Abstract
Purpose
We have revealed that the cause of postoperative dyspnea and/or dysphagia after occipito-cervical (O-C) fusion is mechanical stenosis of the oropharyngeal space and the O-C2 alignment, rather than total or subaxial alignment, is the key to the development of dyspnea and/or dysphagia. The purpose of this study was to confirm the impact of occipito-C2 angle (O-C2A) on the oropharyngeal space and to investigate the chronological impact of a fixed O-C2A on the oropharyngeal space and dyspnea and/or dysphagia after O-C fusion.
Materials and methods
We reviewed 13 patients who had undergone O-C2 fusion, while retaining subaxial segmental motion (OC2 group) and 20 who had subaxial fusion without O-C2 fusion (SA group). The O-C2A, C2–C6 angle and the narrowest oropharyngeal airway space were measured on lateral dynamic X-rays preoperatively, when dynamic X-rays were taken for the first time postoperatively, and at the final follow-up. We also recorded the current dyspnea and/or dysphagia status at the final follow-up of patients who presented with it immediately after the O-C2 fusion.
Results
There was no significant difference in the mean preoperative values of the O-C2A (13.0 ± 7.5 in group OC2 and 20.1 ± 10.5 in group SA, Unpaired t test, P = 0.051) and the narrowest oropharyngeal airway space (17.8 ± 6.0 in group OC2 and 14.9 ± 3.9 in group SA, Unpaired t test, P = 0.105). In the OC2 group, the narrowest oropharyngeal airway space changed according to the cervical position preoperatively, but became constant postoperatively. In contrast, in the SA group, the narrowest oropharyngeal airway space changed according to the cervical position at any time point. Three patients who presented with dyspnea and/or dysphagia immediately after O-C2 fusion had not resolved completely at the final follow-up. The narrowest oropharyngeal airway space and postoperative dyspnea and/or dysphagia did not change with time once the O-C2A had been established at O-C fusion.
Conclusions
The O-C2A established at O-C fusion dictates the patient’s destiny in terms of postoperative dyspnea and/or dysphagia. Surgeons should pay maximal attention when establishing the O-C2A during surgery, because their careless decision for the O-C2A may cause persistent dysphagia or a life-threatening consequence. We recommend that the O-C2A in O-C fusion should be kept at least at more than the preoperative O-C2A in the neutral position.
Keywords: Upper airway, Cervical fusion, Cervical alignment, Dysphagia, Complication
Introduction
Postoperative dyspnea and/or dysphagia (D/D) after occipito-cervical (O-C) fusion are rare but pose an obstacle to activities of daily living and are occasionally life-threatening [1–4]. Although, the true cause of D/D has not been elucidated, cervical flexed alignment has been thought to be a major factor, in addition to pharyngeal edema or a postoperatively enlarged tongue [5–7].
However, we have revealed that the cause of postoperative D/D after O-C fusion is mechanical stenosis of the oropharyngeal space and the O-C2 alignment alone, and not total cervical alignment or subaxial alignment, is the key to the development of D/D. Miyata et al. [8] showed that reduction of the O-C2 angle (O-C2A) after O-C fusion was associated with postoperative D/D, and demonstrated that the O-C2A had a great impact on D/D and the oropharyngeal space, where both respiratory and swallowing functions are involved. They demonstrated that if the O-C2A was reduced by more than 10° compared with the preoperative neutral position, all patients developed D/D after surgery. Ota et al. [9] investigated the association between cervical alignment and the oropharyngeal space in normal volunteers. They demonstrated that the narrowest oropharyngeal airway space (nPAS) was strongly associated with the O-C2A, whereas the C2–C6 angle (C2–C6A) was not significantly associated with the nPAS. They also showed that a decrease in the O-C2A of 10° caused a reduction of the nPAS in the neutral position of approximately 37 %.
