Abstract
Remyelination occurs in multiple sclerosis (MS) lesions but is generally considered to be insufficient. One of the major challenges in MS research is to understand the causes of remyelination failure and to identify therapeutic targets that promote remyelination. Activation of pancreatic endoplasmic reticulum kinase (PERK) signaling in response to endoplasmic reticulum stress modulates cell viability and function under stressful conditions. There is evidence that PERK is activated in remyelinating oligodendrocytes in demyelinated lesions in both MS and its animal model, experimental autoimmune encephalomyelitis (EAE). In this study, we sought to determine the role of PERK signaling in remyelinating oligodendrocytes in MS and EAE using transgenic mice that allow temporally controlled activation of PERK signaling specifically in oligodendrocytes. We demonstrated that persistent PERK activation was not deleterious to myelinating oligodendrocytes in young, developing mice or to remyelinating oligodendrocytes in cuprizone-induced demyelinated lesions. We found that enhancing PERK activation, specifically in (re)myelinating oligodendrocytes, protected the cells and myelin against the detrimental effects of interferon-γ, a key proinflammatory cytokine in MS and EAE. More important, we showed that enhancing PERK activation in remyelinating oligodendrocytes at the recovery stage of EAE promoted cell survival and remyelination in EAE demyelinated lesions. Thus, our data provide direct evidence that PERK activation cell-autonomously enhances the survival and preserves function of remyelinating oligodendrocytes in immune-mediated demyelinating diseases.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease in the central nervous system (CNS). Regeneration of oligodendrocytes and subsequent remyelination are necessary to restore neurological function in patients with MS. Although the remyelination process occurs in the CNS of patients with MS, this remyelination is generally considered to be insufficient.1,2 Increasingly, the evidence suggests that endoplasmic reticulum (ER) stress, which is caused by the accumulation of unfolded or misfolded proteins in the ER, plays a role in inflammatory diseases, including MS and its animal model, experimental autoimmune encephalomyelitis (EAE).3–5 Activation of pancreatic ER kinase (PERK) in response to ER stress promotes a stress-resistant state through the global attenuation of protein translation and the induction of numerous cytoprotective genes. PERK activation, however, also triggers an apoptotic program to eliminate ER-stressed cells.6,7 Although PERK activation has been observed in oligodendrocytes in MS and EAE demyelinated lesions,8–10 the role that PERK signaling plays in remyelinating oligodendrocytes during the remyelination process remains elusive.
Recently, we generated PLP/Fv2E-PERK transgenic mice that allow temporally controlled activation of PERK signaling specifically in oligodendrocytes. By using the unique mouse model, we found that enhanced activation of PERK signaling specifically in mature oligodendrocytes protected the cells and myelin against inflammatory attack in EAE mice.11 Mature oligodendrocytes in adult mice are only responsible for the slow replenishment of myelin components. In contrast, remyelinating oligodendrocytes must synthesize enormous amounts of membrane lipid and protein molecules to assemble myelin sheaths. The cells have extremely high metabolic rates.4,12 This feature makes remyelinating oligodendrocytes in MS and EAE demyelinated lesions more vulnerable to inflammatory attack and ER stress than mature oligodendrocytes.12,13 Our previous studies have shown that ER stress, induced by interferon-γ (IFN-γ), protects mature oligodendrocytes and myelin against inflammatory attack during the acute stage of EAE, but causes remyelinating oligodendrocyte apoptosis and remyelination failure at the recovery stage of EAE.8,14 Thus, in this study, we sought to dissect the precise role of PERK signaling in remyelinating oligodendrocytes in immune-mediated demyelinating diseases exploiting PLP/Fv2E-PERK mice.
The immune cytokine, IFN-γ, is thought to be a major contributing factor to poor remyelination in MS lesions.1,13 Our previous studies have shown that the presence of IFN-γ in the CNS causes hypomyelination in young, developing mice, and remyelination failure in the cuprizone and EAE models.14,15 In this study, we demonstrate that persistent activation of PERK signaling does not affect the viability or function of (re)myelinating oligodendrocytes under normal conditions. Moreover, we show that enhancing PERK activation specifically in (re)myelinating oligodendrocytes attenuates IFN-γ–induced hypomyelination in young, developing mice and IFN-γ–induced remyelination failure in the cuprizone model. More important, we find that enhancing PERK activation specifically in remyelinating oligodendrocytes promotes remyelination in the EAE model. Our findings may lead to the development of therapeutic interventions to improve remyelination in patients with MS.
