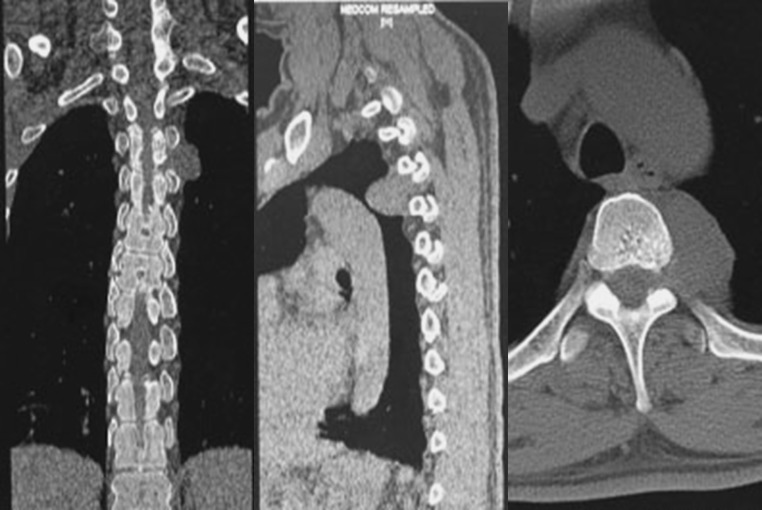
Tumours were classified by examining CT and MRI images of the tumour and surrounding tissue. The images obtained were also used for surgical planning. We initially established the SAs classification as Type I (intraspinal) and Type II (dumbbell-shaped). Type I tumours were characterised by lesions confining to the spinal canal. Type I tumours were further subclassified as Type IA (without lipomatosis) and Type IB (with lipomatosis). Unlike the findings previously described in the literature that SAs were protean, the signals of SAs in the MRI examination were basically the same. The fat content appears hyperintense on T1- and T2-weighted images, meanwhile the vascular component appears isointense to the cord in T1 weighted images, and appears hyperintense on T2-weighted images. Because SAs have a mixture of fat and vascular component, the tumours have basically the same and specific imaging findings. Type IA tumours were characterised by a mainly homogeneous lesion which was isointense on T1 images and hyperintense on T2 images. On fat suppression sequences, the signals were not only diminished, but also had been enhanced. After injection of contrast medium, SAs were strongly enhanced on fat-saturated images (Fig. 1). For Type IB tumours, the portions rich in vessels were consistent with the description above, whereas there was lipomatosis in its upper and/or lower segments which appear hyperintense on T1- and T2-weighted images. On fat suppression sequences, lipomatosis were weakened signals, and no enhancement performance was observed (Fig. 2). Type II tumours were characterised by tumours expanding out of the intervertebral foramina (Fig. 3).
Fig. 1.
Type IA (without lipomatosis) tumour. a In sagittal T1-weighted images, the tumour was basically homogeneous medium signal. b The tumour was T2WI high signal. c In fat suppression images, the signal was still high. d Intense homogeneous enhancement
Fig. 2.
Type IB (with lipomatosis) tumour . a In sagittal T1-weighted images, the tumour’s signal was inhomogeneous, isointense signal was present in center part, and high signal in the surrounding part. b In T2WI, signal was present slightly high in center part, and hyperintense in the surrounding part. c In fat suppression images, the signal was total reversed. d The central part had intense homogeneous enhancement, while the surrounding part maintain low signal
Fig. 3.
Type II tumours (dumbbell-shaped) Tumours expanding out of the intervertebral foramina
Pathology and diagnosis
All tumours were pathological examined by two independent, experienced pathologists. Histologically, SAs are composed of mature adipose tissue and plentiful blood vessels, features of which are either normal or mimicking capillary angiomas. The fatty tissue is of the mature type and shows no remarkable findings. A thin capsule may surround the lesion. Atypia, pleomorphism, mitotic figures and karyotypical abnormalities were all not found. Observing fibrin thrombus can help diagnosis.
Statistical analysis
All data were presented as means ± SDs. Statistical differences between variables were compared using independent-samples T tests. All statistical assessments were considered significant if P < 0.05. Statistical analyses were performed using SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Demographic characteristics
A total of 21 patients (12 men and 9 women) met the inclusion criteria. The mean patient age was 52 ± 13 years (range 19–74 years); while the mean history of the disease before diagnosis was 31 months (range 12 h–30 years). According to the previously classified methods, there were 17 cases of non-infiltrating spinal angiolipoma and 4 cases of infiltrating spinal angiolipoma. Spinal angiolipoma locations included the thoracic segment (n = 15), lumbar segment (n = 3), lower cervical segment (n = 2), and thoracolumbar segment (n = 1), and from another point of view, it included the extradural position (n = 19) and dumbbell shape (n = 2). Among these, one patient had concomitant intradural schwannoma, and five patients had concomitant multiple vertebral hemangioma (Table 2). No patient received another therapy method except for surgery.