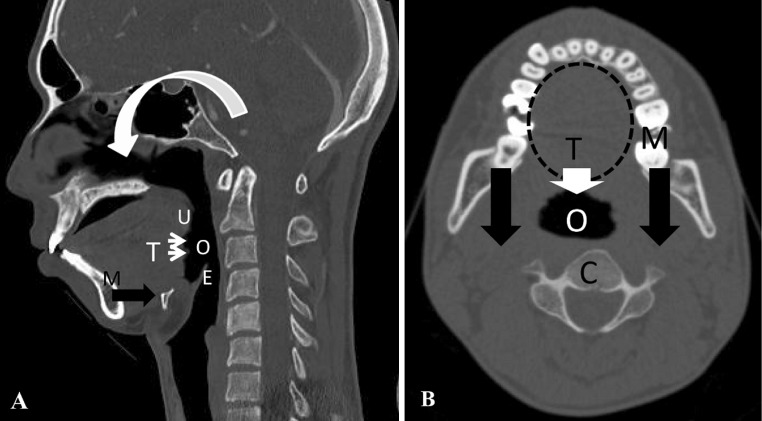
The mechanism of postoperative oropharyngeal stenosis caused by a reduction in the O-C2A has been speculated to be as follows (Fig. 1). Soft tissue surrounds the oropharyngeal space, of which the anterior tongue root is the largest component. This soft tissue is surrounded by bony structures: the mandible anteriorly and laterally and the cervical spine posteriorly. A reduction in the O-C2A makes the mandible shift posteriorly. This reduces the volume of the bony container around the oropharyngeal region, resulting in local airway stenosis [8–10]. A reduction in the oropharyngeal space causes dysphagia or, occasionally, dyspnea as a more severe form [8]. Research from another group has also supported our hypothesis. Ataka et al. [10] reported that obstructive sleep apnea in patients with rheumatoid arthritis (RA) with an upper cervical spine lesion improved after O-C fusion when the O-C2A was fixed in a more extended position, because it caused enlargement of the oropharyngeal space.
Fig. 1.
Schematic drawing of the proposed mechanism of oropharyngeal stenosis. U uvula, E epiglottis, M mandible, T tongue root, O oropharynx, C cervical spine. a Sagittal computed tomography reconstruction of the cervical spine. Reduction in the O-C2A flexes the maxilla (curved white arrow), which makes the mandible shift posteriorly (black arrow) with the tongue root (thin white arrows). b Movement of the tongue root (while arrow) caused by the backward shift of the mandible (black arrow) results in oropharyngeal stenosis
Given this background, we hypothesized that the oropharyngeal space or nPAS, once established by O-C2 fusion, will not change according to the cervical position at any postoperative point, so that any D/D occurring after O-C fusion cannot be resolved. On the other hand, in subaxial fusion procedures that retain O-C2 segmental motion, the postural change of the oropharyngeal space should be maintained for all cervical positions at all postoperative time.
All previous studies focused on the changes in the O-C2A and oropharyngeal space just after the surgery or after a short follow-up period, and also followed the patient’s D/D status after the O-C fusion for a short time only [8, 10]. As far as we know, no study has assessed the chronological changes in the oropharyngeal space established at the O-C fusion, or in postoperative D/D status. Therefore, one of the aims of this study was to establish these outcomes for patients undergoing subaxial fusion as a control group. Another objective was to investigate the current D/D status of those patients who presented with D/D immediately after the O-C2 fusion.
Materials and methods
Our hospital’s institutional review board approved this study.
As a study group, 16 patients who underwent posterior O-C2 fusion surgery while retaining subaxial cervical motion at our institution from April 1997 to September 2010, using a rigid occipital plate and a screw–rod system, were reviewed retrospectively. O-C2 fusions using a semirigid fixation technique, such as wiring, were excluded from this study, since some change in the O-C2A might have occurred before bone union was completed. Three patients were excluded from this study because one lacked postoperative dynamic lateral X-rays and two could not be followed for more than 1 year. The cases finally analyzed were the remaining 13 patients who underwent O-C2 fusion (OC2 group: 6 men and 7 women; mean age 61.2 years; range from 29 to 84 years). The diagnoses included RA (n = 9), O-C1 assimilation (n = 3), destructive spondyloarthropathy (n = 1).
As a control group, 38 patients who received subaxial fusion surgery during the same period including anterior and/or posterior cervical spinal fusion (ACF and/or PCF) without O-C2 fusion were reviewed. We defined this group as comprising patients having continuous fusion from C2 or C3 as the most cranial vertebra to C6 or below as the most distal vertebra. Eighteen patients were excluded from this study because of absent or inadequate imaging studies (n = 13), or a follow-up of less than 1 year (n = 5). This resulted in a group of 20 patients with subaxial fusion (SA group: 11 men and 9 women; mean age 64.6 years; range from 44 to 80 years). In the SA group, original diagnoses included nonunion after ACF (n = 3), cervical spondylotic myelopathy (n = 9), destructive spondyloarthropathy (n = 3), cervical spondylotic amyotrophy (n = 2), RA (n = 1), ossification of the posterior longitudinal ligament (n = 1), and a tumor (n = 1). The fused levels were as follows: C2–6 (n = 4), C2–7 (n = 3), C2–Th1 (n = 1), C3–6 (n = 8), C3–7 (n = 3), and C3–Th1 (n = 1). The mean number of fused levels was 3.9 (range 3–6). ACF was performed in 6 cases, PCF was done in 8 cases, and the remaining 6 patients underwent combined ACF and PCF.
Patients were excluded if they had preoperative dysphagia, dyspnea, and dysphonia in both groups. The mean follow-up period was 45 months (range from 13 to 72 months) in the OC2 group and 52.3 months (range from 12 to 120 months) in the SA group.