Materials and Methods
Mice, Cuprizone Treatment, and EAE Immunization
All mouse lines used were on the C57BL/6J background. PLP/Fv2E-PERK transgenic mice were maintained by mating with C57BL/6J mice.11 GFAP/tTA;TRE/IFN-γ;PLP/Fv2E-PERK triple transgenic mice were produced by crossing line 110 GFAP/tTA mice14–16 with PLP/Fv2E-PERK mice, and then crossing the resulting progeny with line 184 TRE/IFN-γ mice.14–16 To repress the expression of the IFN-γ transgene in the astrocytes of GFAP/tTA;TRE/IFN-γ;PLP/Fv2E-PERK mice, 0.05 mg/mL doxycycline was added to the drinking water and provided ad libitum from conception.14–16 To activate the Fv2E-PERK transgene in the oligodendrocytes of PLP/Fv2E-PERK mice, the mice were given daily i.p. injections of AP20187 (Ariad Pharmaceuticals, Cambridge, MA); controls were injected with vehicle (4% ethanol, 10% PEG-400, and 2.0% Tween-20 in water) only.11
To induce demyelination with cuprizone, 6-week–old male mice were fed a diet of mouse chow containing 0.2% cuprizone (Sigma-Aldrich, St. Louis, MO) for up to 6 weeks. Subsequently, mice were returned to a normal diet for 3 weeks to allow remyelination to occur.14 To induce EAE, 7-week–old female mice were injected s.c. in the flank and at the tail base with 200 μg of myelin oligodendrocyte glycoprotein 35 to 55 peptide–emulsified incomplete Freund’s adjuvant (BD Biosciences, San Jose, CA) supplemented with 600 μg of Mycobacterium tuberculosis (strainH37Ra; BD Biosciences). Two i.p. injections of 400 ng pertussis toxin (List Biological Laboratories, Denver, CO) were given 24 and 72 hours later. Clinical scores (0 indicates healthy; 1, flaccid tail; 2, ataxia and/or paresis of hind limbs; 3, paralysis of hind limbs and/or paresis of forelimbs; 4, tetra paralysis; and 5, moribund or death) were recorded daily.8,11
All animal procedures were conducted in complete compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of South Alabama (Mobile) and the University of Minnesota (Minneapolis).
Real-Time PCR
Deeply anesthetized mice were perfused with ice-cold PBS. RNA was isolated from the brain or the spinal cord using TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNaseI (Invitrogen) to eliminate genomic DNA. Reverse transcription was performed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). TaqMan real-time PCR was performed with iQSupermix (Bio-Rad Laboratories) on a Bio-Rad iQ Real-Time PCR detection system, as previously described.8,14,17
Western Blot Analysis
Tissues harvested from mice were rinsed in ice-cold PBS and were homogenized using a motorized homogenizer, as previously described.15,18 After incubating on ice for 15 minutes, the extracts were cleared by centrifugation at 18,000 × g for 30 minutes, twice. The protein content of each extract was determined by DC Protein Assay (Bio-Rad Laboratories). Extracts (40 μg) were separated by SDS-PAGE and transferred to nitrocellulose. The blots were incubated with a primary antibody against myelin basic protein (MBP; 1:1000; Sternberger Monoclonals, Berkeley, CA), proteolipid protein (PLP; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), or β-actin (1:5000; Sigma-Aldrich). This was followed by a horseradish peroxidase–conjugated secondary antibody; after incubation with the ECL Detection Reagents (GE Healthcare Biosciences, Pittsburgh, PA), the chemiluminescent signal was detected. The intensity of the recorded chemiluminescence signal was quantified with Kodak 1D Image Analysis software version 3.6 (Kodak, Hercules, New Haven, CT).
IHC and TUNEL Staining
Anesthetized mice were perfused through the left cardiac ventricle with 4% paraformaldehyde in PBS. The tissues were removed, postfixed with paraformaldehyde, cryopreserved in 30% sucrose, embedded in optimal cutting temperature compound, and frozen on dry ice. Frozen sections were cut using a cryostat (10 μm thick). For immunohistochemistry (IHC), the sections were treated with −20°C acetone, blocked with PBS containing 10% goat serum and 0.1% Triton X-100, and incubated overnight with the primary antibody diluted in blocking solution. Fluorescein, Cy3, or enzyme-labeled secondary antibodies (Vector Laboratories, Burlingame, CA) were used for detection. IHC detection of CC1 (Adenomatus Polyposis Coli 7, 1:50; EMD Biosciences, Gibbstown, NJ), platelet-derived growth factor α receptor (PDGFαR; 1:100; Millipore, Temecula, CA), phosphorylated translation initiation factor 2α (p-eIF2α; 1:100; Cell Signaling Technology, Danvers, MA), MBP (1:1000; Sternberger Monoclonals), CD3 (1:50; Santa Cruz Biotechnology), CD11b (1:50; Millipore), and aspartoacylase (ASPA; 1:1000; kindly provided by Dr. M.A. Aryan Namboodiri at Uniformed Services University of the Health Sciences, Bethesda, MD) were performed. Fluorescent-stained sections were mounted with Vectashield mounting medium with DAPI (Vector Laboratories) and visualized with a Nikon A1 confocal microscope (Nikon Instruments, Inc., Melville, NY), an Olympus FV1000 confocal microscope (Olympus America, Inc., Center Valley, PA), or a Zeiss Axioskop 2 fluorescence microscope (Carl Zeiss Microscopy, Thornwood, NY).
TUNEL staining was performed using the ApopTag kit (Chemicon, Temecula, CA), according to the manufacturer’s instructions. The sections were mounted with Vectashield mounting medium with DAPI (Vector Laboratories) and visualized with a Nikon A1 confocal microscope or an Olympus FV1000 confocal microscope.
