Abstract
A major limitation in the pharmacological treatment of pulmonary arterial hypertension (PAH) is the lack of pulmonary vascular selectivity. Recent studies have identified a tissue-penetrating homing peptide, CARSKNKDC (CAR), which specifically homes to hypertensive pulmonary arteries but not to normal pulmonary vessels or other tissues. Some tissue-penetrating vascular homing peptides have a unique ability to facilitate transport of co-administered drugs into the targeted cells/tissues without requiring physical conjugation of the drug to the peptide (bystander effect). We tested the hypothesis that co-administered CAR would selectively enhance the pulmonary vascular effects of i.v. vasodilators in Sugen5416/hypoxia/normoxia-exposed PAH rats. Systemically administered CAR was predominantly detected in cells of remodeled pulmonary arteries. Intravenously co-administered CAR enhanced pulmonary, but not systemic, effects of the vasodilators, fasudil and imatinib, in PAH rats. CAR increased lung tissue imatinib concentration in isolated PAH lungs without increasing pulmonary vascular permeability. Sublingual CAR was also effective in selectively enhancing the pulmonary vasodilation by imatinib and sildenafil. Our results suggest a new paradigm in the treatment of PAH, using an i.v./sublingual tissue-penetrating homing peptide to selectively augment pulmonary vascular effects of nonselective drugs without the potentially problematic conjugation process. CAR may be particularly useful as an add-on therapy to selectively enhance the pulmonary vascular efficacy of any ongoing drug treatment in patients with PAH.
Pulmonary arterial hypertension (PAH) remains a highly fatal syndrome, despite recent advancements in its treatment.1,2 New candidate drugs, such as Rho kinase inhibitors3–6 and tyrosine kinase inhibitors,7,8 have shown promise in providing improved treatment for PAH. However, the clinical application of these agents has generally been hampered by their systemic toxicity/adverse effects.9–12 Inhalation is a relatively selective drug delivery method for the lungs,13 but there are several problems with the clinical use of aerosol inhalation in the treatment of PAH.14 In fact, insufficient inhalation due to technical problems may be a major limitation for the effectiveness of inhaled Rho kinase inhibitor, fasudil, in patients with PAH.15
In numerous diseases, the blood vessels in the affected organ express disease-specific cell surface markers (vascular zip codes).16 For example, tumor blood vessels express distinctive markers not present in vessels of normal tissues.16 These target organ–specific molecular structures are detectable by systemically administered homing peptides,16,17 which find their way to the desired location by binding to their receptor expressed in the blood vessels of the target organ. Promising results are accumulating for the use of homing peptides to deliver drugs selectively to tumors.16–18 This potentially useful targeting technology has not yet been applied to the treatment of PAH, although vascular selectivity would be desired in PAH.
We have recently reported tumor-homing peptides that specifically bind to tumor vessels and then extravasate into the tumor tissue, carrying a payload with them.18,19 Even more important, a tumor-penetrating peptide can transport co-administered drugs into the target tissue without chemically coupling the compound with the peptide (bystander effect).20 This novel mechanism involves homing peptide–induced activation of an endocytic trans-tissue transport pathway, named CendR-pathway, in the target tissue of the peptide.18–20 The bystander effect provides the advantage of promoting delivery of a drug to its target tissue without the coupling of the drug to the peptide required in conventional drug targeting.20
CARSKNKDC (CAR) is a vascular homing peptide originally identified as being capable of homing to the neovasculature in injured, regenerating tissues.21 CAR requires heparan sulfate on the target cells for cell binding and uptake into cells,21 suggesting that this peptide may recognize a unique sulfation pattern of heparan sulfate proteoglycans on the target cells. When CAR is fused together with the anti-fibrotic protein, decorin, as a recombinant fusion protein, it accumulates in the wound and penetrates deep into wound granulation tissue.22,23 More recently, we have shown that CAR also selectively accumulates in the walls of hypertensive pulmonary arteries in monocrotaline- and Sugen5416/hypoxia/normoxia-induced (SU/Hx/Nx) PAH rats, but not in normotensive pulmonary arteries.24 This study also showed that CAR penetrates beyond the vascular endothelium; it accumulates in the entire wall of hypertensive pulmonary arteries and in the surrounding lung parenchyma.24
The tissue-penetrating activity of CAR prompted us to examine whether CAR might also induce a bystander effect, and if it could be used to selectively transport co-administered drugs to CAR’s homing destination (ie, PAH tissue). We have tested the effect of CAR co-administration on three vasodilators: i) the Rho kinase inhibitor, fasudil; ii) the tyrosine kinase inhibitor, imatinib; and iii) the phosphodiesterase type 5 inhibitor, sildenafil. They were tested on blood pressure in PAH rats. We have also determined whether i.v. administration of CAR could be replaced by sublingual dosing.