Radiographic assessment
We reviewed dynamic lateral plain X-rays to measure O-C2A, C2–C6A and the nPAS preoperatively, when dynamic X-rays were taken for the first time postoperatively (a mean of 8.8 months after the operation in the OC2 group and a mean of 12.2 months in the SA group), and at the final follow-up.
The O-C2A, C2–C6A, and nPAS parameters have been documented elsewhere [9, 11]. Briefly, the O-C2A indicates the angle between McGregor’s line and the inferior vertebral endplate line of C2. C2–C6A indicates the angle between the inferior endplates of the C2 and C6 vertebral bodies. If the C6 vertebra had been included in an ACF procedure, we used the C2–C7 angle for analysis. In both measurements, a positive value indicates lordosis at the local segment. The nPAS was defined as the shortest anteroposterior distance from the posterior pharyngeal wall to the back of the tongue between the levels of the uvula tip and the tip of the epiglottis (Fig. 2) [9].
Fig. 2.
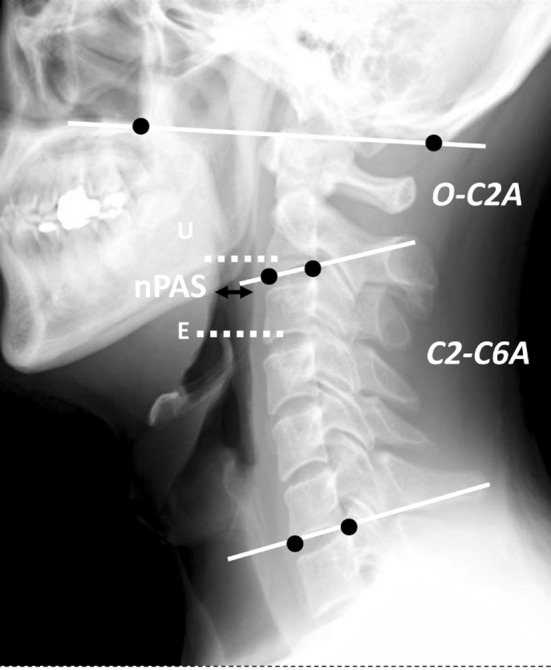
Representative radiographic measurements. E epiglottis tip, U uvula tip. The O-C2A represents the angle between McGregor’s line and the inferior endplate of C2. The C2–C6A represents the angle between the inferior endplates of C2 and C6. In both, a positive value indicates lordosis at the local segment. ‘nPAS’ represents the narrowest anteroposterior distance of the oropharynx between the tips of the uvula and epiglottis (double black arrow)
The difference in the O-C2A (dOC2A), that is the differences between the values in the flexion or extension (X position) and the value in the neutral position, was calculated for each cervical position (flexion or extension) at each time point.
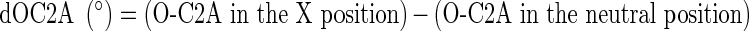 |
To normalize data for the variability of patients in terms of the pharyngeal space, the difference in the nPAS according to each cervical position (%dnPAS) was calculated from the following formula:
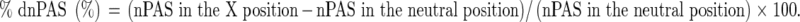 |
We represented the maximal amount of postural change of the nPAS at each time point as Δ nPAS. This was defined as the following formula:
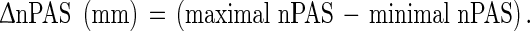 |
The maximal and minimal values were chosen among the three different positions: neutral, flexion, or extension at each time point. We calculated the ΔO-C2A and ΔC2–C6A values using a similar formula. All linear and angular parameters were calculated with a precision of 0.1 mm and 0.1° using the Centricity PACS system (version 2.0; GE Healthcare, Milwaukee, WI, USA).
Clinical assessment
We investigated the occurrence of postoperative D/D in both groups, and interviewed at the final follow-up who presented with D/D immediately after the operation about the severity of the swallowing difficulty. All of them were asked to select one of three replies about their subjective D/D status: improved, unchanged, or deteriorated compared with shortly after the operation.
Statistical analysis
Values are expressed as the mean ± standard deviation. Differences in baseline characteristics were tested using Student’s t test for continuous variables and with the χ2test for categorical variables. Nonnormally distributed variables were compared using the Mann–Whitney nonparametric U test. Spearman correlation coefficients (ρ) were used to evaluate any associations between dOC2A and %dnPAS. Differences in variation of the ΔnPAS in both groups were analyzed using an analysis of covariance (ANCOVA), and the Wilcoxon signed-rank test with Bonferroni’s correction. P < 0.05 was considered statistically significant. We used SPSS software (version12.0; SPSS Inc., Chicago, IL, USA) for all analyses.
