Abstract
Renin is a newly discovered constituent of mast cells. Given that mast cells play a major role in IgE-mediated allergic hypersensitivity, we investigated whether activation of the high-affinity IgE receptor FcεRI elicits release of mast-cell renin. Cross-linking of FcεRI on the surface of mature bone marrow–derived mast cells elicited release of enzymatically active renin protein. The angiotensin I–forming activity of the renin protein was completely blocked by the selective renin inhibitor BILA 2157, which excludes formation of angiotensin I by proteases other than renin. FcεRI-mediated mast-cell renin release was inhibited by dexamethasone and potentiated by the proinflammatory mediator PGE2. Furthermore, cross-linking of mast-cell FcεRI in ex vivo murine hearts passively sensitized with monoclonal anti-DNP IgE also resulted in mast-cell degranulation and overflow of renin. Our findings indicate that IgE-mediated allergic hypersensitivity provokes release of renin from both cultured and resident cardiac mast cells, a process likely to be exacerbated in a chronic inflammatory background. Given the widespread distribution of mast cells, and the presence of angiotensinogen and angiotensin-converting enzyme in many tissues, renin release in immediate hypersensitivity reactions could result in local angiotensin II generation and multiorgan dysfunctions.
Mast cells (MCs) express and secrete active renin.1–3 MCs are known to play a major role in IgE-mediated allergic reactions.4,5 Whether immediate hypersensitivity can elicit MC renin release is a question of major basic and translational importance, given the ubiquitous presence of MCs3,6,7 and the multiplicity of angiotensin-induced organ dysfunctions.8 To address this question, we used murine bone marrow–derived MCs (BMMCs) and MCs in ex vivo murine hearts. Here, we report the novel finding that specific cross-linking of the high-affinity FcεRI receptor on the surface of BMMCs and heart MCs elicits the release of enzymatically active renin protein. This process is inhibited by glucocorticoids and is enhanced by PGE2. Similarly, cross-linking of MC FcεRI in ex vivo murine hearts resulted in MC degranulation and overflow of renin.
Materials and Methods
Isolation of Murine BMMCs
BMMCs were cultured as described previously.9 In brief, bone marrow was obtained from femurs and tibias of C57BL/6J mice euthanized by cervical dislocation under light CO2 anesthesia as approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee. Bone marrow cells were cultured in RPMI 1640 medium (Life Technologies, Carlsbad, CA) containing 10 U/mL antibiotics (penicillin–streptomycin), 10% heat-inactivated fetal calf serum, 55 μmol/L 2-mercaptoethanol, and 20 ng/mL each recombinant murine IL-3 and stem cell factor (SCF) (PeproTech, Rocky Hill, NJ). Bone marrow cells were counted and placed in culture at a cell density of 0.5 × 106 cells/mL. Cell medium was changed every 3 to 4 days, and nonadherent cells were transferred to a new flask. Mature BMMCs obtained after 4 weeks of culture stained positive for Toluidine Blue; moreover, >90% of cells expressed both c-Kit and FcεRI. All experiments were performed with BMMCs cultured for 4 to 7 weeks. Cells were plated on coverslips and incubated in 0.05% Toluidine Blue (Sigma-Aldrich, St. Louis, MO) for 10 minutes at 37°C. Cells were then fixed with 4% paraformaldehyde for 15 minutes at room temperature. Coverslips were mounted on glass slides using VectaMount medium (Vector Laboratories, Burlingame, CA) and were examined under an Eclipse 80i microscope (Nikon Instruments, Melville, NY).
Flow Cytometry
To assess the expression of c-Kit and FcεRI on BMMC cell surface, aliquots of cells cultured for 5 weeks were centrifuged and incubated for 10 minutes with PBS (Sigma-Aldrich) containing 0.1% bovine serum albumin and purified rat anti-mouse CD16/CD32 (Fc Block; BD Biosciences, San Jose, CA) on ice. At the end of the incubation, fluorescein isothiocyanate–conjugated monoclonal anti-mouse FcεRI (eBioscience, San Diego, CA) and phycoerythrin-conjugated monoclonal anti-mouse CD117 (c-Kit; BD Biosciences) were added to the cells for 30 minutes on ice. After a PBS washing, labeled cells were analyzed with a BD LSR II flow cytometer (BD Biosciences).