Materials and Methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of South Alabama (Mobile). PAH was experimentally induced in adult male Sprague-Dawley rats (weighing 204 ± 31 and 323 ± 30 g at the time of Sugen5416 injection and the hemodynamic experiments, respectively; n = 49) by s.c. injection of Sugen5416 (20 mg/kg; Cayman, Ann Arbor, MI) and exposure to hypoxia (10% O2) for 3 weeks. The rats were then returned to normoxia (21% O2) for an additional 2 weeks (5 weeks after the SU injection; SU/Hx/Nx PAH rats).5
Hemodynamic Measurements in Catheterized Rats
All rats were placed on controlled heating pads after they were anesthetized with i.p. 30 mg/kg pentobarbital sodium. Hemodynamic measurements were performed under normoxic conditions, as previously described, with minor modifications.25 Briefly, polyvinyl catheters (internal diameter, 0.28 mm) were inserted into the right ventricle (RV) via the right jugular vein for measurement of RV systolic pressure (RVSP). RVSP, instead of pulmonary arterial pressure, was measured, because the pulmonary artery cannot be routinely catheterized in these severely pulmonary hypertensive rats that have an altered structure of the RV chamber. In cases when we have successfully catheterized the artery, we confirmed that there was no difference between RVSP and systolic pulmonary arterial pressure (data not shown). A microtipped P-V catheter (1.4 Fr; Millar Instruments, Houston, TX) was inserted into the right carotid artery to measure systolic systemic arterial pressure (SAP). In some cases, the catheter was advanced into the left ventricle to measure cardiac output, which was derived from a pressure-volume curve. The cardiac index (CI) was calculated by dividing cardiac output by body weight. The signals were continuously recorded by an MPVS-300 system with PowerLab/4SP, A/D converter (AD Instruments, Colorado Springs, CO), and a personal computer. An additional polyvinyl catheter was inserted into the left jugular vein for the injection of drugs.
IHC Data
Two hours after i.v. injection of 3 mg/kg fluorescein-labeled CAR in SU/Hx/Nx rats, lungs were inflated with formalin-agarose mixture at a constant pressure of 20 cm H2O before the fixation. Immunohistochemical (IHC) staining was performed using rabbit anti-fluorescein isothiocyanate antibody (Invitrogen, Carpenteria, CA), followed by labeled polymer–horseradish peroxidase anti-rabbit IgG secondary antibody (Dako Envison+System-HRP), as described previously.24
Isolated Perfused Lungs and Assessment of Endothelial Permeability
The techniques of lung isolation, ventilation, and constant-flow perfusion with physiological salt solution (Earle’s balanced salt solution; Sigma, St. Louis, MO) have been described previously.5 After the blood was flushed out of the lungs with 20 mL of physiological salt solution, the lungs were perfused with a recirculated volume of 30 mL. The filtration coefficient (Kf) was measured under zone 3 conditions, as previously described.26 Kf was calculated as the rate of weight gain 13 to 15 minutes after a 7 to 10 cm H2O increase in pulmonary venous pressure, normalized per gram of lung dry weight. Kf, the product of specific endothelial permeability and surface area for exchange, is a sensitive measure of lung endothelial permeability when surface area is fully recruited.26
Measurement of Imatinib Concentration by Mass Spectrometry
Lung tissue imatinib concentrations were measured by MicroConstants, Inc., (San Diego, CA) in isolated salt solution–perfused lungs from SU/Hx/Nx rats. After equilibration for 20 minutes, 3 μmol/L imatinib or 3 μmol/L imatinib and 1 mg/30 mL CAR mixture was added to the perfusate reservoir. The lungs were flushed with 30 mL of Earle’s balanced salt solution 5 minutes after the drug administration to remove any residual drugs and were then snap frozen and kept at −80°C. The lungs were homogenized by placing the tissue into 2-mL Matrix tubes from MP Biomedicals (Solon, OH) and adding PBS, pH 7.4, to the tissue, with a 3:1 ratio (v/w). The samples were processed to a smooth homogenate using a Fastprep-24 (MP Biomedicals). Homogenized tissue samples were diluted at least 10-fold using rat plasma with sodium heparin as the anticoagulant. Samples (50.0 μL) containing imatinib with imatinib-d8 as the internal standard were processed simultaneously with spiked standards in rat plasma by adjusting the pH with a 3% ammonia solution and extracted using a solvent mixture of methyl tert butyl ether/hexane (4 mL; 3:1, v/v) and vortex mixing for 5 minutes. The samples were centrifuged to separate the aqueous and organic layers and subsequently kept at −70°C until the lower aqueous layer was frozen. The organic portions were poured into new tubes containing 25.0 μL of 4% propylene glycol in isopropyl alcohol and dried in a TurboVap set at 40°C under a stream of nitrogen gas. The extracts were reconstituted with 50 mmol/L ammonium formate, 0.1% formic acid in water:0.1% formic acid in acetonitrile (400 μL; 10:90, v/v). Analysis was performed on a system composed of an Agilent 1100 high-performance liquid chromatography system coupled to a Waters Quattro Ultima mass spectrometer. The high-performance liquid chromatography system was fitted with an Allure PFP Propyl column (100 × 2.1 mm in diameter, 5 μm) maintained at 40°C. The mobile phase was 50 mmol/L ammonium formate and 0.1% formic acid in water/0.1% formic acid in acetonitrile (11:89, v/v). Imatinib and the internal standard were monitored using transitions of 494.71 > 394.47 and 503.96 > 395.28, respectively. The cone voltage was 50 V, and the collision energy was 23 eV.
