Abstract
Purpose
The pathomechanisms of pain resulting from lumbar disc herniation have not been fully elucidated. Prostaglandins and cytokines generated at the inflammatory site produce associated pain; however, non-steroidal anti-inflammatory drugs and steroids are sometimes ineffective in patients. Tetrodotoxin-sensitive voltage-gated sodium (NaV) channels are related to sensory transmission in primary sensory nerves. The sodium channel NaV1.7 has emerged as an attractive analgesic target. The purpose of this study was to evaluate pain-related behavior and expression of NaV1.7 in dorsal root ganglia (DRG) after combined sciatic nerve compression and nucleus pulposus (NP) application in rats.
Methods
Rats were divided into three groups and underwent either sciatic nerve compression with NP for 2 s using forceps (n = 20), sham operation with neither compression nor NP (n = 20), or no operation (controls, n = 20). Mechanical hyperalgesia was measured every second day for three weeks using von Frey filaments. NaV1.7 expression in L5 DRG was examined 7 and 14 days after surgery using immunohistochemistry. The number of neurons immunoreactive for NaV1.7 was compared among the three groups.
Results
Mechanical hyperalgesia was found over the 14-day observation in the nerve compression plus NP application group, but not in the sham-operated or control groups (P < 0.05). NaV1.7 expression in L5 DRG was up-regulated in the nerve compression plus NP application group, compared with sham-operated and control rats (P < 0.01).
Conclusions
Our results indicate that nerve compression plus NP application produces pain-related behavior. We conclude that NaV1.7 expression in DRG neurons may play an important role in mediating pain from sciatic nerves after compression injury and exposure to NP.
Keywords: Rat, Pain, Nerve, Compression, NaV1.7, Nucleus pulposus
Introduction
Radicular pain is a common symptom of lumbar disc herniation, which is caused by mechanical compression and inflammation of nerve roots in animals and humans [1–3]. Prostaglandins and cytokines generated at the inflammatory site produce associated pain in rats [4, 5]. In humans suffering from lumbar disc herniation, non-steroidal anti-inflammatory drugs are somewhat effective for pain because these drugs decrease inflammation at the site of the herniation; however, the effect is insufficient [6].
Recently, cytokines such as interleukins 1 and 6 (IL-1, IL-6) and tumor necrosis factor-alpha (TNF-α) have been strongly linked to radicular pain [2, 7]. Cohen et al. [8] reported a safety study of transforaminal epidural injection of the TNF-α inhibitor etanercept for the treatment of sciatica caused by disc herniation in 24 patients and found clinical effectiveness for pain due to disc herniation. A single intravenous infusion of the TNF-α inhibitor infliximab was reported to be effective in treating sciatic pain caused by lumbar disc herniation [9]. On the other hand, intravenous infusion of infliximab was compared to a placebo by a Finnish group in the first randomized controlled trial of this inhibitor, and the results were disappointing [10, 11].
Voltage-gated sodium (NaV) channels are transmembrane proteins that conduct current responsible for the rapid depolarization of excitable cells [12]. To date, nine functionally distinct mammalian NaV channel alpha subunits (NaV1.1–NaV1.9) have been cloned, functionally expressed, and characterized [13]. In recent years, the tetrodotoxin-sensitive sodium channel NaV1.7 has emerged as an analgesic target and polymorphisms in the Na(V)1.7 channel may influence susceptibility to pain [14, 15].
The SNC9A gene encoding the alpha subunit of NaV 1.7 causes painful inherited neuropathies such as primary erythromelalgia and paroxysmal extreme pain disorder [16–18], whereas truncation and loss-of-function mutations in SCN9A result in a congenital insensitivity to all forms of pain [19]. In the orthopedic field, the only study published to date investigating genetic factors that contribute to painful knee osteoarthritis (OA) has implicated the SCN9A gene [20]. Furthermore, Valdes et al. [21] have reported that an amino acid change (R1150 W) in the NaV1.7 α-chain is associated with multiple regional pain in knee OA patients. Black et al. [22] reported that Nav1.3, Nav1.7, and Nav1.8 are expressed in painful human neuromas.
However, changes in the expression of NaV1.7 in primary sensory nerves in a model of lumbar disc herniation have not been reported. The purpose of this study was to evaluate pain-related behavior and the expression of NaV1.7 in dorsal root ganglia (DRG) after sciatic nerve compression and application of nucleus pulposus (NP) in rats.