Western Blotting
BMMCs were lysed using radioimmunoprecipitation assay buffer (Sigma-Aldrich). KNRK rat kidney cell lysate was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). BMMC and KNRK samples for electrophoresis (50 μg/lane) and Western blotting were prepared as described previously.10 Polyvinylidene difluoride membranes were probed with anti-mouse renin (Santa Cruz) at a dilution of 1:500. Anti-goat IgG horseradish peroxidase (HRP)–linked secondary antibody (EMD Millipore, Billerica, MA) was used at a 1:5000 dilution. Anti–β-actin HRP-conjugated antibody (Alpha Diagnostic International, San Antonio, TX) was used at a 1:30,000 dilution. Proteins of interest were detected using Immobilon Western chemiluminescent HRP substrate (EMD Millipore).
FcεRI-Mediated BMMC Degranulation
In brief, after 16 hours of sensitization with 0.125 μg/mL anti-DNP IgE (Sigma-Aldrich), 1 × 105 BMMCs per sample were washed and resuspended in Ringer’s buffer (pH 7.4). Equal cell volumes were aliquoted in 96 wells and challenged with 10 ng/mL antigen [dinitrophenylated human serum albumin (DNP-HSA)] for 20 minutes at 37°C in the absence or presence of 1 μmol/L PGE2 (R&D Systems, Minneapolis, MN). Other cells were preincubated with 2 nmol/L dexamethasone (Sigma-Aldrich) for 5 minutes before antigen challenge. When MC activators were used, cells were incubated with 1 μmol/L A23187 calcium ionophore, 300 μg/mL compound 48/80 (c48/80), or 1 mmol/L ATP (all from Sigma-Aldrich) for 20 minutes at 37°C. At the end of the incubation, samples were placed on ice and centrifuged at 300 × g for 5 minutes. Supernatants were collected and used to measure the β-hexosaminidase (β-HEX) release. Cell pellets were lysed with 0.5% Triton X-100, and total lysates were used to determine total β-HEX content. β-HEX release was expressed as the percentage ratio between supernatant and total β-HEX (where total β-HEX is equal to the sum of β-HEX content in supernatant and total lysate).
Measurement of β-HEX
β-HEX was measured as described previously.11 Samples of BMMC supernatants, total lysates, and coronary overflow (20 μL) were placed in 96-well plates. Substrate solution (50 μL; p-nitrophenyl-N-acetyl-β-D-glucosaminide, 1.3 mg/mL in 0.1 mol/L citrate buffer, pH 4.5) were added to each well and incubated for 90 minutes at 37°C. The reaction was stopped by adding 150 μL of 0.2 mol/L glycine (pH 10.7). Optical density was read at 405 nm on a spectrophotometric plate reader using SoftMax Pro version 4.8 (Molecular Devices, Sunnyvale, CA).
Measurement of Histamine
Histamine released from BMMCs was measured using an enzyme immunoassay kit, according to the manufacturer’s instructions (Beckman Coulter, Brea, CA). In brief, after stimulation, supernatants and total lysates were collected from BMMCs as described above for the β-HEX assay. Absorbance was read in a microplate reader (Molecular Devices, Sunnyvale, CA) at 405 nm, and histamine levels were determined by comparison with a calibration curve. Histamine release was expressed as the percent ratio between supernatant and total histamine.
Measurement of Renin Protein
Mouse renin 1 was measured in BMMC supernatants using an enzyme-linked immunosorbent assay kit (RayBiotech, Norcross, GA) according to the manufacturer’s instructions. In brief, 3 × 105 BMMCs were stimulated as described above for the β-HEX assay, and 100 μL from each supernatant was added to wells coated with anti-mouse renin 1 and incubated for 16 hours at 4°C. Absorbance was read in a microplate reader (Molecular Devices, Sunnyvale, CA) at 450 nm, and the renin protein concentration was calculated by comparison with a standard curve.