For cuprizone-treated mice, coronal sections of the fornix region of the corpus callosum, corresponding to Sidman sections 241 to 251, were selected for use, and all comparative analyses were restricted to the midline corpus callosum.19 We quantified immunopositive cells by counting positive cells within the median of the corpus callosum, confined to an area of 0.15 mm2. We scored each slide immunostained for MBP on a scale of 0 to 4. A score of 0 indicated complete demyelination, and a score of 4 indicated normal myelination in the corpus callosum of adult mice.14 For EAE mice, we counted immunopositive cells within the anterior funiculus directly medial to the anterior median fissure in the lumbar spinal cord and confined to an area of 0.1 mm2, as described in our previous articles.8,11
Toluidine Blue Staining and Electron Microscopy Analysis
Mice were anesthetized and perfused with PBS containing 4% paraformaldehyde and 2.5% glutaraldehyde. For cuprizone-treated mice, brains were sliced into sections (1 mm thick). The section corresponding to the region of the fornix was trimmed, processed for electron microscopic (EM) analysis, and oriented so that a cross section of the corpus callosum was achieved. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and analyzed as described previously.14 For EAE mice, the lumbar spinal cord was processed and embedded. Thin sections were cut, stained with toluidine blue, and analyzed as described previously.11 Moreover, ultrathin sections were cut, stained with uranyl acetate and lead citrate, and analyzed as described previously.11 We counted the total number of axons, calculated the total percentage of remyelinated axons, and measured the diameter of axons and the thickness of myelin sheaths using NIH ImageJ software version 1.45S (http://rsb.info.nih.gov/ij, last accessed November 1, 2012; NIH, Bethesda, MD), as described previously.14,17
Statistics
Data are expressed as means ± SD. For quantitative histological analyses, the average number for each mouse was used for statistical analysis. If the data followed the gaussian distribution, multiple comparisons were statistically evaluated by the one-way analysis of variance test, followed by Tukey’s test, and comparison of two groups was statistically evaluated by two-tailed t-test using SigmaStat 3.1 software (Hearne Scientific Software, Chicago, IL). If the data did not follow the gaussian distribution, a multiple comparison was statistically evaluated by the Kruskal-Wallis test, followed by a Dunns test, and comparison of two groups was statistically evaluated by the Mann-Whitney test using SigmaStat 3.1 software. Phenotypic differences among the groups were statistically evaluated by the χ2 test using SigmaStat 3.1 software. The relationship between myelin thickness and axon diameter was statistically evaluated by a linear regression using SigmaStat 3.1 software. P < 0.05 was considered significant.
Results
Persistent Activation of PERK Signaling Does Not Affect the Function of Myelinating Oligodendrocytes in Young, Developing Mice