Materials and methods
All protocols for animal procedures were approved by the Ethics Committees of Chiba University in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996 revision).
Harvesting of NP from lumbar intervertebral discs of donor rats
Ten 6-week-old male Sprague–Dawley rats (200–250 g) were anesthetized with intraperitoneal (i.p.) injections of sodium pentobarbital (40 mg/kg). NP was harvested from lumbar intervertebral discs (L2/3 to L4/5) and was used in the following experiments.
Rat nerve compression + NP model
Sixty 6-week-old male Sprague–Dawley rats (200–250 g) were anesthetized with sodium pentobarbital (40 mg/kg, i.p.), and their left sciatic nerves were exposed. The sciatic nerves were compressed, and NP was applied once for 2 s with 2-mm-wide smooth forceps (NP + nerve compression group; n = 20). The sciatic nerve was pinched gradually, and the pinching was stopped if the rat showed cramps in its hind paw. In sham-operated rats, the left sciatic nerves were exposed, but the sciatic nerves were not pinched, and NP was not applied (sham-operated group, n = 20). Another group of rats (control group, n = 20) received no surgery and were used as controls. Of the 60 rats used in these studies, 30 were used only for the assessment of pain-related behavior, and a separate group of 30 was used only for immunohistochemical analysis.
Evaluation of mechanical hyperalgesia
Tactile hyperalgesia was evaluated in 10 rats each from the control, sham-operated, and NP + nerve compression groups. There were no animal dropouts on any day. Left hind paw mechanical pain thresholds were assessed before surgery and during 20 days after surgery using von Frey filaments with a bending force of 1.20 g (noxious stimulation). The von Frey filament was applied to the plantar surface of the left hind paw for five trials at approximately 5-min intervals. The responses to these stimuli were scored as follows: 0, no response; 1, withdrawal from the von Frey filament; and 2, immediate flinching or licking of the hind paw. The scores from the five trials in each animal were added to give a total score, from 0 to 10 for each animal, and the average nociceptive score was calculated for each group [23]. Mean nociceptive score = Σ total score for each animal/number of animals (n = 10).
Immunohistochemical detection of NaV1.7 in L5 DRG
The expression of NaV1.7 was evaluated in 10 rats each from the control, sham-operated, and NP + nerve compression groups. At 7 days (each group, n = 5) and 14 days (each group, n = 5) after surgery, rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and perfused transcardially with 250 mL of 4 % paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). The left L5 DRG was resected and cut into 10-μm sections on a cryostat. Sections were mounted on poly-l-lysine-coated slides and were incubated for 60 min in a blocking solution containing 0.3 % Triton X-100 and 5 % skim milk in 0.01 M phosphate buffered saline at room temperature. Sections were processed for immunohistochemical detection of NaV1.7 by incubation with rabbit antibody to NaV1.7 (1:1,000; Alomone Labs Ltd., Jerusalem, Israel) for 20 h at 4 °C, followed by incubation with goat anti-rabbit Alexa 488-fluorescein-conjugated antibody (1:400; Molecular Probes, Eugene, OR). The sections were examined using a fluorescence microscope (Nikon, Japan). Immunoreactive cells in 10 sections from each ganglion were counted at 400× magnification using a counting grid. The number of NaV1.7-immunoreactive cells per 0.0225 mm2 was counted in DRG sections and averaged for each animal in a blinded manner. Observers were also blinded to the condition of the animal.
Statistical analysis
Hind paw withdrawal latencies were compared using one-way analysis of variance (ANOVA) for repeated measurements. For multiple comparisons, we used Dunnett’s post hoc test. Comparison of the number of NaV 1.7-immunoreactive neurons among groups was made using a one-way ANOVA followed by Dunnett’s post hoc test. Values of P < 0.05 were considered statistically significant, and error bars indicate standard error of the mean (SEM).