Table 2.
Basic information of patients with spinal angiolipomas
| Patient | Image type | Sex | Age (years) | BMI | Disease history (months) | Level | Location | Axial position | Preop JOA score | Postop JOA score | Infiltrating or not | Concomitant disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IA | M | 50 | – | 60 | L3–L4 | Extradural | Dorsal | 3 | – | No | – |
| 2 | IA | M | 53 | 26.0 | 12 | T4–T7 | Extradural | Dorsal | 8 | 10 | No | – |
| 3 | IA | M | 58 | 28.6 | 24 | T9–T10 | Extradural | Dorsal | 7 | 11 | No | – |
| 4 | IA | F | 41 | 29.0 | 1 | T5–T6 | Extradural | Dorsal | 9 | – | No | – |
| 5 | IA with haemorrhage | F | 19 | – | 12 h | C3–C6 | Extradural | Dorsal | – | – | No | – |
| 6 | IA with haemorrhage | F | 26 | – | 2 weeks | C4–C6 | Extradural | Dorsal and left | – | – | No | – |
| 7 | IA | F | 63 | 27.8 | 12 | T7–T10 | Extradural | Dorsal | 0 | 10 | No | Multiple vertebral hemagioma (vertebral body of T10, L1, L2) |
| 8 | IA | F | 62 | 24.0 | 12 | T8–10 | Extradural | Dorsal | 9 | 11 | No | |
| 9 | IA | F | 74 | 24.1 | 6 | L4–L5 | Extradural | Left | 8 | 11 | No | Schwannoma,L5S1 |
| 10 | IA | M | 55 | 25.7 | 6 | T3–T5 | Extradural | Right | 9 | 11 | No | Multiple vertebral hemagioma (vertebral body of T1, T4) |
| 11 | IA | M | 62 | 25.2 | 360 | L4–L5 | Extradural | Dorsal | 10 | 11 | Yes | – |
| 12 | IB | M | 61 | 28.2 | 18 | T11–L3 | Extradural | Dorsal | 9 | – | Yes | – |
| 13 | IB | F | 43 | 30.4 | 24 | T7–T9 | Extradural | Dorsal and right | 9 | 10 | No | Vertebral hemagioma (vertebral body and accessories of T9) |
| 14 | IB | M | 57 | 29.3 | 8 | T5–T8 | Extradural | Dorsal | 8 | – | No | Multiple vertebral hemagioma (vertebral body of T6, T12, L1 and left accessories of T9) |
| 15 | IB | F | 69 | 29.4 | 24 | T4–T6 | Extradural | Dorsal and right | 10 | 10 | No | – |
| 16 | IB | M | 62 | 25.8 | 24 | T4–T6 | Extradural | Dorsal | 4 | 8 | No | Multiple vertebral hemagioma (vertebral body of T1, T7, T9, L2) |
| 17 | IB | F | 47 | 28.1 | 2 | T2–T3 | Extradural | Dorsal | 7 | 10 | Yes | – |
| 18 | II | F | 50 | – | 24 | T2–T4 | Dumbbell-shape | Dorsal and left | – | – | No | – |
| 19 | II | M | 37 | – | 5 | T4–T7 | Dumbbell-shape | Dorsal | – | – | No | – |
| 20 | – | F | 51 | – | 24 | T4–T5 | Extradural | Dorsal | – | – | – | – |
| 21 | – | F | 59 | – | 12 | T8–T11 | Extradural | – | – | – | – | – |
Radiological manifestations and tumour classification
Two patients lacking of radiological information were excluded from tumour classification. Finally, a total of 17 patients had Type I tumours, whereas two patients had Type II tumours. Of the patients with Type I tumours, eleven had Type IA tumours and six had Type IB tumours.
Pathology and diagnosis
Histological examination of the 21 tumours disclosed angiolipoma. It was a special tumour composed of mature lipocytes with delicate proliferating blood vessels (Fig. 4). Atypia, pleomorphism, and mitotic figures of both adipose and angiomatous component were not encountered.