Results
The excellent reproducibility and repeatability of the O-C2A, C2–C6A, and nPAS parameters measured on lateral cervical X-rays have been documented elsewhere [9, 11]. Briefly, the radiographs were measured twice with a 1-week interval by the same 2 spine surgeons, independently. Reliability was evaluated by calculating the intraclass correlation coefficient (ICC). The inter- and intraobserver ICCs values of the radiographic parameters for all cervical positions were more than 0.93.
The patient’s demographic data for both groups are shown in Table 1. There were no significant differences between the two groups in terms of age, male-to-female ratio, or mean follow-up period.
Table 1.
Summary of patients demographic data (mean ± SD)
| Group OC2 | Group SA | P | |
|---|---|---|---|
| Case number | 13 | 20 | |
| Sex male:female | 6:7 | 11:9 | 0.728a |
| Mean age (years) | 61.2 ± 15.2 | 64.6 ± 9.3 | 0.522b |
| Term to the first dynamic X-rays since operation (m) | 8.8 ± 2.4 | 12.2 ± 7.0 | 0.171b |
| Final F/U (m) | 45.0 ± 18.6 | 52.3 ± 32.0 | 0.512b |
| Diagnosis | RA (n = 9) | CSM (n = 9), nonunion after ACF (n = 3), | |
| O-C1 assimilation (n = 3) | |||
| DSA (n = 3), CSA (n = 2), RA (n = 1) | |||
| DSA (n = 1) | OPLL (n = 1), tumor (n = 1) |
Final F/U final follow-up, RA rheumatoid arthritis, DSA destructive spondyloarthropathy, CSM cervical spondylotic myelopathy, ACF anterior cervical spinal fusion, CSA cervical spondylotic amyotrophy, OPLL ossification of the posterior longitudinal ligament
aχ2 test
bMann–Whitney U test
Preoperative morphological measurements data are shown in Table 2. There was significant difference between the 2 groups only in ΔO-C2A value, reflecting the fact that the cases in the OC2 group had upper cervical lesions.
Table 2.
Preoperative morphological measurements (mean ± SD)
| OC2 group | SA group | P | |
|---|---|---|---|
| O-C2A (°) | 13.0 ± 7.5 | 20.1 ± 10.5 | 0.051a |
| ΔO-C2A (°) | 13.9 ± 9.2 | 26.2 ± 9.4 | 0.001a* |
| C2–C6A (°) | 12.8 ± 9.5 | 5.7 ± 14.0 | 0.131a |
| ΔC2–C6A (°) | 31.3 ± 12.9 | 29.9 ± 13.1 | 0.771a |
| nPAS (mm) | 17.8 ± 6.0 | 14.9 ± 3.9 | 0.105a |
| ΔnPAS (mm) | 7.0 ± 5.1 | 9.7 ± 4.9 | 0.169b |
ΔnPAS = (maximal nPAS − minimal nPAS). The maximal and minimal values were chosen from among the three different positions: neutral, flexion, or extension
O-C2A occipito-C2 angle, C2-C6A C2-C6 angle, nPAS represents the narrowest anteroposterior distance of the oropharynx
* Statistically significant
aUnpaired t test
bMann–Whitney U test
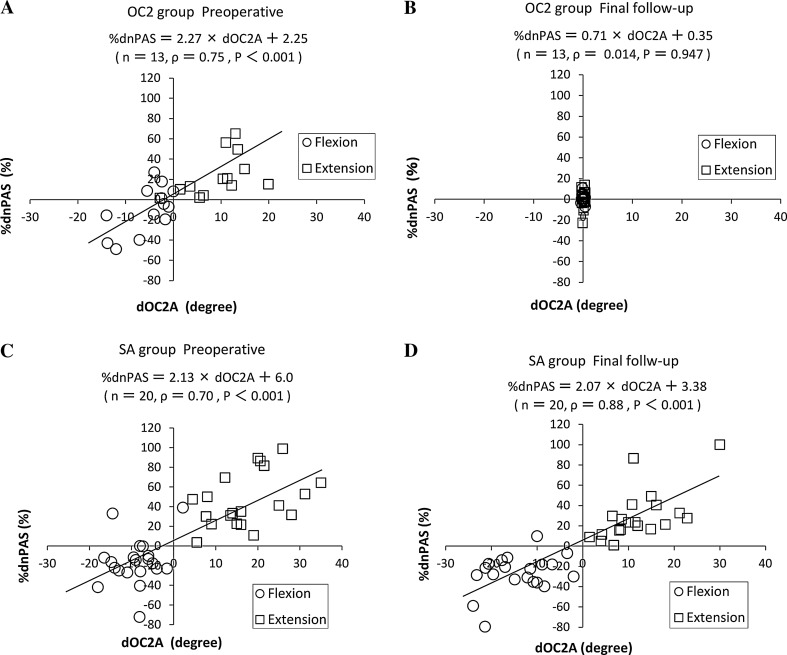
Figure 3 shows the association between the dOC2A and %dnPAS preoperatively and at the final follow-up. In the OC2 group, the preoperative scatter diagram shows that there was a positive linear correlation (Fig. 3a: Spearman’s ρ = 0.75, P < 0.001), whereas no significant correlation was found in the final follow-up (Fig. 3b: Spearman’s ρ = 0.014, P = 0.947). The %dnPAS became constant compared with its preoperative distribution, even though the C2–C6 motion was preserved. In the SA group, a strong positive linear correlation of dOC2A with %dnPAS was found at both time points (preoperative: Spearman’s ρ = 0.70, P < 0.001; final follow-up: Spearman’s ρ = 0.88, P < 0.001; Fig. 3c, d).