Results
Mechanical hyperalgesia caused by NP + nerve compression
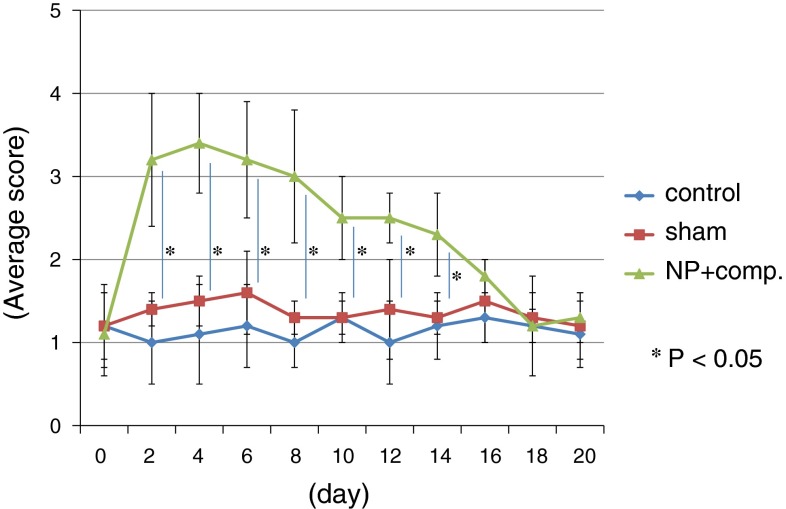
Through day 14, animals in the NP + nerve compression group displayed a level of mechanical hyperalgesia that was significantly greater than that exhibited by animals in the control and sham-operated groups (P < 0.05) (Fig. 1). There was no significant difference in pain score between the control and sham-operated groups (Fig. 1).
Fig. 1.
Comparison of mechanical hyperalgesia among the control, sham-operated, and NP + compression groups. Data are shown as mean ± SEM. A high score indicates increased pain
NaV1.7 expression in DRG
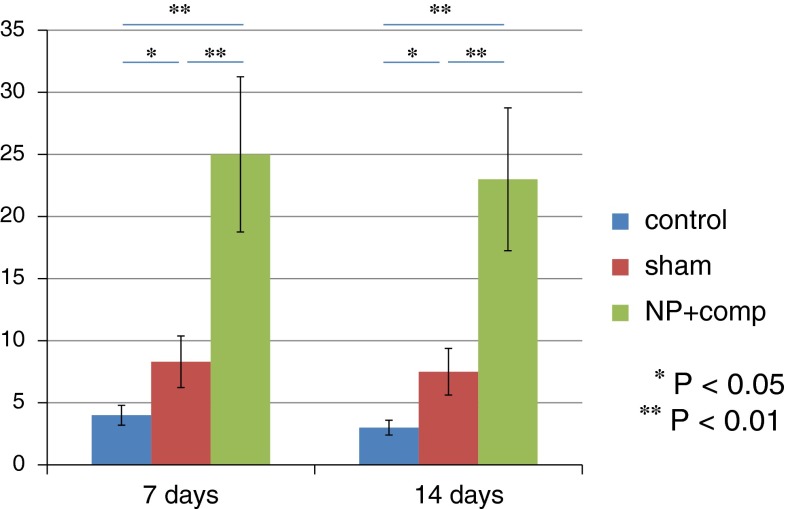
No evidence of NaV1.7-immunoreactivity was detected in DRG neurons from rats in the control group, but NaV1.7-immunoreactivity was observed in small and intermediate-size DRG neurons from rats in the sham-operated and NP + nerve compression groups (Fig. 2). The number of NaV1.7-immunoreactive DRG neurons in the NP + nerve compression group was significantly greater than that in the control and sham-operated rats on days 7 and 14 (P < 0.01) (Fig. 3).
Fig. 2.
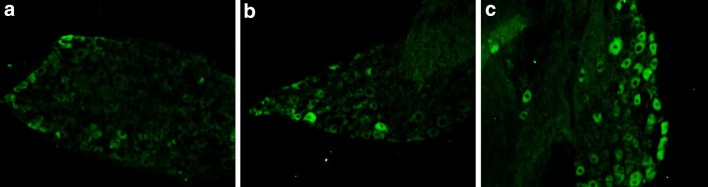
NaV1.7 immunoreactivity in DRG neurons from control (a), sham-operated (b), and NP + compression (c) groups on day 7
Fig. 3.