Measurement of Renin Activity
Renin activity was measured, as described previously,11 in supernatants from BMMCs (treated as described above) using a radioimmunoassay kit (DiaSorin, Stillwater, MN; Saluggia, Italy), according to the manufacturer’s instructions. Porcine angiotensinogen substrate (Sigma-Aldrich) was added to the supernatants. The reaction for angiotensin I production was conducted for 1.5 hours in the presence or absence of 100 nmol/L of the highly selective renin inhibitor BILA 2157 (BILA).2,12 Total protein concentration was measured using a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Results were normalized to total protein.
Perfusion of Mouse Hearts ex Vivo
Seven C57BL/6J male mice, 10 to 12 weeks old (Jackson Laboratory, Bar Harbor, ME), were passively sensitized with an intraperitoneal injection of murine anti-DNP IgE (6 μg/200 μL PBS; Sigma-Aldrich). Three other mice (nonsensitized controls) received an intraperitoneal injection of PBS alone. Twenty-four hours later, all mice were intraperitoneally injected with 100 IU heparin, anesthetized with CO2, and euthanized by cervical dislocation as approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee. The isolated hearts were transferred to a Langendorff apparatus (Radnoti, Monrovia, CA). The aorta was cannulated with a flanged 20-gauge stainless steel needle. The heart was perfused through the aorta in a retrograde mode at a constant pressure of 100 cm H2O with modified Krebs–Henseleit buffer containing (mmol/L) NaCl, 120; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25; glucose, 11; pyruvic acid, 2; and EDTA, 0.5. The perfusion fluid was bubbled with 95% O2–5% CO2 at 37°C, to achieve a pH of 7.4. After a 20-minute equilibration period, hearts were challenged with antigen (rapid intra-aortic injection of 200 μg DNP-HSA in 300 μL Krebs–Henseleit buffer). Samples of coronary flow effluent were collected every 2 minutes. The samples were assayed for β-HEX, renin activity, and histamine. For the β-HEX measurements, samples of coronary effluent were concentrated 8- to 10-fold by centrifugal filtration (EMD Millipore).
Statistical Analysis
Unpaired t-test was used throughout the study. P < 0.05 was considered statistically significant. Data are expressed as means ± SEM.
Results
Renin Is Expressed in BMMCs
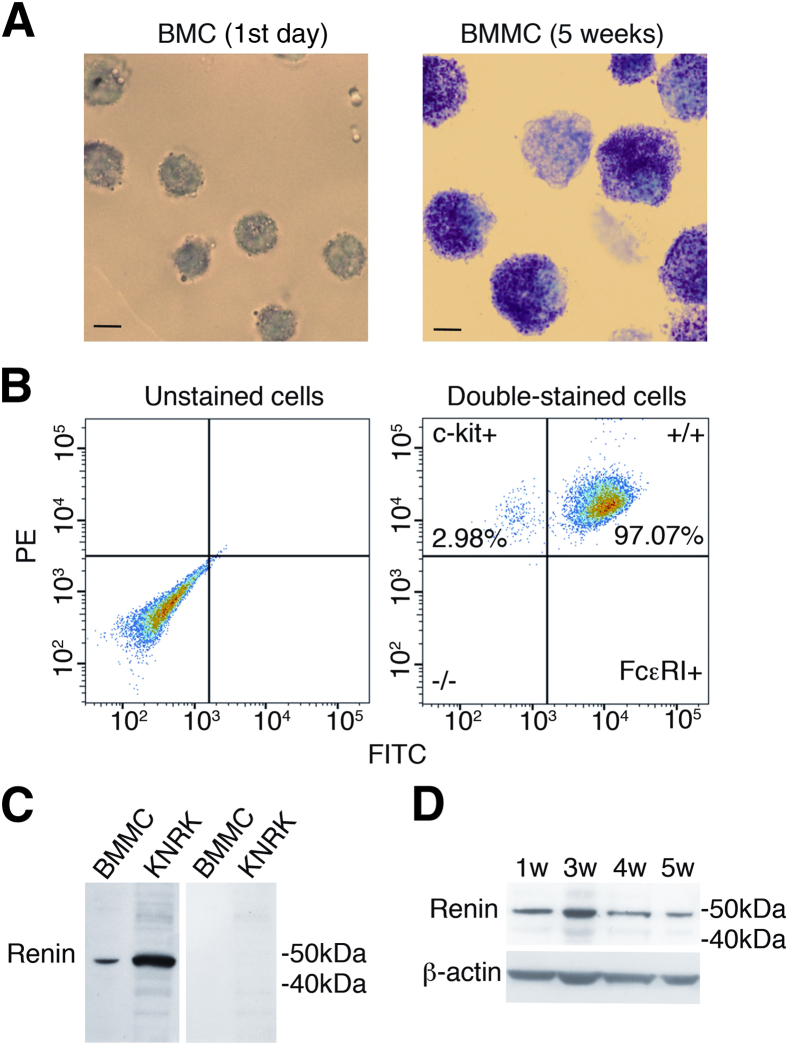
Murine bone marrow cells matured into typical MCs on in vitro culture with SCF and IL-3 for 5 weeks, at which point they exhibited the classical Toluidine-Blue granular staining, were double-positive (>90%) for the FcεRI and c-Kit receptors, and contained renin protein (Figure 1). Notably, renin protein was present in bone marrow cells at the time of their isolation and continued to be present during the differentiation phase (Figure 1).