In response to ER stress, PERK is activated through dimerization and autophosphorylation, enabling it to phosphorylate eIF2α.20,21 Phosphorylation of eIF2α inhibits global protein synthesis, but stimulates the expression of certain stress-induced genes, such as CCAAT enhancer binding protein homologous protein (CHOP) and growth arrest and DNA damage 34 (GADD34), by promoting the translation of the cytosolic transcription factor ATF4. We have generated PLP/Fv2E-PERK transgenic mice that allow temporally controlled activation of PERK signaling specifically in oligodendrocytes in the absence of ER stress.11 These mice express Fv2E-PERK, an artificial PERK that is generated by fusing the eIF2α kinase effector domain of PERK to a polypeptide containing two modified FK506 binding domains (Fv2E),22 under the control of the mouse PLP transcriptional control region.23,24 We have demonstrated that PLP/Fv2E-PERK mice express the transgene product Fv2E-PERK specifically in oligodendrocytes and that the activity of Fv2E-PERK is tightly controlled by administration of the dimerizer AP20187.11
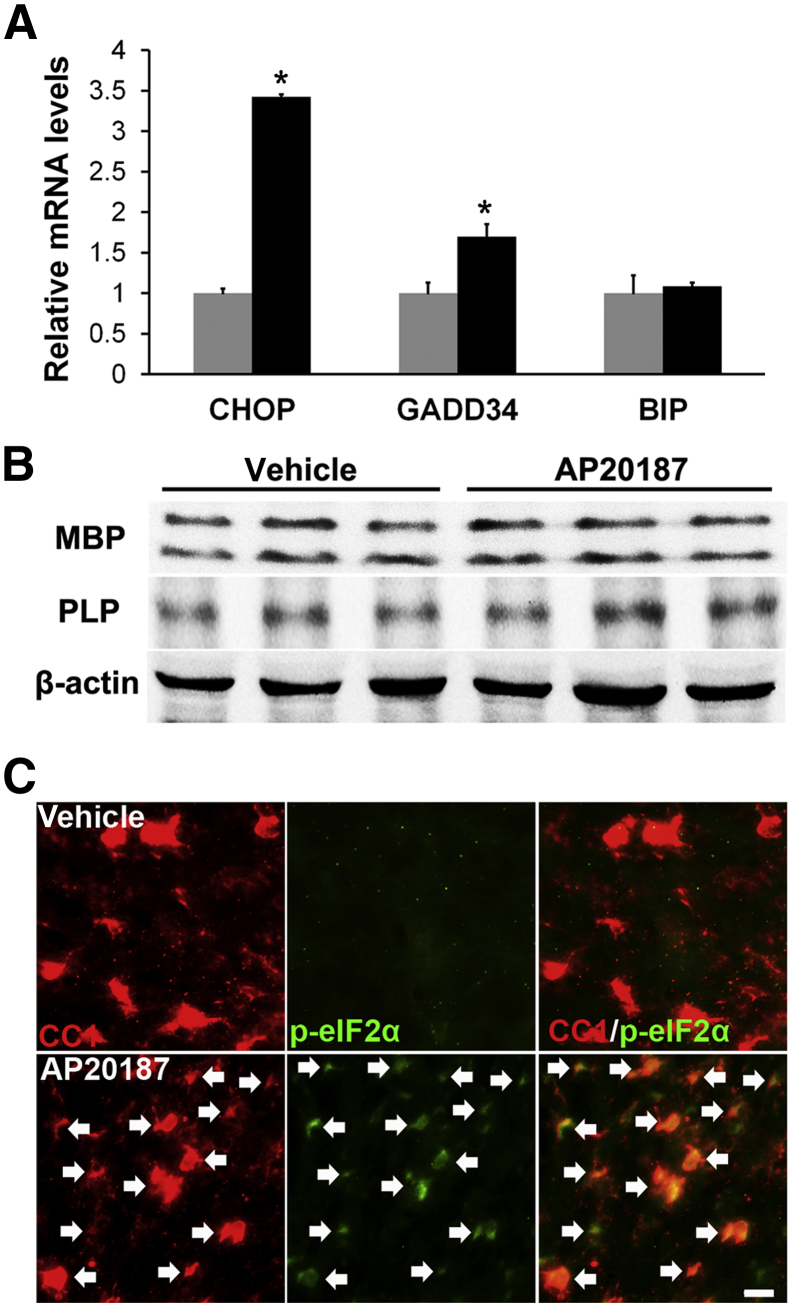
Robust myelination occurs in the mouse CNS between postnatal day (P) 7 and P21.25 To determine whether PERK activation affects the myelinating function of oligodendrocytes, PLP/Fv2E-PERK mice were treated with 2 mg/kg AP20187 daily starting on P12 and ending on P21. AP20187-treated transgenic mice were indistinguishable from littermate transgenic mice treated with vehicle and from untreated wild-type mice. Real-time PCR analysis showed that AP20187 treatment significantly enhanced the expression of the PERK-responsive genes CHOP and GADD34 in the CNS of the transgenic mice (Figure 1A), but did not alter the expression of binding immunoglobulin protein, an ER stress-inducible gene not regulated by PERK signaling.26 Meanwhile, Western blot analysis showed that AP20187 treatment did not change the levels of MBP and PLP in the CNS of 21-day-old PLP/Fv2E-PERK mice (Figure 1B). CC1 (a marker for oligodendrocytes) and p-eIF2α double immunostaining showed that AP20187 treatment markedly increased the level of p-eIF2α in most oligodendrocytes in the CNS of 21-day-old PLP/Fv2E-PERK mice (Figures 1C and 2I). Interestingly, we found that AP20187 treatment did not change the number of oligodendrocytes in the CNS of 21-day-old PLP/Fv2E-PERK mice (Figures 1C and 2J). Collectively, these data indicate that AP20187 treatment results in PERK activation in oligodendrocytes at non-physiological levels in transgenic mice with enforced expression of Fv2E-PERK specifically in oligodendrocytes, and that persistent activation of PERK signaling specifically in myelinating oligodendrocytes does not affect their viability or function.
Figure 1.
Persistent activation of PERK signaling in myelinating oligodendrocytes does not affect developmental myelination. A: Real-time PCR analysis shows that AP20187 treatment significantly increases the expression of CHOP and GADD34 in the spinal cord of 21-day-old PLP/Fv2E-PERK mice, but does not affect the expression of binding immunoglobulin protein (BIP). N = 3 animals. Error bars represent SD. B: Western blot analysis shows that AP20187 treatment does not affect the protein levels of MBP and PLP in the brain of 21-day-old PLP/Fv2E-PERK mice. N = 3 animals. C: CC1 and p-eIF2α double immunostaining shows that p-eIF2α immunoreactivity is undetectable in oligodendrocytes in the brain of 21-day-old PLP/Fv2E-PERK mice treated with vehicle, but becomes detectable in oligodendrocytes (arrows) in AP20187-treated mice. Black bars, AP20187; gray bars, vehicle. N = 3 animals. ∗P < 0.05. Scale bar = 20 μm (C).