Effects of I.V. CAR on Vasodilators
Bolus Injection
We examined short-term hemodynamic effects of the co-administration of CAR and fasudil or imatinib in catheterized SU/Hx/Nx PAH rats. After baseline measurements, a mixture of 3 mg/kg CAR (dose based on previous homing studies21,24) and 0.1, 1, 3, and 10 mg/kg fasudil at 15-minute intervals or 3 mg/kg CAR and 5 and 20 mg/kg imatinib at 15-minute intervals was administered via left jugular vein, and the parameters were continuously monitored. At the end of measurements, the hearts were dissected for calculation of RV/left ventricle + septum weight ratio.
Continuous Infusion
We first determined an infusion rate of 10 mg/mL fasudil that caused minimal reductions in SAP and RVSP in SU/Hx/Nx rats. We then infused fasudil i.v. with and without 3 mg/mL CAR at the determined rate of 5 mL/minute using an infusion pump in SU/Hx/Nx PAH rats and monitored the parameters over 30 minutes.
Effects of Sublingual CAR on Vasodilator Activity
We examined the effects of sublingually administered CAR on imatinib- and sildenafil-induced vasodilation in SU/Hx/Nx rats. Fifteen minutes after sublingual administration of 3 mg/kg fluorescein-conjugated CAR, or the same volume of the saline vehicle, 5 and 20 mg/kg imatinib at 15-minute intervals or 3 and 30 mg/kg sildenafil at 15-minute intervals were given i.v. in anesthetized and catheterized rats. After the hemodynamic measurements, the rats were euthanized, and blood was flushed with 30 mL of PBS. Organs were fixed for subsequent IHC analysis.
Pulmonary Vascular Permeability
Pulmonary vascular permeability was estimated by measuring the Kf26 in isolated perfused lungs from SU/Hx/Nx rats. After 20 minutes of equilibration, Kf values were measured before and 30 minutes after the addition of 1 mg/30 mL CAR mixture.
Statistical Analysis
Values are means ± SEM. Comparisons between groups were made with the Student’s unpaired t-test. Differences were considered significant at P < 0.05.
Results
Occlusive Pulmonary Arterial Lesions in SU/Hx/Nx PAH Rats Are Homing Targets of CAR
We used the SU/Hx/Nx rat model of PAH to study the effect of CAR co-administration on the activity of vasodilators. The SU/Hx/Nx rats had a high RVSP (102 ± 3 mm Hg; n = 33) compared with control rats (24 ± 1 mm Hg; n = 8). Small pulmonary arteries and arterioles displayed severe, occlusive, neointimal lesions in association with the expression of the endothelial cell marker, von Willebrand factor, as reported previously for the PAH model.25,27 Consistent with a previous study,24 i.v. administered CAR was detected in remodeled pulmonary arteries of SU/Hx/Nx rats (Figure 1, H and I), but not in normal pulmonary arteries (Figure 1, A and B). CAR was distributed in all layers of the remodeled arteries. CAR produced a much lower signal, if any, in other organs (liver, spleen, and heart), except for the kidney, where the peptide is excreted (Figure 1, C–G and J–N). The intensive CAR signal in the kidney was found only in tubules, but not in other structures, such as arteries and glomeruli (Figure 1, E and L). This supports the previous studies21,24 that the presence of CAR in the kidneys is not the result of homing, but the fact that all proteins/peptides with a molecular weight of <60 kDa are excreted through the kidneys.
Figure 1.