Figure 1.
Granular staining and expression of c-Kit, FcεRI, and renin in BMMCs. A: Characteristic Toluidine Blue–stained murine bone marrow cells at isolation and differentiated BMMCs after 5 weeks in culture with IL-3 and SCF. B: Fluorescence-activated cell-sorting analysis shows c-Kit-PE and FcεRI–fluorescein isothiocyanate coexpression (>90%) on the BMMC surface after 5 weeks in culture. C: Representative Western blots with anti-mouse renin antibody show a renin-positive band in total protein lysate (50 μg/lane) of BMMCs (5-week culture) and KNRK rat kidney cells used as positive control (left lanes) and lack of immunoreactive bands after direct incubation of total BMMC and KNRK lysates with the secondary antibody (right lanes). D: Representative Western blots of total lysates isolated from BMMCs at 1, 3, 4, and 5 weeks (w) in culture. Scale bars: 5 μm (A). BMC, bone marrow cells; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
BMMC Renin Release by Classical Degranulating Agents
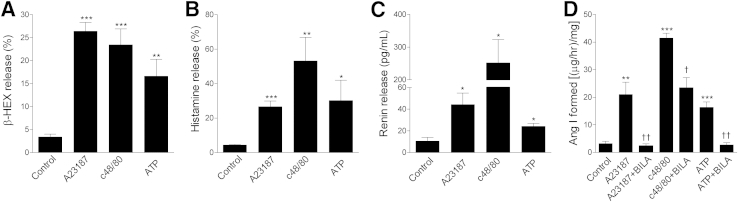
Exposure of BMMCs to canonical degranulating agents (1 μmol/L Ca2+ ionophore A23187,13 c48/80,14 and 1 mmol/L ATP15) elicited degranulation (approximately fivefold to eightfold increase in β-HEX release, relative to control), and release of histamine (approximately 7- to 13-fold increase) and renin protein (approximately 2- to 25-fold increase) (Figure 2). Released renin was enzymatically active, because it cleaved angiotensin I from angiotensinogen (approximately 5- to 14-fold increase in angiotensin I formed, relative to control) (Figure 2D). Notably, the formation of angiotensin I in the supernatant of MCs incubated with A23187, c48/80, or ATP or subjected to FcεRI aggregation was prevented by 100 nmol/L of the highly selective renin inhibitor BILA (Figures 2 and 3),1,2,12 which demonstrates that proteases other than renin were not involved in the formation of angiotensin I.
Figure 2.
Classical MC activators elicit degranulation and release of histamine and renin from mature BMMCs. BMMCs were incubated for 20 minutes at 37°C with A23187 (1 μmol/L), c48/80 (300 μg/mL), or ATP (1 mmol/L). A–C: Release of β-HEX (A), histamine (B), and renin protein (C). Percentages represent the percent ratio between supernatant and total β-HEX or histamine. D: Renin activity as affected by the three MC activators in the absence or presence of the renin inhibitor BILA (100 nmol/L) was measured in BMMC supernatant in terms of angiotensin I (Ang I) formation (μg/hour per mg of total protein). Data are expressed as means ± SEM. Number of experiments (left to right): n = 10, 7, 6, and 10 (A); n = 7, 14, 5, and 6 (B); n = 4, 5, 4, and 7 (C); and n = 6, 5, 8, 4, 3, 13, and 6 (D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus control. †P < 0.01, ††P < 0.001 versus absence of BILA.