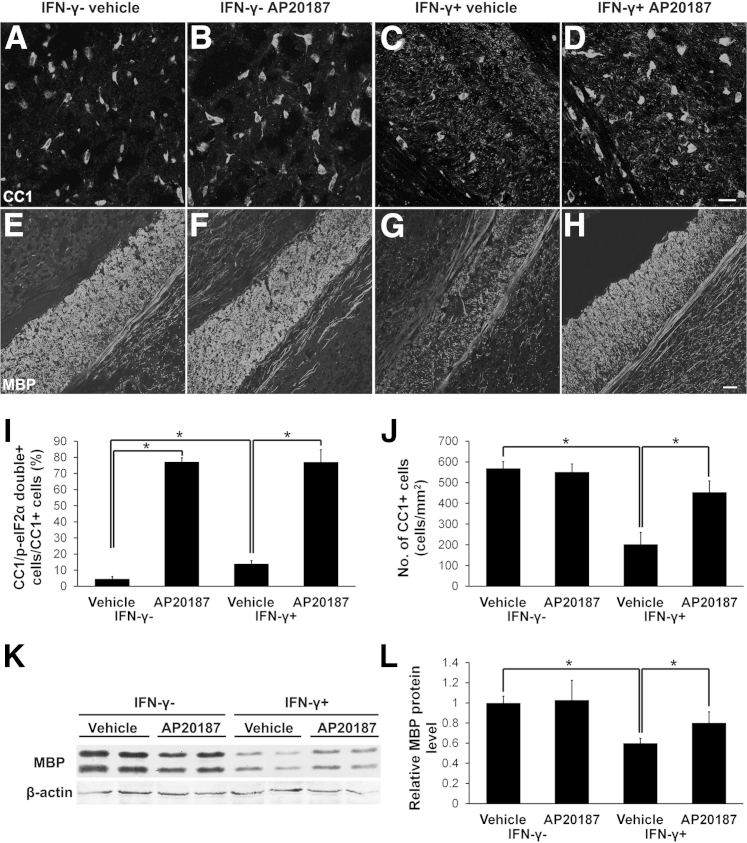
Figure 2.
Enhancing PERK activation in myelinating oligodendrocytes protects the cells and myelin against IFN-γ in young, developing mice. A–D and J: CC1 immunostaining shows that AP20187 treatment does not alter the number of oligodendrocytes in the corpus callosum of 21-day-old control mice, but significantly attenuates IFN-γ–induced oligodendrocyte loss in IFN-γ–expressing mice. N = 3 animals. E–H: MBP immunostaining shows that AP20187 treatment does not affect the degree of myelination in the corpus callosum of 21-day-old control mice, but ameliorates IFN-γ–induced myelin loss in IFN-γ–expressing mice. N = 3 animals. I: CC1 and p-eIF2α double immunostaining shows that the presence of IFN-γ in the CNS moderately increases the percentage of CC1-positive cells that are double positive for CC1 and p-eIF2α in the corpus callosum of 21-day-old vehicle-treated mice (IFN-γ+; vehicle mice versus IFN-γ−; vehicle mice), and that >80% of oligodendrocytes are p-eIF2α positive in the corpus callosum of 21-day-old AP20187-treated mice (IFN-γ−; AP20187 mice and IFN-γ+; AP20187 mice). N = 3 animals. K and L: Western blot analysis shows that AP20187 treatment does not alter the level of MBP in the brain of 21-day-old control mice, but significantly attenuates the reduction of MBP level in mice expressing IFN-γ. The MBP protein level is reported relative to β-actin. N = 3 animals. I, J, and L: Error bars represent SD. ∗P < 0.05. Scale bars: 20 μm (A–D); 50 μm (E–H).
Enhancing PERK Activation in Myelinating Oligodendrocytes Ameliorates IFN-γ–Induced Hypomyelination
IFN-γ is thought to be a key proinflammatory cytokine in MS and EAE.13,27 We have generated transgenic mice that allow controllable delivery of IFN-γ to the CNS using the tetracycline-controllable system.16 Our previous studies have shown that the presence of IFN-γ in the CNS during development causes oligodendrocyte loss and hypomyelination and that PERK signaling enhances the viability of myelinating oligodendrocytes in IFN-γ–expressing mice.15,18 It has been well documented that the presence of IFN-γ in the CNS reduces the viability of oligodendrocytes by activating T cells and microglia/macrophages.28,29 Moreover, recent studies have shown that PERK signaling influences the activity of T cells and macrophages.30,31 Thus, it is not clear whether the protective effects of PERK signaling on oligodendrocytes in IFN-γ–expressing mice are the result of its actions in oligodendrocytes or in inflammatory cells.
To determine the cell-autonomous role of PERK signaling in myelinating oligodendrocytes in response to IFN-γ, GFAP/tTA mice were crossed with PLP/Fv2E-PERK mice, and the resulting progeny were crossed with TRE/IFN-γ mice to obtain GFAP/tTA;TRE/IFN-γ;PLP/Fv2E-PERK triple transgenic mice. The triple transgenic mice were treated with doxycycline from conception to repress transgenic IFN-γ expression. One group of the triple transgenic mice released from doxycycline at embryonic day 14 (E14) was treated with 0.5 mg/kg AP20187 daily starting on P12 (IFN-γ+; AP20187 mice). A second group of the triple transgenic mice released from doxycycline at E14 was treated with vehicle (IFN-γ+; vehicle mice). The triple transgenic mice never released from doxycycline were treated with either AP20187 or vehicle to serve as controls (IFN-γ−; AP20187 mice and IFN-γ−; vehicle mice, respectively). IFN-γ+; vehicle mice exhibited tremor and ataxia, as described in our previous articles.15,17,18 Interestingly, IFN-γ+; AP20187 mice displayed a significantly milder tremoring phenotype than IFN-γ+; vehicle mice (Table 1).