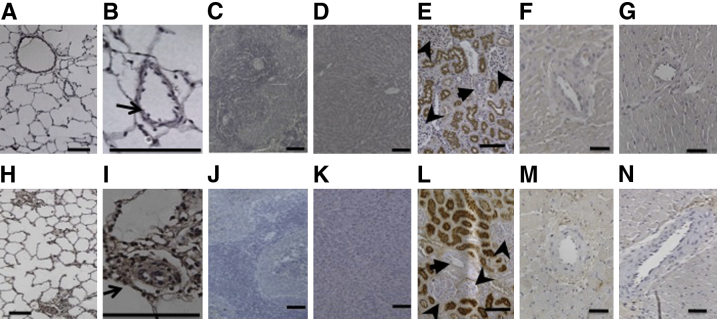
Distribution of CAR in tissues of a normal (A–G) and an SU/Hx/Nx PAH (H–N) rat. Samples were collected 2 hours after i.v. injection of 3 mg/kg body weight fluorescein-labeled CAR. A and H: Low-power magnification images of lung tissue. B and I: High-power magnification images of a normal and severely remodeled pulmonary artery. C–G and J–N: Low-power magnification images of tissues from spleen (C and J), liver (D and K), kidney (E and L), and right (F and M) and left (G and N) ventricles. Arrows indicate renal arteries; arrowheads, glomeruli. Scale bars: 100 μm.
Hemodynamic Effects of Co-Administered CAR on Vasodilators
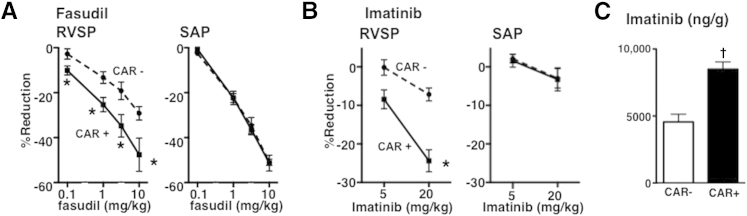
Short-term bolus i.v. injections of fasudil alone caused marked dose-dependent reductions in both RVSP and SAP in anesthetized PAH rats (Figure 2A). Intravenously injecting 3 mg/kg CAR alone had no effects on RVSP, SAP, or CI in PAH rats (RVSP, from 99 ± 17 to 102 ± 19 mm Hg; SAP, from 160 ± 8 to 159 ± 7 mm Hg; CI, from 123 ± 39 to 129 ± 40 mL/minute per kilogram; n = 3). Co-administered CAR significantly enhanced the short-term pressure-lowering effects of fasudil on RVSP, but not on SAP (Figure 2A). Co-administered CAR with 0.1 mg/kg fasudil had no effect on CI when compared with fasudil alone (Table 1).
Figure 2.
Enhancing effects of CAR on fasudil- and imatinib-induced pulmonary vasodilation and on lung tissue imatinib accumulation. Dose-response curves to fasudil (A) and imatinib (B) (i.v., bolus), mixed with and without 3 mg/kg CAR for RVSP and SAP in SU/Hx/Nx PAH rats. Values are means ± SEM. N = 5 to 6. ∗P < 0.05 versus without CAR. C: Imatinib content (ng) in 1 g lung tissue after addition of 3 mmol/L imatinib with (+) and without (−) 1 mg CAR to 30 mL perfusate of isolated perfused lungs from SU/Hx/Nx rats. N = 5 and 4 for CAR+ and CAR−, respectively. †P < 0.05 versus CAR–.
Table 1.
Cardiac Index Before and After i.v. Injection of Agents
| Agent | Before injection | After injection |
|---|---|---|
| CAR (n = 5) | 123 ± 39 | 129 ± 40 |
| Fasudil (n = 3) | 116 ± 7 | 129 ± 7 |
| CAR + fasudil (n = 3) | 90 ± 7 | 103 ± 6 |
Values are means ± SEM mL/minute per kilogram.
We next studied whether CAR would potentiate the activity of vasodilators that act through a different molecular mechanism than the Rho kinase inhibition. Short-term i.v. bolus injections of imatinib alone induced a dose-dependent and pulmonary preferential reduction in blood pressure (Figure 2B), which is in agreement with our previous report,7 although this effect may be limited to the hypertensive pulmonary circulation.28 Similar to what was observed with fasudil, co-administered CAR also effectively enhanced imatinib-induced pulmonary, but not systemic, vasodilation, resulting in a near pulmonary-specific vasodilation at a dose of 20 mg/kg (Figure 2B). Co-administered CAR with 20 mg/kg imatinib had no effects on CI when compared with imatinib alone (Table 1).
Imatinib Concentration in Isolated PAH Lungs
To examine whether CAR increases drug transport into the hypertensive lungs, we measured tissue concentrations of imatinib in isolated, salt solution–perfused PAH lungs. Co-administered CAR markedly increased imatinib levels in PAH lung tissue compared with imatinib given alone (Figure 2C).