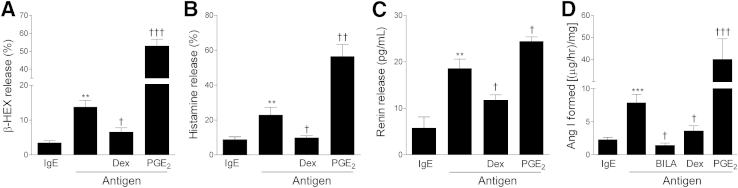
Figure 3.
FcεRI aggregation elicits the release of renin from BMMCs, modulated by dexamethasone (Dex) and PGE2. A–D: BMMCs were sensitized for 16 hours with murine anti-DNP IgE and then were challenged with DNP-HSA antigen for 20 minutes at 37°C in the presence or absence of 2 nmol/L dexamethasone (Dex), 1 μmol/L PGE2. In D, in the absence or presence of 100 nmol/L BILA to assess renin-induced angiotensin I formation. For release of β-HEX (A) and histamine (B), percentages represent the percent ratio between supernatant and total β-HEX or histamine. Renin activity (D) was measured in BMMC supernatant with antigen alone or with antigen and BILA, Dex, or PGE2. Data are expressed as means ± SEM. Number of experiments (left to right): n = 8, 21, 8, and 9 (A); n = 8, 21, 18, and 9 (B); n = 4, 4, 5, and 4 (C); and n = 16, 12, 3, 6, and 6 (D). ∗∗P < 0.01; ∗∗∗P < 0.001 versus control (IgE). †P < 0.05, ††P < 0.01, and †††P < 0.001 versus antigen.
BMMC Renin Release by FcεRI Cross-Linking
After passive sensitization of BMMCs with murine anti-DNP IgE, challenge with DNP-HSA also caused degranulation (approximately fourfold increase in β-HEX release, relative to control), release of histamine (approximately 2.5-fold increase), and release of renin protein (approximately threefold increase), as well as an increase in angiotensin I–forming activity (approximately fourfold increase) (Figure 3).
Given that anti-inflammatory glucocorticoids are known to prevent IgE-mediated MC degranulation,17 we assessed whether they also modulate MC renin release. When IgE-sensitized MCs were challenged with specific antigen in the presence of 2 nmol/L dexamethasone, β-HEX and histamine release were reduced by approximately 50% to 60%, whereas the release of renin protein and angiotensin I–forming activity decreased by approximately 40% and 60%, respectively (Figure 3).