Fig. 4.
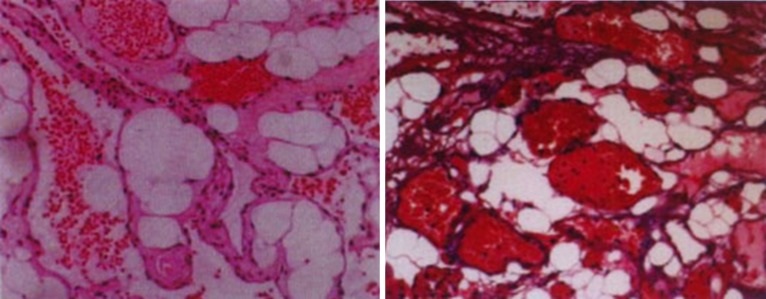
Representative hematoxylin and eosin stained spinal angiolipomas samples (magnification = ×10)
Immunohistochemical study was done in two patients, and the result was: SMA (+), CD31 (+), Elastic fiber staining (+), S-100 (−) (patient No. 15); CD34 (+), CD31 (+), S-100 (+), ki-67 (+<1 %), CD56 (−), Inhibin-a (−), NSE (−), ck mixed (−), EMA (−), GFAP (−) (patient No. 20). Fibrin thrombus can be found sometimes.
Surgery
All of 21 patients received total resection. Laminectomy was carried out in 13 patients, unilateral hemilaminectomy in six patients, recapping laminoplasty in one patient and internal fixation in one patient. Generally speaking, the tumours involving fewer segments were removed en bloc, and the ones involving more segments were taken out in a piecemeal but not an intralesional manner.
Type IA tumours
This type had rich vascular tissue in the tumours, and always had a clear boundary, even in the infiltrating type. Adhesions between tumours and dura mater were not serious, the bone damage was also caused by tumours’ expansive oppression, hardened edge of the bone often exists, boundaries between the two masses were basically clear, dissection of the tumour and then en bloc resection of tumour is not difficult.
For the infiltrating SAs, insufficient surgical field may be a barrier to achieve en bloc resection of tumours. However, in this case, the tumours can be cut into blocks and then removed. No matter whether the tumour was infiltrating or not, full resection of the tumours was achieved by simple posterior approach in these 13 patients.
Considering the slight adhesions, a complete surgical dissection can be accomplished by making a smaller incision and restricted bony opening. Unilateral hemilaminectomy, for example, was a choice.
Type IB tumours
This type had lipomatosis in its upper and/or lower segments. However, many neurosurgeons and orthopedists were not aware of the significance of its existence. In the vast majority of cases, operators only dealt with the part rich in vessels, left the lipomatosis in its original shape and so did our six patients.
Type II tumours
The lesion involved the spinal canal inside and outside, and forms a dumbbell-shaped structure. So, unilateral vertebral arch often needs to be removed to provide adequate operative space. In patient 18, the tumour adhered to pleura membranes, but blunt dissection of the tumour was still able to implement. After removing the sternocostal join, the mass was entirely resected.
Clinical evaluation and follow-up
Table 3 summarises the overall outcomes for the 21 patients. The mean BMI of group IA was less than group IB (26.3 ± 2.0 vs 28.5 ± 1.6; P = 0.04). Surgery provided all of them with symptomatic relief to some degree. The mean preoperative JOA score was pretty close between the IA and IB group (7.0 ± 3.3 vs 7.8 ± 2.1; P = 0.60), whereas the IA had better prognosis (10.7 ± 0.5 vs 9.5 ± 1.0; P = 0.02). The mean recovery rate was 93.9 ± 12.5 % with IA group and 45.5 ± 32.1 % with IB group. Chemotherapy and radiotherapy were not routine adjuvant treatments for those patients who underwent subtotal resection.
Table 3.