Table 1.
IFN-γ+; AP20187 Mice Display a Significantly Milder Tremor than IFN-γ+; Vehicle Mice
| Variable | No tremor (%) | Mild tremor (%) | Tremor (%) | Severe tremor (%) |
|---|---|---|---|---|
| IFN-γ−; vehicle mice (n = 30 animals) | 100 | 0 | 0 | 0 |
| IFN-γ−; AP20187 mice (n = 15 animals) | 100 | 0 | 0 | 0 |
| IFN-γ+; vehicle mice (n = 27 animals) | 0 | 7.4 | 3.7 | 88.9 |
| IFN-γ+; AP20187 mice (n = 12 animals) | 0 | 14.3 | 42.9∗ | 42.9∗ |
Mild tremor indicates tremor only in the tail; tremor, tremor in the tail and body; and severe tremor, whole body tremor and occasional seizure.
P < 0.05.
As expected, CC1 and p-eIF2α double immunostaining showed that AP20187 treatment increased the level of p-eIF2α in oligodendrocytes in control mice and IFN-γ–expressing mice (Figure 2I). We also found that, although AP20187 treatment did not affect oligodendrocyte numbers in the CNS of 21-day-old control animals (Figure 2, A, B, and J), it did attenuate oligodendrocyte loss in the CNS of 21-day-old IFN-γ–expressing mice (Figure 2, C, D, and J). Moreover, MBP immunostaining and Western blot analyses showed that AP20187 treatment did not affect the myelination process in the CNS of control animals, but significantly alleviated myelin loss in the CNS of 21-day-old IFN-γ–expressing mice (Figure 2, E–H, K, and L). In addition, PDGFαR immunostaining showed that AP20187 treatment did not significantly alter the number of oligodendrocyte progenitor cells (OPCs) in the CNS of 21-day-old control mice or IFN-γ–expressing mice (data not shown). Collectively, these data suggest that enhancing PERK activation in myelinating oligodendrocytes protects the cell and myelin against the cytotoxicity of IFN-γ, resulting in the attenuation of the tremoring phenotype in the mice.
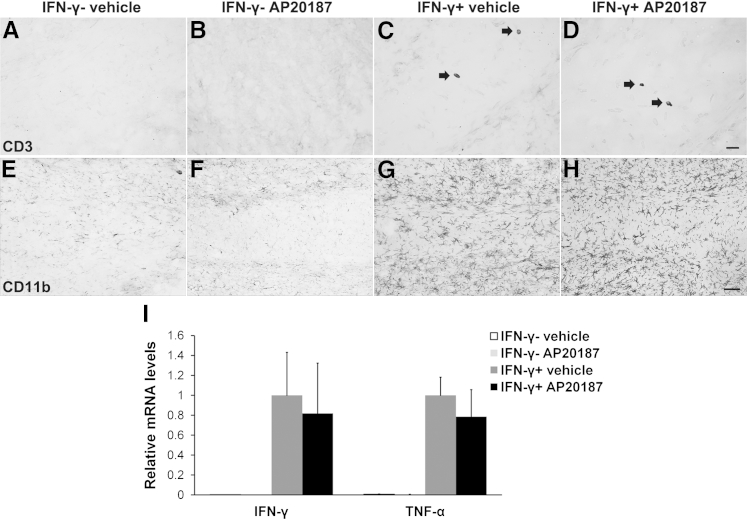
Our previous study demonstrated that inflammatory cells (T cells and microglia/macrophages) do not express the transgene product Fv2E-PERK in PLP/Fv2E-PERK mice.11 Moreover, recent studies have shown that AP20187 has no noticeable effect on the functions of inflammatory cells.11,32 Therefore, we further sought to exclude the possibility that AP20187 treatment influences the IFN-γ–induced inflammatory response in GFAP/tTA;TRE/IFN-γ;PLP/Fv2E-PERK mice. As expected, we found that AP20187 treatment did not significantly affect the expression of IFN-γ in the CNS of control mice or IFN-γ–expressing mice (Figure 3I). Consistent with our previous studies,17,18 we found that the presence of IFN-γ in the CNS moderately recruited T cells into the CNS, markedly activated microglia/macrophages, and dramatically increased the expression of TNF-α (Figure 3). Nevertheless, AP20187 treatment did not significantly alter T-cell recruitment, microglia/macrophage activation, or TNF-α expression in the CNS of 21-day-old control mice or IFN-γ–expressing mice. Therefore, it is unlikely that AP20187 treatment attenuates the IFN-γ–induced oligodendrocyte loss and hypomyelination in GFAP/tTA;TRE/IFN-γ;PLP/Fv2E-PERK mice by altering the inflammatory response. Taken together, these results demonstrate that PERK activation cell-autonomously preserves the survival and function of myelinating oligodendrocytes in response to IFN-γ.
Figure 3.