Fig. 3.
A scatter diagram showing the association between the dOC2A and the %dnPAS. dOC2A (°) = (O-C2A in the X position) − (O-C2A in the neutral position). %dnPAS (%) = (nPAS in the X position − nPAS in the neutral position)/(nPAS in the neutral position) × 100. ‘X position’ indicates flexion or extension. a OC2 group: preoperative values. b OC2 group: final follow-up values. c SA group: preoperative values. d SA group: final follow-up values
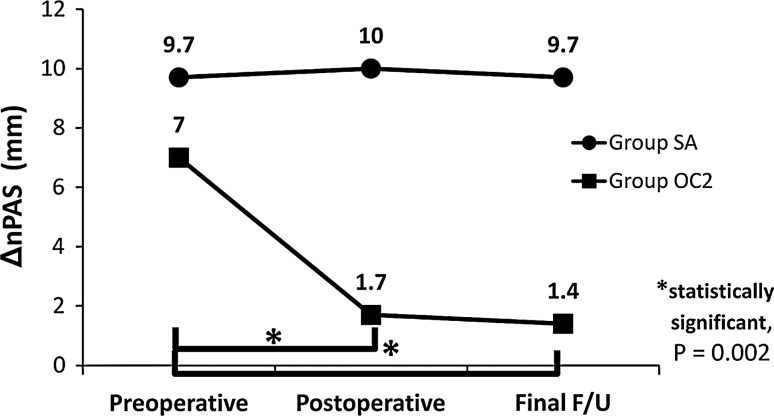
Figure 4 shows chronological changes in the ΔnPAS. The ΔnPAS value in the OC2 group at the first postoperative point and at the final follow-up was significantly smaller than preoperatively (P = 0.002), and there was no significant difference between the first postoperative point and the final follow-up (P = 0.1). On the other hand, there were no significant differences in the value of ΔnPAS among preoperative, postoperative, and the final follow-up points in the SA group. These findings demonstrate that the postural change in nPAS diminished dramatically, and became constant once the O-C2 is fixed. A representative case in each group is presented in Fig. 5.
Fig. 4.
This line graph shows the chronological changes in the ΔnPAS between the 2 groups. Final F/U final follow-up, *statistically significance
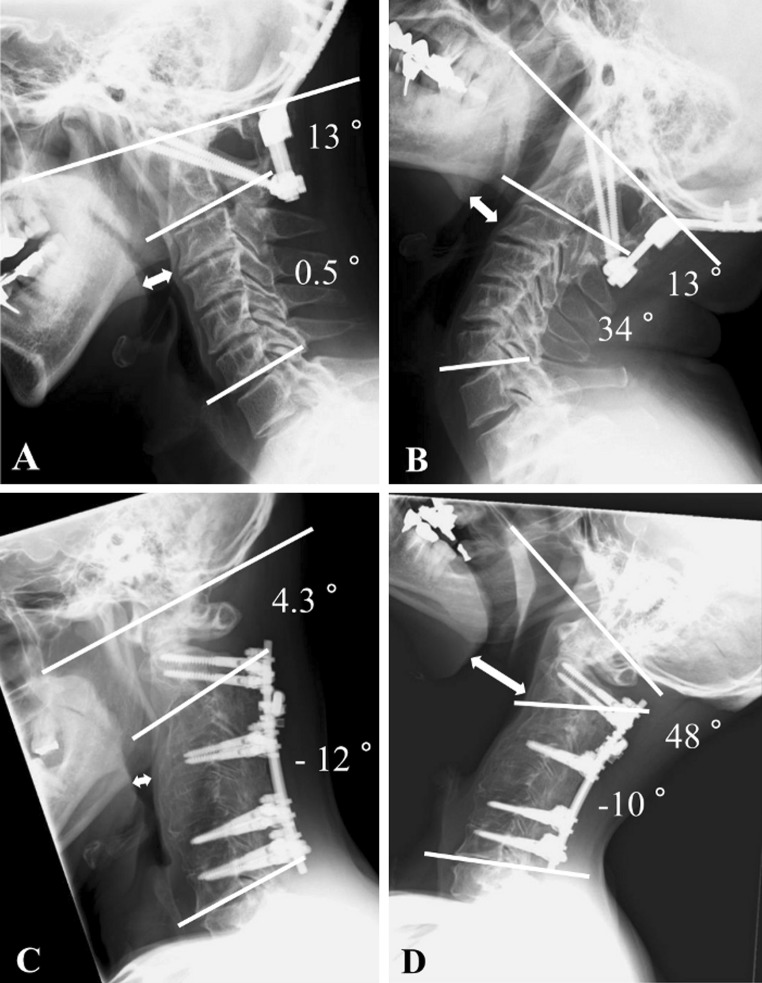
Fig. 5.