The average number of NaV1.7-immunoreactive DRG neurons on days 7 and 14 in the control, sham-operated, and NP + compression groups. Data are shown as mean ± SEM
Discussion
In the current study, the combination of sciatic nerve compression and application of NP produced long-lasting pain-related behavior in rats. In addition, this procedure resulted in a significant up-regulation of NaV1.7 expression in DRG neurons. We conclude that NaV1.7 expression in DRG neurons may play an important role in mediating pain from sciatic nerves subjected to compression injury and exposure to NP.
Radicular pain caused by lumbar disc herniation is induced by mechanical compression and inflammation [1, 2]. Recently, cyclooxygenase-2, TNF-α, and cytokines such as IL-1 and IL-6 have been strongly linked to the effects of NP on nerve roots in the lumbar spine [2, 24]. These studies have been performed in models using the application of NP to nerve roots to mimic the effect of disc herniation in humans. It has also been reported that nerve growth factor and brain-derived neurotrophic factor, which are related to inflammatory pain, and TNF-α are activated in the spinal cord and DRG following lumbar disc herniation, and that their expression is closely associated with pain [25–27]. The administration of TNF-α inhibitors to animal models with lumbar disc herniation has been shown to prevent the injury-related reduction in nerve conduction velocity and also seems to limit the nerve-fiber injury, intracapillary thrombus formation, and intraneural edema [28]. In this regard, cytokines and growth factors have an important role in pain transmission caused by compression and exposure to NP.
As mentioned above, these models mimic the exposure to NP encountered in human lumbar disc herniation. On the other hand, in the current study, we used a rat model of sciatic nerve compression plus inflammation by NP. Several authors have reported on the validity of this model. Sakuma used this model to evaluate pain behavior using the CatWalk system, a computer-assisted apparatus for analyzing gait that provides an automated means for assessing gait function during pain [29]. Their analysis showed significant differences compared to controls in four pain parameters. Norimoto et al. [30] reported up-regulation of TNF-α and calcitonin gene-related peptide in DRG neurons during the acute pain phase in the same model. The mechanical allodynia was ameliorated by the TNF-α inhibitor etanercept. Finally, Yamashita et al. [31] used TNF-α knockout mice and examined the importance of TNF-α in the response to NP in nerve injury-related pain. They concluded that TNF-α, as a component of NP, was important for the production of radicular pain accompanied by long-lasting degeneration of DRG neurons after sciatic nerve injury. The model used in this study evokes the same pain-related behavior, cytokine expression, and changes in neurotransmitters in DRG neurons as models in which NP is applied to the nerve root. Therefore, we conclude the model in the current study is a valid disc herniation model.
The role of NaV1.7 in a disc herniation model has not been reported previously. The current model includes elements of inflammation and compressive nerve injury, as mentioned above. Peripheral inflammation increases the expression of NaV1.7 in peripheral nerves and DRG [32–34]. Inflammation in rat hind paws was shown to induce NaV up-regulation in axons of digital nerves [32], and peripheral inflammation following carrageenan injection was reported to increase the expression of NaV1.7 in DRG neurons [33]. In addition, the expression of NaV1.7 in a distinct population of DRG innervating the rat knee joint was increased in a model of chronic inflammatory joint pain [34]. Furthermore, some authors have demonstrated a decrease in inflammatory hyperalgesia in response to herpes vector-mediated knockdown of NaV1.7 in primary afferents. These results provide clear evidence for the involvement of increased NaV1.7 expression in nociceptive neurons in the development of inflammatory hyperalgesia [35, 36]. Furthermore, NaV1.7 has an important role in nerve injury pain. Shields et al. [37] reported that NaV1.7 in DRG are implicated in injury-induced neuronal hyperexcitability in a burn-injury model. They suggested NaV1.7-blocking drugs may be effective in burn patients. In this regard, an increase in the expression of NaV1.7 in DRG neurons in the current study may be caused by both compression of sciatic nerve and inflammation due to NP. Therefore, blockade of NaV1.7 may be a therapeutic strategy for the treatment of pain in patients with lumbar disc herniation.
There are some limitations of this study. Despite changes in the expression of NaV1.7, we found no histological changes in the sciatic nerves after the application of NP. Second, we only used the NaV1.7 sodium channel subtype as a marker, and did not examine other NaV channels. Further study will be needed to strengthen our hypothesis.
In conclusion, the combination of sciatic nerve compression and application of NP produced pain-related behavior and increased NaV1.7 expression in rat DRG neurons. Blockade of NaV1.7 may be a therapeutic strategy for the treatment of pain in patients with lumbar disc herniation in the future.