Continuous I.V. Infusion
To test whether a continuous infusion of a vasodilator at low concentrations in the presence of CAR could enhance pulmonary and reduce systemic effects of the vasodilator, we i.v. administered a mixture of fasudil plus CAR at a low infusion rate in PAH rats. We found that infusion of a mixture of CAR and fasudil produced an augmented and sustained reduction in RVSP (32.3% ± 7.2%; n = 3), with a much smaller decrease in SAP (11.7% ± 1.7%; n = 3) (Supplemental Figure S1). In contrast, infusion of fasudil alone appeared to reduce SAP more than RVSP (18.9% ± 4.8% versus 10.1% ± 6.1%; n = 4 each) (Supplemental Figure S1), although the difference was not statistically significant.
Lack of CAR Effects on Pulmonary Vascular Permeability
CAR (1 mg/30 mL perfusate) did not increase the filtration coefficient in isolated salt solution–perfused PAH lungs (from 0.026 ± 0.003 to 0.024 ± 0.007 mL/minute per cm H2O per gram weight; n = 3). In support of these findings, no sign of lung edema formation was observed histologically.
Effects of Sublingually Administered CAR on Vasodilators
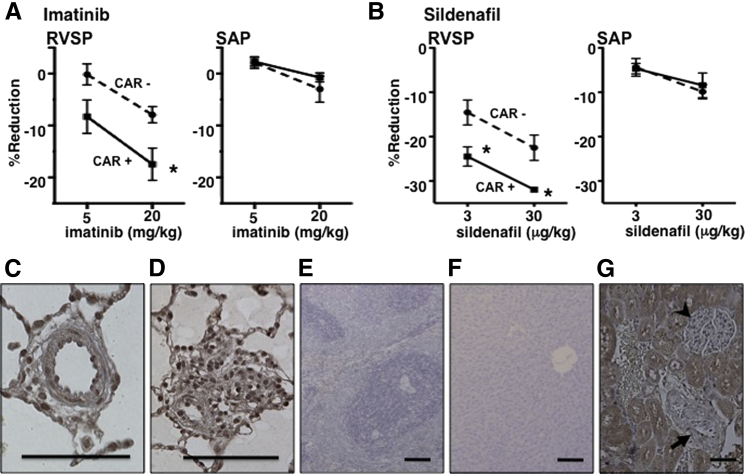
We next tested whether CAR was active when given sublingually. Sublingual administration of CAR effectively augmented the short-term pulmonary vasodilatory effect of imatinib (Figure 3A). The activity of sildenafil was similarly augmented by sublingual CAR (Figure 3B). Histological analysis of the tissues revealed a CAR signal in all layers of the remodeled pulmonary arteries 1 hour after sublingual administration of CAR to PAH rats (Figure 3, C and D). Similar to the findings with i.v. injections, a much lower signal, if any, was found for CAR in other organs (liver and spleen), except for the excretion route, kidneys (Figure 3, E–G).
Figure 3.
Effects of sublingually administered CAR. Effects of sublingually administered 3 mg/kg CAR on i.v. (bolus) imatinib-induced (A) and sildenafil-induced (B) reduction in RVSP and SAP in SU/Hx/Nx PAH rats. Values are means ± SEM. N = 4 to 6. ∗P < 0.05 versus without CAR. Distribution of sublingually administered CAR: a pulmonary artery with medial thickening (C), a small pulmonary artery with neointimal occlusion (D), liver (E), spleen (F), and kidney (G) from an SU/Hx/Nx rat. Arrow indicates renal artery; arrowhead, glomerulus. Scale bars: 100 μm.
Discussion
We show herein that simple co-administration of the vascular homing peptide, CAR, which targets PAH vasculature, significantly enhances the short-term blood pressure–lowering effects of vasodilator drugs on RVSP, but not on SAP, in PAH rats. Our results potentially provide a new paradigm for target tissue–specific pharmacological intervention in PAH (Supplemental Figure S2).
CAR is a cell- and tissue-penetrating homing peptide, as shown by the accumulation of CAR inside cultured cells, penetrating deep into granulation tissue in wounds and into the walls of remodeled pulmonary vessels in PAH rats.21–24 The homing of CAR is specific for hypertensive pulmonary vessels,24 because it does not accumulate in normal tissues, including normal pulmonary arteries.21,24 We initially tried to make use of the tissue-penetrating properties of CAR in drug delivery by coupling a drug (fasudil) to the peptide. However, the coupling inactivated the drug (M. Oka and E. Ruoslahti, unpublished results). We then tested a different strategy, the bystander effect (ie, co-administration of the drug with the peptide with no physical coupling of the two to each another).20 Our approach was inspired by the recent demonstration that another tissue-penetrating peptide (iRGD; single amino acid code: CRGDKGPDC) could be used in this manner to enhance drug delivery into its target organ, tumors.20 There are some differences in the tissue-penetration mechanism of the two peptides: iRGD activates an endocytic trans-tissue transport pathway dependent on neuropilin-1,18–20 whereas CAR uses another, yet to be defined, pathway, which involves binding to the heparan sulfate proteoglycans on the cell surface.21 However, the functions of the two peptides appear to be similar because we find that, as has been shown for various drugs co-administered with iRGD in tumors,20 CAR enhances the accumulation of co-administered imatinib in its target tissue, the PAH vasculature (Supplemental Figure S2).