Enhancing PERK activation specifically in oligodendrocytes does not alter the inflammatory response in IFN-γ–expressing mice. A–D: CD3 immunostaining shows few infiltrating T cells (arrows) in the corpus callosum of 21-day-old IFN-γ+; vehicle mice compared with control IFN-γ−; vehicle mice or IFN-γ−; AP20187 mice. Moreover, the numbers of infiltrating T cells observed in IFN-γ+; AP20187 mice are comparable to those in IFN-γ+; vehicle mice. N = 3 animals. E–H: CD11b immunostaining shows that the presence of IFN-γ dramatically activates microglia/macrophages in the corpus callosum of 21-day-old IFN-γ+; vehicle mice compared with control IFN-γ−; vehicle mice or IFN-γ−; AP20187 mice. The level of microglia/macrophage activation is comparable in IFN-γ+; AP20187 and IFN-γ+; vehicle mice. N = 3 animals. I: Real-time PCR analysis reveals dramatically increased levels of IFN-γ and TNF-α in the brain of 21-day-old IFN-γ+; vehicle mice compared with control IFN-γ−; vehicle mice or IFN-γ−; AP20187 mice. More important, AP20187 treatment does not significantly change the levels of IFN-γ or TNF-α in the brain of IFN-γ–expressing mice. N = 3 animals. Error bars represent SD. Scale bars: 20 μm (A–D); 50 μm (E–H).
Persistent Activation of PERK Signaling Does Not Affect the Function of Remyelinating Oligodendrocytes Derived from OPCs in Adult Mice
Although the cellular mechanisms responsible for remyelination and developmental myelination share many similarities, multiple lines of evidence suggest they also differ in several important ways.33 Therefore, we investigated whether persistent activation of PERK signaling is detrimental to remyelinating oligodendrocytes derived from OPCs in adult mice using the cuprizone model. When young adult C57BL/6J male mice are fed 0.2% cuprizone in their diet, mature oligodendrocytes in the corpus callosum are lost, which is followed by complete demyelination by 5 weeks of cuprizone exposure. OPCs accumulate within the demyelinated lesion, and mature oligodendrocytes repopulate the area beginning at approximately 6 weeks into the treatment protocol, resulting in complete remyelination after several weeks of cuprizone removal.34 It is generally believed that the cuprizone model is one of the best mouse models to understand the processes of demyelination and remyelination in the CNS. Recently, the cuprizone model is increasingly used to study the mechanisms of remyelination in demyelinating diseases of the CNS, such as MS.35,36
Six-week-old male PLP/Fv2E-PERK mice on the C57BL/6J background were treated with 0.2% cuprizone chow for up to 6 weeks. To determine whether PERK activation in mature oligodendrocytes affects cuprizone-induced demyelination, PLP/Fv2E-PERK mice were treated with 0.5 mg/kg AP20187 or vehicle daily starting 2 days before cuprizone exposure. In accordance with previous studies,34 there was almost complete oligodendrocyte loss and demyelination in the corpus callosum of vehicle-treated mice after 5 weeks of cuprizone treatment (data not shown). We found that AP20187 treatment did not alter oligodendrocyte loss or demyelination in the corpus callosum of PLP/Fv2E-PERK mice after 5 weeks of cuprizone treatment (data not shown). Therefore, these data suggest that PERK activation specifically in mature oligodendrocytes has no effect on cuprizone-induced oligodendrocyte loss and demyelination.
To determine the effects of PERK activation on remyelinating oligodendrocytes, PLP/Fv2E-PERK mice, which had been treated with 0.2% cuprizone chow for 6 weeks, were subsequently returned to a normal diet for 3 weeks to allow remyelination to occur. The Fv2E-PERK was activated in remyelinating oligodendrocytes by the daily administration of 0.5 mg/kg AP20187 starting on the day of cuprizone removal. Controls were injected with vehicle only. As expected, CC1 and p-eIF2α double immunostaining showed that AP20187 treatment markedly increased the level of p-eIF2α specifically in oligodendrocytes in the corpus callosum (Supplemental Figure S1A). Moreover, we found that AP20187 treatment did not significantly affect the number of remyelinating oligodendrocytes or the degree of remyelination in the demyelinated lesions after 3 weeks of cuprizone removal (Supplemental Figure S1 and Figures 4 and 5). Thus, these data indicate that the persistent activation of PERK signaling in remyelinating oligodendrocytes, derived from OPCs in adult mice, does not affect their viability or function.
Figure 4.