Postoperative lateral radiographs in the flexion and extension (a, b). A case in the OC2 group. The nPAS (double white arrow) became almost constant, once the O-C2A had been fixed (nPAS: 14.2 mm in flexion, and 15.4 mm in extension) (c, d). A case with C2–C7 posterior cervical fusion in the SA group. The changes in the nPAS had been maintained even after subaxial fusion (nPAS: 5.3 mm in flexion, and 32.9 mm in extension)
In clinical assessment, although 2 patients in the control group complained of swallowing difficulty after ACF only in the early postoperative period, no patient had subjective dysphagia at the final follow-up. In the study group, 3 patients had D/D immediately after the O-C fusion and continued to the final follow-up (Table 3), and the remaining 10 patients had not complained of D/D through the whole postoperative period. For these D/D patients, we consulted to otolaryngologists as for postoperative D/D pathology. They performed routine examination of laryngopharynx (3 patients), laryngoscopy (2 patients), and esophagography (1 patient). However, no neurological or mucosal pathology were pointed out. All patients did not desire further confirmatory test or treatment for dysphagia, because their symptom was mild.
Table 3.
Summary of data of 3 patients with dyspnea and/or dysphagia after O-C2 fusion
| Case no. | Age (years), sex | Origin | Fusion range | FU (m) | O-C2A variation (°)a | Pre-op nPASb (mm) | Post-op nPASb (mm) | Final F/U nPASb (mm) | Complications | Outcome of the sequelae |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 41, M | O-C1 As, AAS | O-C2 | 84 | −14 | 12.3 | 10.0 | 10.7 | Dysphagia | No change |
| 2. | 56, F | RA, AAS, VS | O-C2 | 51 | −16 | 16.8 | 8.5 | 9.8 | Dysphagia | No change |
| 3. | 73, F | DSA | O-C2 | 35 | −6.5 | 12.4 | 8.8 | 12.0 | Dysphagia | Continued but slightly improved |
O-C1 As O-C1 assimilation, RA rheumatoid arthritis, AAS atlantoaxial subluxation, VS vertical subluxation, DSA destructive spondyloarthropathy
aO-C2A variation = (postoperative O-C2A in the neutral position) − (preoperative O-C2A in the neutral position)
bMeasurement in the neutral position
Discussion
We have argued that the main cause of postoperative D/D after the O-C fusion is fixation with an O-C2A that is smaller than the preoperative neutral position [8]. The absolute value of the O-C2A in a neutral position varies between individuals, and any change from this position is important clinically [9, 12, 13]. The impact of the O-C2A on the oropharyngeal space and D/D after the O-C fusion has been demonstrated from several directions [8–10], and the present study reconfirms it clearly from another perspective. Furthermore, we clarified that the nPAS and postoperative D/D did not change with time once the O-C2A had been established during the O-C fusion.
It is well known that dysphagia sometimes occurs after ACF. Proposed causes include pharyngeal edema, transient local nerve palsy, use of plate fixation, plate prominence, the use of recombinant human bone morphogenetic protein-2, and severe pain [14–18]. The incidence of dysphagia was reported to be 50–56% at 1 month after ACF, but the symptoms resolved gradually over the first few months. At 1 year, only 13–21% of patients were symptomatic [14, 19]. Since the mechanism of dysphagia after the O-C fusion is speculated to be quite different from that after ACF, spontaneous recovery is much less likely, that is, the cause of D/D after the O-C fusion is mainly mechanical stenosis of the airway and it occurs regardless of inflammation, edema, or neuropraxia. Therefore, it should be stressed that once D/D occurs after the O-C fusion, and if the fixed O-C2A is found to be smaller than that in the preoperative neutral position, the surgeon should not hesitate to correct the O-C2A to the preoperative neutral position or slightly more. It would be unfortunate, if the surgeon were to wait for several months, expecting spontaneous recovery, in which time bone union was completed. After the publication of our previous papers [8, 9], we were consulted regarding 2 cases of postoperative dysphagia from other hospitals. We recommended correction of the O-C2 alignment based on measuring the O-C2A. The dysphagia disappeared immediately after the revision surgery in both cases. As Ota et al. [9] demonstrated, a decrease in the O-C2A of only 10° caused a reduction of approximately 37% in the nPAS at the neutral position. This means that even a slight change in the O-C2A will dramatically affect the oropharyngeal space.