Acknowledgments
Conflict of interest
None.
References
- 1.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:132–1425. [PubMed] [Google Scholar]
- 2.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Toyone T, Takahashi K, Kitahara H, Yamagata M, Murakami M, Moriya H. Visualisation of symptomatic nerve roots. Prospective study of contrast-enhanced MRI in patients with lumbar disc herniation. J Bone Joint Surg Br. 1993;75:529–533. doi: 10.1302/0301-620X.75B4.8331104. [DOI] [PubMed] [Google Scholar]
- 4.Olmarker K, Storkson R, Berge OG. Pathogenesis of sciatic pain: a study of spontaneous behavior in rats exposed to experimental disc herniation. Spine. 2002;27:1312–1317. doi: 10.1097/00007632-200206150-00013. [DOI] [PubMed] [Google Scholar]
- 5.Olmarker K, Nutu M, Storkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine. 2003;28:1635–1641. doi: 10.1097/01.BRS.0000083162.35476.FF. [DOI] [PubMed] [Google Scholar]
- 6.Hatori M, Kokubun S. Clinical use of etodolac for the treatment of lumbar disc herniation. Curr Med Res Opin. 1999;15:193–201. doi: 10.1185/03007999909114091. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Saito T, Moriya H, Takahashi K. Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine. 2007;32:159–167. doi: 10.1097/01.brs.0000251437.10545.e9. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SP, Bogduk N, Dragovich A, Buckenmaier CC, 3rd, Griffith S, Kurihara C, Raymond J, Richter PJ, Williams N, Yaksh TL. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110:1116–1126. doi: 10.1097/ALN.0b013e3181a05aa0. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimäki J, Paimela L, Kyllönen E, Lindgren KA, Tervonen O, Seitsalo S, Hurri H. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine. 2004;29:2115–2119. doi: 10.1097/01.brs.0000141179.58778.6c. [DOI] [PubMed] [Google Scholar]
- 10.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Järvinen S, Niinimäki J, Veeger N, Seitsalo S, Hurri H. The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine. 2005;30:2724–2728. doi: 10.1097/01.brs.0000190815.13764.64. [DOI] [PubMed] [Google Scholar]
- 11.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Bowman C, Hammond A, Kirkham B, Järvinen S, Niinimäki J, Veeger N, Haapea M, Torkki M, Tervonen O, Seitsalo S, Hurri H. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine. 2006;31:2759–2766. doi: 10.1097/01.brs.0000245873.23876.1e. [DOI] [PubMed] [Google Scholar]
- 12.Rupasinghe DB, Knapp O, Blomster LV, Schmid AB, Adams DJ, King GF, Ruitenberg MJ (2012) Localization of Nav 1.7 in the normal and injured rodent olfactory system indicates a critical role in olfaction, pheromone sensing and immune function. Channels (Austin):103–10 [DOI] [PubMed]
- 13.King GF, Escoubas P, Nicholson GM (2008) Peptide toxins that selectively target insect Na(V) and Ca(V) channels. Channels (Austin) 2:100–16. PMID:18849658. doi:10.4161/chan.2.2.6022 [DOI] [PubMed]
- 14.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007;30:555–563. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Estacion M, Harty TP, Choi JS, Tyrrell L, Dib-Hajj SD, Waxman SG. A sodium channel gene SCN9A polymorphism that increases nociceptor excitability. Ann Neurol. 2009;66:862–866. doi: 10.1002/ana.21895. [DOI] [PubMed] [Google Scholar]
- 16.Waxman SG, Dib-Hajj S (2005) Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med 11:555–62. PMID:16278094. doi:10.1016/j.molmed.2005.10.004 [DOI] [PubMed]
- 17.Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM, Rees M (2006) SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52:767–74. PMID:17145499. doi:10.1016/j.neuron.2006.10.006 [DOI] [PubMed]
- 18.Drenth JP, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 2007;117:3603–3609. doi: 10.1172/JCI33297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG (2006) An SCN9A channelopathy causes congenital inability to experience pain. Nature 444:894–8. PMID:17167479. doi:10.1038/nature05413 [DOI] [PMC free article] [PubMed]