PGE2 is known to modulate FcεRI-mediated MC activation.18 We therefore assessed whether this eicosanoid also modulates MC renin release. When IgE-sensitized MCs were challenged with the specific antigen in the presence of 1 μmol/L PGE2, the release of β-HEX, histamine, renin protein, and angiotensin I–forming activity increased approximately fourfold, 2.5-fold, 30%, and fivefold, respectively (Figure 3). This suggests that MC renin release and consequent RAS activation can be magnified in the course of inflammatory responses in which PGE2 is copiously produced.19
FcεRI Cross-Linking Induces Renin Release from Resident Tissue MCs
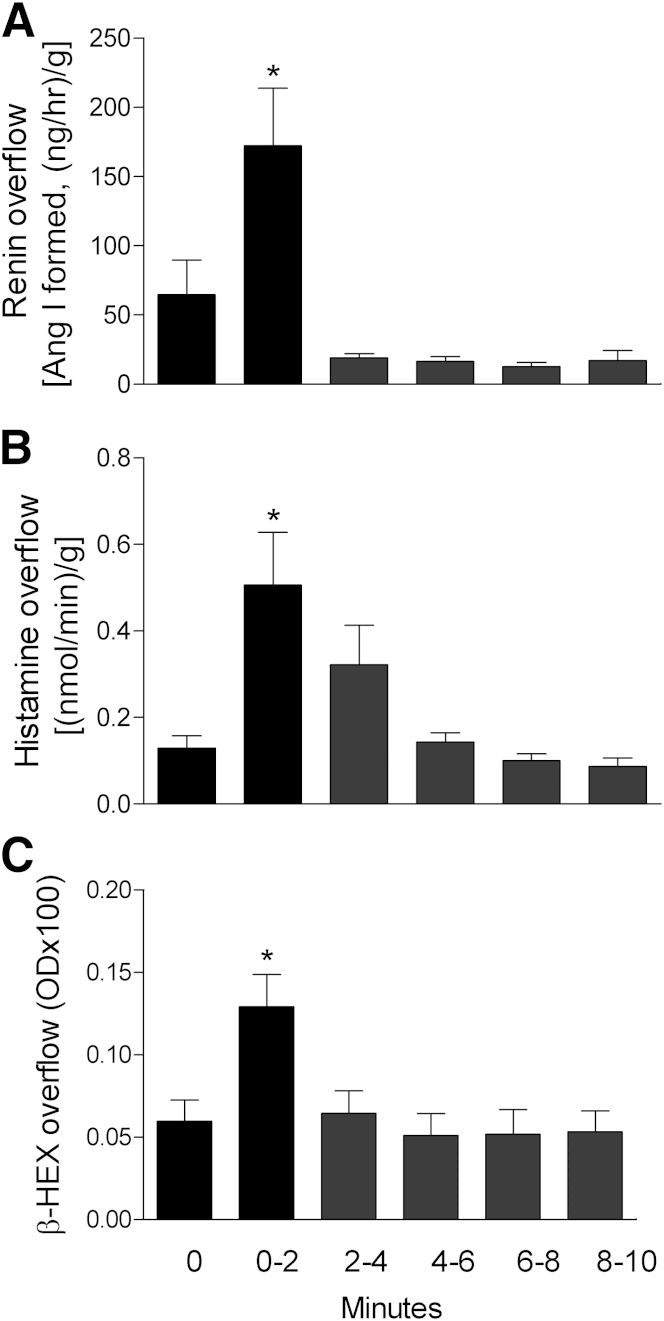
We next asked whether resident tissue MCs also release renin after FcεRI cross-linking. We used Langendorff-perfused hearts excised from mice passively sensitized with monoclonal anti-DNP IgE. Antigen challenge of these hearts resulted in substantial resident MC degranulation, as demonstrated by a marked increase in the coronary overflow of β-HEX, histamine, and renin activity in the first 2 minutes after antigen challenge (two-, four-, and threefold increase, respectively) (Figure 4). Notably, no release of β-HEX or histamine and no renin activity occurred after antigen challenge of hearts from control nonsensitized mice.
Figure 4.
FcεRI cross-linking on tissue-resident MCs elicits the release of renin from ex vivo mouse hearts. Time course of overflow of active renin (indicative of angiotensin I formation) (A), histamine (B), and β-HEX (marker of MC degranulation) (C) from Langendorff-perfused murine hearts. Mice were passively sensitized in vivo with 6 μg of murine monoclonal anti-DNP IgE; 24 hours later, hearts were excised and challenged with 200 μg of DNP-HSA. Overflow fractions were collected every 2 minutes after antigen injection. Data are expressed as means ± SEM. n = 5 to 7 hearts. *P < 0.05 versus coronary effluent preceding antigen, unpaired t-test. OD, optical density at 405 nm.
Discussion
Here, we report the novel finding that cross-linking of FcεRI-bound IgE antibodies on the surface of BMMCs and tissue-resident MCs elicits the release of enzymatically active renin protein. This process is inhibited by glucocorticoids and is enhanced by the proinflammatory mediator PGE2. To our knowledge, this is the first time that FcεRI aggregation has been shown to elicit MC renin release.