Recent refinements in instrumentation, such as the plate–screw–rod system, and the advent of strong anchoring techniques, such as Magerl or pedicle screws, have enabled us to obtain very rigid fixation with excellent clinical results. However, these systems do not allow for slight postoperative changes in the O-C2A, probably decreasing the chance of a spontaneous recovery from D/D after the O-C fusion. In the conventional semirigid fixation techniques, such as onlay bone grafting with halo-vest fixation or sublaminar segmental wiring techniques, it is possible that postoperative D/D will improve spontaneously by allowing the patient to achieve unconscious adjustment of the fixed position. Therefore, the O-C2A determined during surgery might now be the most important factor in avoiding postoperative D/D.
The present study had several weaknesses and limitations, including its retrospective study design, and relatively small sample size with many cases being excluded. Another limitation of our study is that dysphagia is a subjective manifestation, so different individuals might view even the same condition differently, and some cases might be underreported [20]. Therefore, in the absence of universal questionnaires to evaluate the degree of dysphagia in patients undergoing cervical spine surgery, it is best to evaluate the swallowing function objectively both pre- and postoperatively, for example using the Bazaz grading system and Dysphagia Short Questionnaire [15, 21]. Another limitation of this study was the difference between groups in the ratio of patients with RA, because it has been recognized that RA is a major risk factor for postoperative narrowing of the airway [22–24]. However, it has been speculated that mechanical stenosis caused by upper cervical alignment also plays a major role in postoperative airway complications in such patients [10, 25].
Considering the ease of exposure of the operative field, we might generate a flexed position of the O-C2A during the surgery. Moreover, it is very difficult to notice slight changes in the O-C2 alignment macroscopically. Therefore, the surgeon must check the O-C2A using a C-arm after the insertion of all cervical screws and, if necessary, change the patient’s head position just before the final occipital plate-rod fixation.
Surgeons should be aware that the O-C2A established at the O-C fusion dictates the patient’s destiny in terms of postoperative D/D and should pay full attention when establishing it during the surgery. Furthermore, they should not hesitate to correct the O-C2A should they encounter D/D in a patient after the O-C fusion with a reduced O-C2A.
Acknowledgments
The authors are grateful to all the staff of the Kyoto University for allowing them to study their patients and for their assistance.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this paper.
References
- 1.Matsuyama Y, Kawakami N, Yoshihara H, et al. Long-term results of occipitothoracic fusion surgery in RA patients with destruction of the cervical spine. J Spinal Disord Tech. 2005;18(suppl 1):S101–S106. doi: 10.1097/01.bsd.0000127700.29969.e6. [DOI] [PubMed] [Google Scholar]
- 2.Tagawa T, Akeda K, Asanuma Y, et al. Upper airway obstruction associated with flexed cervical position after posterior occipitocervical fusion. J Anesth. 2011;25(1):120–122. doi: 10.1007/s00540-010-1069-0. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida M, Neo M, Fujibayashi S, Nakamura T. Upper-airway obstruction after short posterior occipitocervical fusion in a flexed position. Spine (Phila Pa 1976) 2007;32(8):E267–E270. doi: 10.1097/01.brs.0000259977.69726.6f. [DOI] [PubMed] [Google Scholar]
- 4.Ichinose K, Kozuma S, Fukuyama S, et al. A case of airway obstruction after posterior occipito-cervical fusion (in Japanese) Masui. 2002;51(5):513–515. [PubMed] [Google Scholar]
- 5.Meakem TD, Meakem TJ, Rappaport W. Airway compromise from prevertebral soft tissue swelling during placement of halo-traction for cervical spine injury. Anesthesiology. 1990;73(4):775–776. doi: 10.1097/00000542-199010000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Dark A, Armstrong T. Severe postoperative laryngeal oedema causing total airway obstruction immediately on extubation. Br J Anaesth. 1999;82(4):644–646. doi: 10.1093/bja/82.4.644. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Hsieh PF, Huang HH, Chan KC. Upper airway obstruction after cervical spine fusion surgery: role of cervical fixation angle. Acta Anaesthesiol Taiwan. 2008;46(3):134–137. doi: 10.1016/S1875-4597(08)60008-9. [DOI] [PubMed] [Google Scholar]