- 20.Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, Drenth JP, Dai F, Wheeler J, Sanders F, Wood L, Wu TX, Karppinen J, Nikolajsen L, Männikkö M, Max MB, Kiselycznyk C, Poddar M, Te Morsche RH, Smith S, Gibson D, Kelempisioti A, Maixner W, Gribble FM, Woods CG. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci USA. 2010;107:5148–5153. doi: 10.1073/pnas.0913181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdes AM, Arden NK, Vaughn FL, Doherty SA, Leaverton PE, Zhang W, Muir KR, Rampersaud E, Dennison EM, Edwards MH, Jameson KA, Javaid MK, Spector TD, Cooper C, Maciewicz RA, Doherty M (2011) Role of the Nav1.7 R1150 W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res (Hoboken):440–4 [DOI] [PubMed]
- 22.Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 2008;64:644–653. doi: 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]
- 23.Takasaki I, Andoh T, Shiraki K, Kuraishi Y. Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Pain. 2000;86:95–101. doi: 10.1016/S0304-3959(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 24.Ohtori S, Takahashi K, Aoki Y, Doya H, Ozawa T, Saito T, Moriya H. Spinal neural cyclooxygenase-2 mediates pain caused in a rat model of lumbar disk herniation. J Pain. 2004;5:385–391. doi: 10.1016/j.jpain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K. Distribution and appearance of tumor necrosis factor-alpha in the dorsal root ganglion exposed to experimental disc herniation in rats. Spine. 2004;29:2235–2241. doi: 10.1097/01.brs.0000142223.30453.e5. [DOI] [PubMed] [Google Scholar]
- 26.Obata K, Tsujino H, Yamanaka H, Yi D, Fukuoka T, Hashimoto N, Yonenobu K, Yoshikawa H, Noguchi K. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain. 2002;99:121–132. doi: 10.1016/S0304-3959(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 27.Onda A, Murata Y, Rydevik B, Larsson K, Kikuchi S, Olmarker K. Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine. 2004;29:1857–1861. doi: 10.1097/01.brs.0000137054.08788.b2. [DOI] [PubMed] [Google Scholar]
- 28.Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26:863–869. doi: 10.1097/00007632-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 29.Sakuma T, Kamoda H, Miyagi M, Ishikawa T, Arai G, Eguchi Y, Suzuki M, Oikawa Y, Sakuma Y, Kubota G, Inage K, Saino T, Orita S, Yamauchi K, Inoue G, Takahashi K, Ohtori S. Comparison of CatWalk analysis and von Frey testing for pain assessment in a rat model of nerve crush plus inflammation. Spine (Phila Pa 1976) 2013;38(15):E919–E924. doi: 10.1097/BRS.0b013e318297bfb6. [DOI] [PubMed] [Google Scholar]
- 30.Norimoto M, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Orita S, Eguchi Y, Sugiura A, Ochiai N, Takaso M, Takahashi K. Direct application of the TNF-alpha inhibitor, etanercept, does not affect CGRP expression and phenotypic change of DRG neurons following application of nucleus pulposus onto injured sciatic nerves in rats. Spine (Phila Pa 1976) 2008;33(22):2403–2408. doi: 10.1097/BRS.0b013e31818441a2. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita M, Ohtori S, Koshi T, Inoue G, Yamauchi K, Suzuki M, Takahashi K. Tumor necrosis factor-alpha in the nucleus pulposus mediates radicular pain, but not increase of inflammatory peptide, associated with nerve damage in mice. Spine (Phila Pa 1976) 2008;33(17):1836–1842. doi: 10.1097/BRS.0b013e31817bab2a. [DOI] [PubMed] [Google Scholar]
- 32.Coggeshall RE, Tate S, Carlton SM. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett. 2004;355:45–48. doi: 10.1016/j.neulet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain. 2008;12:564–572. doi: 10.1016/j.ejpain.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeomans DC, Levinson SR, Peters MC, Koszowski AG, Tzabazis AZ, Gilly WF, Wilson SP. Decrease in inflammatory hyperalgesia by herpes vector-mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum Gene Ther. 2005;16:271–277. doi: 10.1089/hum.2005.16.271. [DOI] [PubMed] [Google Scholar]
- 37.Shields SD, Cheng X, Uçeyler N, Sommer C, Dib-Hajj SD, Waxman SG (2012) Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury. J Neurosci 10819–10832 [DOI] [PMC free article] [PubMed]