Clinical evaluation data of IA and IB Type SAs
| Patient | IA | IB | P | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
| BMI | – | 26.0 | 28.6 | 29.0 | 27.8 | 24.0 | 24.1 | 25.7 | 25.2 | 28.2 | 30.4 | 29.3 | 29.4 | 25.8 | 28.1 | |
| Means ± SDs | 26.3 ± 2.0 | 28.5 ± 1.6 | 0.04* | |||||||||||||
| Preop JOA score | 3 | 8 | 7 | 9 | 0 | 9 | 8 | 9 | 10 | 9 | 9 | 8 | 10 | 4 | 7 | |
| Means ± SDs | 7.0 ± 3.3 | 7.8 ± 2.1 | 0.60 | |||||||||||||
| Postop JOA score | – | 10 | 11 | – | 10 | 11 | 11 | 11 | 11 | – | 10 | – | 10 | 8 | 10 | |
| Means ± SDs | 10.7 ± 0.5 | 9.5 ± 1.0 | 0.02* | |||||||||||||
| Recovery rate | – | 66.7 % | 100.0 % | – | 90.9 % | 100.0 % | 100.0 % | 100.0 % | 100.0 % | – | 50.0 % | – | 0.0 % | 57.1 % | 75.0 % | |
| Means ± SDs | 93.9 ± 12.5 % | 45.5 ± 32.1 % | 0.005* | |||||||||||||
* Indicates statistical significance (P < 0.05)
Discussion
Herein, we have retrospectively reviewed the clinical findings regarding diagnosis, surgical treatment, and outcomes for 21 cases of SAs from a single institution over the past decade. To our knowledge, our report is the largest single centre report on SAs and hence, we believe that it is a valuable contribution to the existing literature on this rare form of SAs.
Demographic characteristics
In many aspects, our cohort of patients with SAs are consistent with previous literatures: SAs predominate among the 40- to 60-year age group [7–9], duration of symptoms is usually long in SAs [10], SAs generally arise from the posterior epidural space at the thoracic levels and usually extend over several vertebral bodies [11].
However, there are some differences, too. We summarise the differences as three interesting phenomenon: (1) This article reports two cervical SAs (c3–c6 and c4–c6). For the tumours occurring in the cervical segments, the patients’ age is 19 and 26, the history of disease before diagnosis is 12 h and 2 weeks, respectively. Besides the two patients, all suffered from epidural haemorrhage which was confirmed by surgery. The two patients were very dissimilar to others. Groen’s one article [12] which discussed spontaneous spinal epidural hematomas also showed the related interesting phenomenon: all vertebral segments can be affected by spontaneous spinal epidural hematomas, with a predominance of the C6-around area (Fig. 4). Though I do not have rigorous evidences to support a hypothesis right now, I still think it is a phenomenon worth exploring. (2) Although some literature suggested that a female predominance was observed in SAs [13], in our cohort of patients, there were more men (n = 12) than women (n = 9). Of course, this may be due to the result in a single center selection bias. (3) There were 23.8 % cases (5/21) that had the coexistence of vertebral hemangiomas. This seems higher than the prevalence in the general population. On the other hand, Barzin [14] pointed out that the incidence can be influenced by diagnostic tools. The incidence was higher in MRI researches (27 %) than in autopsy and X-ray studies (10–12 %). My samples were relatively small, further studies of larger samples were required to find out the truth.
Clinical presentation
From a clinical point of view, extradural angiolipomas do not differ from other benign space-occupying spinal lesions. Subjective complaints are mostly of sensory disorders and motor deficits below the level of the lesion [15] that often progress to weakness in the lower limbs for long periods with sphincter dysfunction in the later stages [16] (Table 4). Because the SAs are predominately found in the dorsal part of the spinal cord, the appearance of the sensory disorders is ahead of the motor deficits. Duration of symptoms is usually long in both types.
Table 4.
Clinical findings in 21 patients with spinal angiolipomas
| Clinical characteristic | No. of patients | Percentage |
|---|---|---|
| Numbness or paraesthesia | 17 | 81 |
| Leg weakness | 14 | 67 |
| Pain | 8 | 38 |
| Sphincter disturbance | 1 | 5 |
| Ataxia | 1 | 5 |
Some patients presented with a remitting and relapsing course in both groups. These relapses may attribute to the characteristics of vascular lesions. Pregnancy [17, 18] may promote such change. Sudden onset or worsening of neurological symptoms occurred when there is a rapid increase in tumour size due to intratumoral thrombosis, haemorrhage, or a steal phenomenon [19]. In addition, there was a history of weight gain coincident with the onset of symptoms in two cases [20, 21].
Radiological manifestations and tumour classification
In most instances, spinal radiographs were normal with only a few reports of bone involvement, i.e. erosion of the pedicles or vertebral body, trabeculation, widened pedicles and foraminal widening. Computed tomographic examination can give some hints and allow for the assessment of bone degradation compressed by the tumour. Magnetic resonance imaging was particularly useful for determining the features of a neoplasm. Besides, the presentation of SAs did not vary greatly as said before in the literature. They had a substantially uniform composition, so they had special and unique performances in T1, T2, fat-suppress, and enhancement sequences.