The calcium ionophore A2318713 was an effective elicitor of renin release from BMMCs, suggesting that activation of Ca2+ influx and phospholipase C–dependent mobilization of Ca2+ from intracellular stores20 play a role in MC renin release. Given that an increase in intracellular calcium inhibits renin release from juxtaglomerular cells,21 different mechanisms must control renin secretion in MCs and in the juxtaglomerular apparatus. The finding that c48/80 induced release of MC renin, together with β-HEX and histamine, suggests the presence of renin in extrudable granular formations, analogous to mastocytoma cells stimulated with c48/80 or substance P.1,16 Indeed, typical MC degranulation requires the expulsion of membrane-free granules from an intact cell capable of repeated degranulation,14 as we have previously observed in mastocytoma cells repeatedly stimulated to release renin with c48/80.1
Although ATP is known to elicit MC degranulation,15 the present study is the first to show that ATP promotes the release of active renin from MCs. This novel finding is of particular cardiovascular relevance, because sympathetic purinergic nerve terminals release ATP in close proximity to MCs,1,22 likely promoting renin release.
Dexamethasone inhibited FcεRI-induced MC degranulation and, most importantly, it also decreased renin release. A dexamethasone-induced decrease in MC degranulation has been attributed to reduced FcεRI expression on the MC surface,23 and it is reported that dexamethasone diminishes MC degranulation and cytokine production by suppressing FcεRI-induced activation of PI3-kinase.24 Furthermore, nongenomic mechanisms have been invoked in the rapid glucocorticoid-induced inhibition of IgE-mediated MC degranulation and histamine release in a guinea-pig asthma model in vivo and in the RBL-2H3 MC line in vitro. These effects were mediated by a suppression of the increase in intracellular Ca2+ that characterizes MC degranulation.25
The PGE2-induced potentiation suggests that MC renin release and consequent RAS activation can be magnified in the course of inflammatory responses in which PGE2 is copiously produced.19 Given the pervasiveness of PGE2 production in inflammatory reactions,26 and also the PGE2-induced potentiation of renin release from BMMCs, PGE2 can be envisioned as a major player in the activation of local RAS in various organs.
In conclusion, we found that FcεRI cross-linking on BMMCs and tissue-resident MCs elicits the release of enzymatically active renin protein. The angiotensin I–forming activity of the renin protein was completely blocked by the highly selective renin inhibitor BILA, a finding that excludes involvement of other proteases in the formation of angiotensin I. The finding that MC renin release was mimicked by canonical degranulators such as calcium ionophore and c48/80 suggests that MC renin may be stored in granules and that its release may depend on an increase in intracellular Ca2+. Our present findings indicate that IgE-mediated allergic hypersensitivity provokes the release of MC renin, which is potentiated by PGE2 and inhibited by dexamethasone. Given the ubiquitous presence of MCs,3,6,7 and the presence of angiotensinogen and angiotensin-converting enzyme in many tissues,27 renin release in allergic hypersensitivity reactions could result in local angiotensin II formation and angiotensin II–mediated multiorgan dysfunctions, a process likely exacerbated in an inflammatory background.
Acknowledgments
We thank Rajesh K. Singh and Rebal Turjoman for help with BMMC culture and Jason McCormick for help with fluorescence-activated cell sorting analysis.
Footnotes
Supported in part by the NIH (grants R01HL034215 and R37HL47073 to R.L.), the American Heart Association (grant-in-aid 11GRNT5600025 to R.L.), and scientist development (grant 11SDG5710010 to A.D.L.) and the Caja Madrid Foundation (P.A.R.).