Orally administered peptides are readily degraded by digestive tract peptidases, and their oral bioavailability is poor. Thus, direct i.v. injection remains the most common and effective means of administering therapeutic peptides. However, an oral route would be the preferred route because of its high level of patient acceptance and long-term compliance, both factors that increase the therapeutic value of the drug. Peptides that have a cyclic conformation and contain disulfide bonds tend to be more resistant to peptidases than linear peptides.29 Thus, the disulfide-bonded, cyclic structure of CAR may provide a certain degree of resistance to proteases. Because the sublingual region has lower enzyme activity than the digestive tract,30 we tested sublingual administration of CAR and found that CAR effectively augmented the short-term pulmonary vasodilatory effect of imatinib and sildenafil and, similar to intravenously administered CAR, the peptide predominantly accumulated in the PAH vasculature. These results suggest that active CAR peptide can be absorbed via the sublingual mucosa to reach the target organ, hypertensive lungs. Surprisingly, sublingual CAR was as effective as bolus-injected CAR, suggesting the superiority of the sublingual route as the most practical route of CAR administration.
We have shown that CAR homes to hypertensive pulmonary arteries of two different preclinical models of pulmonary hypertension (monocrotaline and SU/Hx/Nx rats).24 Furthermore, CAR binding and uptake to human cells and tissue xenografts has been shown.24 However, additional studies are warranted to examine if CAR works similarly in human PAH as in the rat model of PAH.
Other limitations of our study stem from the peptide nature of CAR, an unknown half-life in circulation, and susceptibility to proteases. These factors could make i.v./oral administration impractical, except in the management of acute crises in a hospital setting. The successful sublingual administration of CAR may provide a solution to these limitations.
We have demonstrated that the vascular homing peptide, CAR, selectively binds to the hypertensive pulmonary arteries, and that it penetrates into the endothelium and fibrotic tissue of the severely remodeled pulmonary arterial wall in a rat model of PAH, which closely mimics the human disorder. Simple co-administration of CAR selectively enhances the pulmonary vascular effects of various systemically administered vasodilators (ie, fasudil, imatinib, and sildenafil). The peptide increases the lung tissue imatinib concentration in isolated perfused PAH lungs, presumably because CAR facilitates transport of co-administered drugs into PAH tissue, without increasing pulmonary vascular permeability. More important, our results suggest that CAR is active when sublingually administered, potentially providing a more practical route of administration of CAR than i.v. injections. These findings potentially have high clinical significance for the treatment of PAH, because CAR enables decreasing drug dosage to reduce its systemic adverse effects without decreasing the pulmonary efficacy of the drug, which has never been achieved by any other means. Our results open the door to a new direction of PAH treatment and warrant further investigations.
Acknowledgments
We thank David Johnson and MicroConstants, Inc., for the analytical work and Dr. Yukimitsu Kuwabara for his technical assistance.
Footnotes
Supported by NIH grants HL106101 and CA125255 (M.K.), CA152327 (E.R.), Department of Defense grant X81XWH-08-2-0032 (E.R.), American Heart Association grant 12080100 (M.K.), Florida Biomed grant 2KF04 (M.K.), the Academy of Finland (T.A.H.J.), the Sigrid Juselius Foundation (T.A.H.J.), the Finnish Medical Foundation (T.A.H.J.), the Instrumentarium Research Foundation (T.A.H.J.), Vascular BioSciences (D.M.), and the Department of Pharmacology and the Center for Lung Biology, University of South Alabama (M.O. and I.F.M.).
Disclosures: D.M. is an employee and shareholder of Vascular BioSciences and VBS Pharmaceuticals, E.R. is a shareholder of VBS Pharmaceuticals, and M.K. and T.A.H.J. are warrant holders of VBS Pharmaceuticals.
Supplemental Data
Hemodynamic effects of i.v. coinfusion of CAR and fasudil. A: Representative pressure tracings of effects of 3 mg/mL CAR and 10 mg/mL fasudil mixture infusion (5 mL/minute) on SAP and RVSP in an SU/Hx/Nx PAH rat. B: Maximum reductions in SAP and RVSP by fasudil + CAR (+CAR) and fasudil alone (−CAR) infusions. Values are means ± SEM. n = 3 for +CAR, and n = 4 for −CAR. ∗P < 0.05 versus SAP +CAR, +P < 0.05 versus RVSP −CAR.