Previously, SAs were divided into infiltrating and non-infiltrating types. The infiltrating type was more complex and hardly reached complete resection. They should be considered and treated differently [22–25]. However, some scholars found out that the two types were basically the same [18], and these literatures were mostly before 1990. More recently, some experts also reported several full-resection cases. In addition, based on the author’s experience, the bone damage of the infiltrating type was caused by tumours’ expansive oppression, boundaries between the two masses were basically clear. Be more patient, total resection of the tumour was still possible.
Also, the outcomes of the infiltrating and non-infiltrating types had no differences [26]. The previous classification is meaningless for surgery design and outcome assessment and a new classification strategy is proposed in this article.
Pathology and diagnosis
Histologically, a tumour composed of mature lipocytes with delicate proliferating blood vessels was seen [27]. The ratio of fat to vessels was variable [3], but the two components were uniformly mixed together. Tumours with an abundance of smooth muscle proliferation were further subclassified as angiomyolipomas [3]. Atypia, pleomorphism, and mitotic figures of both adipose and angiomatous component were never encountered [18].
Treatment options and results
In the present report, we have described a classification system for Spinal angiolipomas, which may be used as a guide for treatment. Type IA tumours always has a clear boundary and involved less vertebral segment, so the treatment method is relatively simple and minimally invasive surgery skills can be tried. Overweight people are more likely to get the Type IB, and its results are slightly worse than Type IA. For this point, I think, the spinal cord compression caused by lipomatosis is the reason. The operators must be aware of the importance of lipomatosis, and make removal of the surrounding fat as much as possible when operating [28]. For the patients whose lipomatosis was not dealt with or cannot be fully removed, a diet therapy aiming at losing weight may be a choice. Fogel et al. [29] reported that the patients who managed conservatively by weight loss improved in 81.8 % of the cases in simplex spinal epidural lipomatosis. Type II tumours are more complex, and internal fixation may be needed. Recurrence is exceptional in SAs.
Conclusions
In summary, we have retrospectively described our experience treating a relatively large number of patients with SAs over a period of approximately 10 years. The diagnosis of SAs should be made with reference to clinical, radiological, and pathological examination findings. SAs should be treated with surgical resection, and most patients have a good prognosis.
Conflict of interest
None.
Contributor Information
Yu Si, Email: siyudr@yahoo.com.
Zhenyu Wang, Email: wzyu502@yahoo.com.
References
- 1.Garg A, Gupta V, Gaikwad S, et al. Spinal angiolipoma: report of three cases and review of MRI features. Australas Radiol. 2002;46:84–90. doi: 10.1046/j.1440-1673.2001.01001.x. [DOI] [PubMed] [Google Scholar]
- 2.Kujas M, Lopes M, Lalam TF, et al. Infiltrating extradural spinal angiolipoma. Clin Neuropathol. 1999;18:93–98. [PubMed] [Google Scholar]
- 3.Fourney DR, Tong KA, Macaulay RJB, et al. Spinal angiolipoma. Can J Neu Sci. 2001;28:82–88. doi: 10.1017/s0317167100052628. [DOI] [PubMed] [Google Scholar]
- 4.Ando K, Imagama S, Wakao N, et al. Examination of the influence of ossification of the anterior longitudinal ligament on symptom progression and surgical outcome of ossification of the thoracic ligamentum flavum: a multicenter study. J Neurosurg Spine. 2012;16:147–153. doi: 10.3171/2011.10.SPINE11296. [DOI] [PubMed] [Google Scholar]
- 5.Association JO Scoring system for cervical myelopathy. J Jpn Orthop Assoc. 1994;68:490–503. [Google Scholar]
- 6.Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Rubin G, Gornish M, Sandbank J, et al. Spinal extradural angiolipoma. Case report and review of the literature. Spine. 1992;17:719–724. doi: 10.1097/00007632-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Turgut M. Spinal angiolipomas: report of a case and review of the cases published since the discovery of the tumour in 1890. Br J Neurosurg. 1999;13:30–40. doi: 10.1080/02688699944159. [DOI] [PubMed] [Google Scholar]