References
- 1.Silver R.B., Reid A.C., Mackins C.J., Askwith T., Schaefer U., Herzlinger D., Levi R. Mast cells: A unique source of renin. Proc Natl Acad Sci USA. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackins C.J., Kano S., Seyedi N., Schäfer U., Reid A.C., Machida T., Silver R.B., Levi R. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid A.C., Silver R.B., Levi R. Renin: at the heart of the mast cell. Immunol Rev. 2007;217:123–140. doi: 10.1111/j.1600-065X.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang T.W., Wu P.C., Hsu C.L., Hung A.F. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Adv Immunol. 2007;93:63–119. doi: 10.1016/S0065-2776(06)93002-8. [DOI] [PubMed] [Google Scholar]
- 5.Kalesnikoff J., Galli S.J. Anaphylaxis: mechanisms of mast cell activation. In: Ring J., editor. Anaphylaxis. Karger; Basel: 2010. pp. 45–56. [DOI] [PubMed] [Google Scholar]
- 6.Galli S.J. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 7.Beaven M.A. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 9.Singh R.K., Mizuno K., Wasmeier C., Wavre-Shapton S.T., Recchi C., Catz S.D., Futter C., Tolmachova T., Hume A.N., Seabra M.C. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS J. 2013;280:892–903. doi: 10.1111/febs.12081. [DOI] [PubMed] [Google Scholar]
- 10.Seyedi N., Mackins C.J., Machida T., Reid A.C., Silver R.B., Levi R. Histamine H3-receptor-induced attenuation of norepinephrine exocytosis: a decreased PKA activity mediates a reduction in intracellular calcium. J Pharmacol Exp Ther. 2005;312:272–280. doi: 10.1124/jpet.104.072504. [DOI] [PubMed] [Google Scholar]
- 11.Koda K., Salazar-Rodriguez M., Corti F., Chan N.Y.-K., Estephan R., Silver R.B., Mochly-Rosen D., Levi R. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation. 2010;122:771–781. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simoneau B., Lavallée P., Anderson P.C., Bailey M., Bantle G., Berthiaume S., Chabot C., Fazal G., Halmos T., Ogilvie W.W., Poupart M.A., Thavonekham B., Xin Z., Thibeault D., Bolger G., Panzenbeck M., Winquist R., Jung G.L. Discovery of non-peptidic P2-P3 butanediamide renin inhibitors with high oral efficacy. Bioorg Med Chem. 1999;7:489–508. doi: 10.1016/s0968-0896(98)00265-x. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein L.M. The mechanism of basophil histamine release induced by antigen and by the calcium ionophore A23187. J Immunol. 1975;114:1692–1699. [PubMed] [Google Scholar]
- 14.Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80–induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971;51:465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlquist R., Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 1974;34:368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- 16.Morrey C., Brazin J., Seyedi N., Corti F., Silver R.B., Levi R. Interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunction. J Pharmacol Exp Ther. 2010;335:76–84. doi: 10.1124/jpet.110.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heiman A.S., Crews F.T. Hydrocortisone selectively inhibits IgE-dependent arachidonic acid release from rat peritoneal mast cells. Prostaglandins. 1984;27:335–343. doi: 10.1016/0090-6980(84)90084-4. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen M., Solle M., Audoly L.P., Tilley S.L., Stock J.L., McNeish J.D., Coffman T.M., Dombrowicz D., Koller B.H. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J Immunol. 2002;169:4586–4593. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi M., Rosenberg D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dedkova E.N., Sigova A.A., Zinchenko V.P. Mechanism of action of calcium ionophores on intact cells: ionophore-resistant cells. Membr Cell Biol. 2000;13:357–368. [PubMed] [Google Scholar]
- 21.Kurtz A. Renin release: sites, mechanisms, and control. Annu Rev Physiol. 2011;73:377–399. doi: 10.1146/annurev-physiol-012110-142238. [DOI] [PubMed] [Google Scholar]
- 22.Bulanova E., Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6:3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi M., Hirai K., Komiya A., Miyamasu M., Furumoto Y., Teshima R., Ohta K., Morita Y., Galli S.J., Ra C., Yamamoto K. Regulation of mouse mast cell surface Fc epsilon RI expression by dexamethasone. Int Immunol. 2001;13:843–851. doi: 10.1093/intimm/13.7.843. [DOI] [PubMed] [Google Scholar]
- 24.Andrade M.V., Hiragun T., Beaven M.A. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 2004;172:7254–7262. doi: 10.4049/jimmunol.172.12.7254. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J., Liu D.F., Liu C., Kang Z.M., Shen X.H., Chen Y.Z., Xu T., Jiang C.L. Glucocorticoids inhibit degranulation of mast cells in allergic asthma via nongenomic mechanism. Allergy. 2008;63:1177–1185. doi: 10.1111/j.1398-9995.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 26.Gomi K., Zhu F.G., Marshall J.S. Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J Immunol. 2000;165:6545–6552. doi: 10.4049/jimmunol.165.11.6545. [DOI] [PubMed] [Google Scholar]
- 27.Paul M., Poyan M.A., Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]