Hypothesized effect of CAR co-administration on fasudil/vasodilatory drugs in PAH. A: When fasudil is given alone as a bolus i.v. injection, fasudil molecules (yellow circles) enter into hypertensive pulmonary and normal systemic arterial smooth muscle cells evenly to cause similar degrees of hypotension throughout the vasculature. B: When CAR is coinjected with fasudil, CAR (red triangles) binds to its receptor (green double triangles) expressed specifically in the PAH vasculature and enhances the transport of fasudil to the target organ, the hypertensive pulmonary arteries. The outcome is the augmented pulmonary-selective vasodilation. The high plasma concentration of fasudil still causes a similar degree of hypotension as fasudil given alone on the systemic side of the circulation. C: When the same dose of fasudil and CAR (as in the bolus injection) are infused slowly over a long period, CAR peptide is able to transport enough fasudil into the hypertensive pulmonary artery to cause a substantial vasodilatory response, whereas the low plasma concentration of fasudil produces a limited degree of systemic vasodilation.
References
- 1.Galie N., Manes A., Negro L., Palazzini M., Bacchi-Reggiani M.L., Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin V.V., Archer S.L., Badesch D.B., Barst R.J., Farber H.W., Lindner J.R., Mathier M.A., McGoon M.D., Park M.H., Rosenson R.S., Rubin L.J., Tapson V.F., Varga J., Harrington R.A., Anderson J.L., Bates E.R., Bridges C.R., Eisenberg M.J., Ferrari V.A., Grines C.L., Hlatky M.A., Jacobs A.K., Kaul S., Lichtenberg R.C., Lindner J.R., Moliterno D.J., Mukherjee D., Pohost G.M., Rosenson R.S., Schofield R.S., Shubrooks S.J., Stein J.H., Tracy C.M., Weitz H.H., Wesley D.J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology foundation task force on expert consensus documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 3.Abe K., Shimokawa H., Morikawa K., Uwatoku T., Oi K., Matsumoto Y., Hattori T., Nakashima Y., Kaibuchi K., Sueishi K., Takeshit A. Long-term treatment with a rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 4.Nagaoka T., Morio Y., Casanova N., Bauer N., Gebb S., McMurtry I., Oka M. Rho/rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–L672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 5.Oka M., Homma N., Taraseviciene-Stewart L., Morris K.G., Kraskauskas D., Burns N., Voelkel N.F., McMurtry I.F. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 6.Parikh V.N., Jin R.C., Rabello S., Gulbahce N., White K., Hale A., Cottrill K.A., Shaik R.S., Waxman A.B., Zhang Y.Y., Maron B.A., Hartner J.C., Fujiwara Y., Orkin S.H., Haley K.J., Barabasi A.L., Loscalzo J., Chan S.Y. Microrna-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe K., Toba M., Alzoubi A., Koubsky K., Ito M., Ota H., Gairhe S., Gerthoffer W.T., Fagan K.A., McMurtry I.F., Oka M. Tyrosine kinase inhibitors are potent acute pulmonary vasodilators in rats. Am J Respir Cell Mol Biol. 2011;45:804–808. doi: 10.1165/rcmb.2010-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schermuly R.T., Dony E., Ghofrani H.A., Pullamsetti S., Savai R., Roth M., Sydykov A., Lai Y.J., Weissmann N., Seeger W., Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussemaker E., Pistrosch F., Forster S., Herbrig K., Gross P., Passauer J., Brandes R.P. Rho kinase contributes to basal vascular tone in humans: role of endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol. 2007;293:H541–H547. doi: 10.1152/ajpheart.00770.2006. [DOI] [PubMed] [Google Scholar]
- 10.Casey D.B., Badejo A.M., Dhaliwal J.S., Sikora J.L., Fokin A., Golwala N.H., Greco A.J., Murthy S.N., Nossaman B.D., Hyman A.L., Kadowitz P.J. Analysis of responses to the rho-kinase inhibitory-27632 in the pulmonary and systemic vascular bed of the rat. Am J Physiol Heart Circ Physiol. 2010;299:H184–H192. doi: 10.1152/ajpheart.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkela R., Grazette L., Yacobi R., Iliescu C., Patten R., Beahm C., Walters B., Shevtsov S., Pesant S., Clubb F.J., Rosenzweig A., Salomon R.N., Van Etten R.A., Alroy J., Durand J.B., Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 12.Murthy S.N., Nossaman B.D., Kadowitz P.J. New approaches to the treatment of pulmonary hypertension: from bench to bedside. Cardiol Rev. 2010;18:76–84. doi: 10.1097/CRD.0b013e3181cbcbf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagaoka T., Fagan K.A., Gebb S.A., Morris K.G., Suzuki T., Shimokawa H., McMurtry I.F., Oka M. Inhaled rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- 14.Melani A.S. Inhalatory therapy training: a priority challenge for the physician. Acta Biomed. 2007;78:233–245. [PubMed] [Google Scholar]