- 8.Miyata M, Neo M, Fujibayashi S, et al. O-C2 angle as a predictor of dyspnea and/or dysphagia after occipitocervical fusion. Spine (Phila Pa 1976) 2009;34(2):184–188. doi: 10.1097/BRS.0b013e31818ff64e. [DOI] [PubMed] [Google Scholar]
- 9.Ota M, Neo M, Aoyama T, et al. Impact of the O-C2 angle on the oropharyngeal space in normal patients. Spine (Phila Pa 1976) 2011;36(11):E720–E726. doi: 10.1097/BRS.0b013e3181f9f714. [DOI] [PubMed] [Google Scholar]
- 10.Ataka H, Tanno T, Miyashita T, Isono S, Yamazaki M. Occipitocervical fusion has potential to improve sleep apnea in patients with rheumatoid arthritis and upper cervical lesions. Spine (Phila Pa 1976) 2010;35(19):E971–E975. doi: 10.1097/BRS.0b013e3181c691df. [DOI] [PubMed] [Google Scholar]
- 11.Izeki M, Neo M, Ito H, et al. Reduction of atlantoaxial subluxation causes airway stenosis. Spine (Phila Pa 1976) 2013;38(9):E513–E520. doi: 10.1097/BRS.0b013e31828b26df. [DOI] [PubMed] [Google Scholar]
- 12.Shoda N, Takeshita K, Seichi A, et al. Measurement of occipitocervical angle. Spine (Phila Pa 1976) 2004;29(10):E204–E208. doi: 10.1097/00007632-200405150-00022. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga S, Onishi T, Sakou T. Significance of occipitoaxial angle in subaxial lesion after occipitocervical fusion. Spine (Phila Pa 1976) 2001;26(2):161–165. doi: 10.1097/00007632-200101150-00010. [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Bazaz R, Furey CG, Yoo J. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J. 2007;7(2):141–147. doi: 10.1016/j.spinee.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976) 2002;27(22):2453–2458. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Riley LH, 3rd, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976) 2005;30(22):2564–2569. doi: 10.1097/01.brs.0000186317.86379.02. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya R, Weir R, Sethi A, et al. Interbody fusion with allograft and rh-BMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342–345. doi: 10.1302/0301-620X.89B3.18270. [DOI] [PubMed] [Google Scholar]
- 18.Haller JM, Iwanik M, Shen FH. Clinically relevant anatomy of high anterior cervical approach. Spine (Phila Pa 1976) 2011;36(25):2116–2121. doi: 10.1097/BRS.0b013e31820408af. [DOI] [PubMed] [Google Scholar]
- 19.Riley LH, 3rd, Vaccaro AR, Dettori JR, Hashimoto R. Postoperative dysphagia in anterior cervical spine surgery. Spine (Phila Pa 1976) 2010;35(9 Suppl):S76–S85. doi: 10.1097/BRS.0b013e3181d81a96. [DOI] [PubMed] [Google Scholar]
- 20.Edwards CC, 2nd, Karpitskaya Y, Cha C, et al. Accurate identification of adverse outcome after cervical spine surgery. J Bone Joint Surg Am. 2004;86(2):251–256. doi: 10.1302/0301-620X.86B2.13878. [DOI] [PubMed] [Google Scholar]
- 21.Martin S, Catarina I, Therese E, Claes O. The dysphagia short questionnaire: an instrument for evaluation of dysphagia—a validation study with 12 months’ follow-up after anterior cervical spine surgery. Spine (Phila Pa 1976) 2012;37(11):996–1002. doi: 10.1097/BRS.0b013e31823a7a5b. [DOI] [PubMed] [Google Scholar]
- 22.Redlund-Johnell I. Upper airway obstruction in patients with rheumatoid arthritis and temporomandibular joint destruction. Scand J Rhumatol. 1988;17(4):273–279. doi: 10.3109/03009748809098796. [DOI] [PubMed] [Google Scholar]
- 23.Keenan MA, Stiles CM, Kaufman RL. Acquired laryngeal deviation associated with cervical spine disease in erosive polyarticular arthritis. Use of the fiberoptic bronchoscope in rheumatoid disease. Anesthesiology. 1983;58(5):441–449. doi: 10.1097/00000542-198305000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Chen JJ, Branstetter BF, 4th, Myers EN. Cricoarytenoid rheumatoid arthritis: an important consideration in aggressive lesions of the larynx. AJNR Am J Neuroradiol. 2005;26(4):970–972. [PMC free article] [PubMed] [Google Scholar]
- 25.Ataka H, Isono S, Yamazaki M, Tanno T, Miyashita T. Sleep-disordered breathing in patients with rheumatoid arthritis and upper cervical lesion (in Japanese) J Spine Res. 2011;2(1):35–42. doi: 10.1097/BRS.0b013e3181c691df. [DOI] [PubMed] [Google Scholar]