- 9.Samdani AF, Garonzik IM, Jallo G, et al. Spinal angiolipoma: case report and review of the literature. Acta Neurochir (Wien) 2004;146:299–302. doi: 10.1007/s00701-003-0196-x. [DOI] [PubMed] [Google Scholar]
- 10.Gelabert-Gonza’lez M, Garc’a-Allut A. Spinal extradural angiolipoma. Report of two cases and review of the literature. Eur Spine J. 2009;18:324–335. doi: 10.1007/s00586-008-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leu NH, Chen CY, Shy CG, et al. MR imaging of an infiltrating spinal epidural angiolipoma. AJNR. 2003;24:1008–1011. [PMC free article] [PubMed] [Google Scholar]
- 12.Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir (Wien) 2004;146:103–110. doi: 10.1007/s00701-003-0160-9. [DOI] [PubMed] [Google Scholar]
- 13.Ghanta RK, Koti K, Dandamudi S. Spinal epidural angiolipoma: A rare cause of spinal cord compression. J Neurosci Rural Pract. 2012;3:341–343. doi: 10.4103/0976-3147.102617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barzin M, Maleki I (2009) Incidence of vertebral hemangioma on spinal magnetic resonance imaging in Northern Iran. Pak J Biol Sci 15;12:542–544 [DOI] [PubMed]
- 15.Miki T, Oka M, Shima M, et al. Spinal angiolipoma. A case report. Acta Neurochir (Wien) 1981;58:115–119. doi: 10.1007/BF01401689. [DOI] [PubMed] [Google Scholar]
- 16.Provenzale JM, McLendon RE. Spinal angiolipomas: MR features. AJNR Am J Neuroradiol. 1996;17:713–719. [PMC free article] [PubMed] [Google Scholar]
- 17.Preul MC, Leblanc R, Tampieri D, et al. Spinal angiolipomas: report of three cases. J Neurosurg. 1993;78:280–286. doi: 10.3171/jns.1993.78.2.0280. [DOI] [PubMed] [Google Scholar]
- 18.Trabulo A, Cerqueira J, Roque P, et al. Spinal angiolipomas revisited: two case reports. Acta Neurochir (Wien) 1996;138:1311–1319. doi: 10.1007/BF01411061. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumi S, Nonaka Y, Abe Y, et al. Spinal angiolipoma in a pregnant woman presenting with acute epidural hemorrhage. J Clin Neurosci. 2011;18:849–851. doi: 10.1016/j.jocn.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Guegan Y, Fardoun R, Launois B, et al. Spinal cord compression by extradural fat after prolonged corticosteroid therapy. J Neurosurg. 1982;56:267–269. doi: 10.3171/jns.1982.56.2.0267. [DOI] [PubMed] [Google Scholar]
- 21.Llewellyn CG. Spinal angiolipoma mimicking extradural lipomatosis. Can Assoc Radiol J. 1996;47:51–53. [PubMed] [Google Scholar]
- 22.Han SR, Yee GT, Choi CY, et al. Infiltrating spinal angiolipoma. J Korean Neurosurg Soc. 2012;52:161–163. doi: 10.3340/jkns.2012.52.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haji FA, Patel YK, Ang LC, et al. A case of mistaken identity: spinal epidural angiolipoma. Can J Neurol Sci. 2011;38:357–359. doi: 10.1017/s0317167100011628. [DOI] [PubMed] [Google Scholar]
- 24.Chotai S, Hur JS, Moon HJ, et al. Spinal angiolipoma–case report. Neurol Med Chir (Tokyo) 2011;51:539–542. doi: 10.2176/nmc.51.539. [DOI] [PubMed] [Google Scholar]
- 25.Dufrenot L, Pelé E, Cursolle JC, et al. Spinal epidural angiolipoma: a case report. Ann Pathol. 2010;30:30–32. doi: 10.1016/j.annpat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Guzey Feyza Karagoz, et al. Lumbar extradural infiltrating angiolipoma: a case report and review of 17 previously reported cases with infiltrating spinal angiolipomas. Spine J. 2007;7:739–744. doi: 10.1016/j.spinee.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Lin JL, Lin F. Two entities in angiolipoma. Cancer. 1974;34:720–727. doi: 10.1002/1097-0142(197409)34:3<720::AID-CNCR2820340331>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 28.Murphey MD, Carroll JF, Flemming DJ, et al. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24:1433–1466. doi: 10.1148/rg.245045120. [DOI] [PubMed] [Google Scholar]
- 29.Guy R. Fogel, Paul Y. Cunningham, JDb et al.(2005) Spinal epidural lipomatosis: case reports, literature review and meta-analysis. Spine J 5:202–211 [DOI] [PubMed]