- 15.Fujita H., Fukumoto Y., Saji K., Sugimura K., Demachi J., Nawata J., Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels. 2010;25:144–149. doi: 10.1007/s00380-009-1176-8. [DOI] [PubMed] [Google Scholar]
- 16.Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans. 2004;32:397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- 17.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012;24:3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugahara K.N., Teesalu T., Karmali P.P., Kotamraju V.R., Agemy L., Girard O.M., Hanahan D., Mattrey R.F., Ruoslahti E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510–520. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teesalu T., Sugahara K.N., Kotamraju V.R., Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci U S A. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugahara K.N., Teesalu T., Karmali P.P., Kotamraju V.R., Agemy L., Greenwald D.R., Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Järvinen T.A.H., Ruoslahti E. Molecular changes in the vasculature of injured tissues. Am J Pathol. 2007;171:702–711. doi: 10.2353/ajpath.2007.061251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Järvinen T.A.H., Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci U S A. 2010;107:21671–21676. doi: 10.1073/pnas.1016233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Järvinen T.A.H., Ruoslahti E. Targeted antiscarring therapy for tissue injuries. Adv Wound Care. 2013;2:50–54. doi: 10.1089/wound.2011.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urakami T., Jarvinen T.A., Toba M., Sawada J., Ambalavanan N., Mann D., McMurtry I., Oka M., Ruoslahti E., Komatsu M. Peptide-directed highly selective targeting of pulmonary arterial hypertension. Am J Pathol. 2011;178:2489–2495. doi: 10.1016/j.ajpath.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe K., Toba M., Alzoubi A., Ito M., Fagan K.A., Cool C.D., Voelkel N.F., McMurtry I.F., Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez D.F., King J.A., Weber D., Addison E., Liedtke W., Townsley M.I. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res. 2006;99:988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taraseviciene-Stewart L., Kasahara Y., Alger L., Hirth P., Mc Mahon G., Waltenberger J., Voelkel N.F., Tuder R.M. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 28.Pankey E.A., Thammasibon S., Lasker G.F., Baber S., Lasky J.A., Kadowitz P.J. Imatinib attenuates monocrotaline pulmonary hypertension and has potent vasodilator activity in the pulmonary and systemic vascular beds in the rat. Am J Physiol Heart Circ Physiol. 2013;305:H1288–H1296. doi: 10.1152/ajpheart.00329.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogdanowich-Knipp S.J., Chakrabarti S., Williams T.D., Dillman R.K., Siahaan T.J. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:530–541. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 30.Patel V.F., Liu F., Brown M.B. Advances in oral transmucosal drug delivery. J Control Release. 2011;153:106–116. doi: 10.1016/j.jconrel.2011.01.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hemodynamic effects of i.v. coinfusion of CAR and fasudil. A: Representative pressure tracings of effects of 3 mg/mL CAR and 10 mg/mL fasudil mixture infusion (5 mL/minute) on SAP and RVSP in an SU/Hx/Nx PAH rat. B: Maximum reductions in SAP and RVSP by fasudil + CAR (+CAR) and fasudil alone (−CAR) infusions. Values are means ± SEM. n = 3 for +CAR, and n = 4 for −CAR. ∗P < 0.05 versus SAP +CAR, +P < 0.05 versus RVSP −CAR.
Hypothesized effect of CAR co-administration on fasudil/vasodilatory drugs in PAH. A: When fasudil is given alone as a bolus i.v. injection, fasudil molecules (yellow circles) enter into hypertensive pulmonary and normal systemic arterial smooth muscle cells evenly to cause similar degrees of hypotension throughout the vasculature. B: When CAR is coinjected with fasudil, CAR (red triangles) binds to its receptor (green double triangles) expressed specifically in the PAH vasculature and enhances the transport of fasudil to the target organ, the hypertensive pulmonary arteries. The outcome is the augmented pulmonary-selective vasodilation. The high plasma concentration of fasudil still causes a similar degree of hypotension as fasudil given alone on the systemic side of the circulation. C: When the same dose of fasudil and CAR (as in the bolus injection) are infused slowly over a long period, CAR peptide is able to transport enough fasudil into the hypertensive pulmonary artery to cause a substantial vasodilatory response, whereas the low plasma concentration of fasudil produces a limited degree of systemic vasodilation.