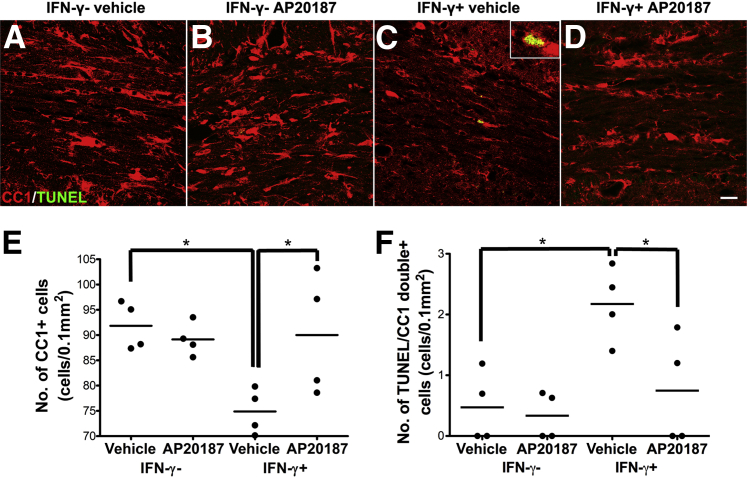
Enhancing PERK activation protects remyelinating oligodendrocytes against the cytotoxicity of IFN-γ. A–D: CC1 and TUNEL double immunostaining shows that there are markedly fewer oligodendrocytes and more apoptotic oligodendrocytes in the corpus callosum of IFN-γ+; vehicle mice after 3 weeks of cuprizone removal compared with control IFN-γ−; vehicle mice or IFN-γ−; AP20187 mice. Interestingly, AP20187 treatment noticeably increases the number of oligodendrocytes and reduces the number of apoptotic oligodendrocytes in IFN-γ+; AP20187 mice compared with IFN-γ+; vehicle mice. Inset: High-magnification image shows a CC1 and TUNEL double-positive cell. N = 4 animals. E: Quantitative analysis of CC1-positive cells shows that AP20187 treatment does not alter the number of oligodendrocytes in the corpus callosum of control mice after 3 weeks of cuprizone removal, but significantly attenuates the reduction of oligodendrocyte numbers in IFN-γ–expressing mice. N = 4 animals. F: Quantitative analysis of CC1 and TUNEL double-positive cells shows that AP20187 treatment does not significantly change the number of apoptotic remyelinating oligodendrocytes in the corpus callosum of control mice after 3 weeks of cuprizone removal, but significantly reduces the amount of IFN-γ–induced apoptosis of remyelinating oligodendrocytes in IFN-γ–expressing mice. N = 4 animals. ∗P < 0.05 (E and F). Scale bar = 20 μm (A–D).
Figure 5.
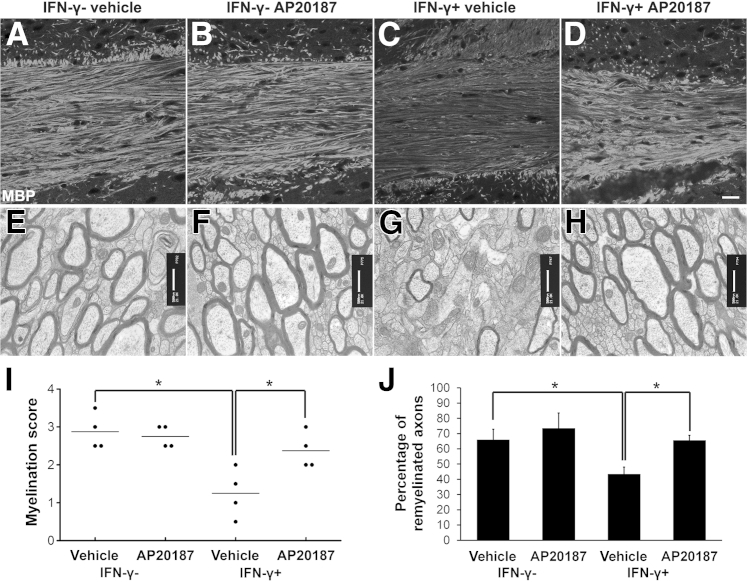
Enhancing PERK activation in remyelinating oligodendrocytes attenuates IFN-γ–induced remyelination failure in cuprizone-induced demyelinated lesions. A–D and I: MBP immunostaining shows that AP20187 treatment does not affect the degree of remyelination in the corpus callosum of control mice after 3 weeks of cuprizone removal, but significantly attenuates IFN-γ–induced remyelination failure in IFN-γ–expressing mice. N = 4 animals. E–H and J: EM analysis shows that AP20187 treatment does not significantly alter the percentage of remyelinated axons in the corpus callosum of control mice after 3 weeks of cuprizone removal, but significantly attenuates the reduced percentage of remyelinated axons observed in IFN-γ–expressing mice. N = 4 animals. Error bars represent SD. ∗P < 0.05. Scale bars: 20 μm (A–D); 500 nm (E–H).
Enhancing PERK Activation in Remyelinating Oligodendrocytes Ameliorates IFN-γ–Induced Remyelination Failure in Cuprizone-Induced Demyelinated Lesions
We have shown that the presence of IFN-γ in the CNS induces remyelinating oligodendrocyte apoptosis and suppresses remyelination in the demyelinated lesions in the cuprizone model, and that PERK haploinsufficiency exacerbates IFN-γ–induced remyelination failure.14 We used the cuprizone model to determine whether enhancing PERK activation in remyelinating oligodendrocytes would have the opposite effect of promoting cell survival and remyelination in response to IFN-γ. Six-week-old male GFAP/tTA;TRE/IFN-γ;PLP/Fv2E-PERK triple transgenic mice that had been maintained on doxycycline from conception were simultaneously treated with 0.2% cuprizone chow and released from doxycycline. The triple transgenic mice were treated with cuprizone chow for 6 weeks, and then returned to a normal diet for 3 weeks. One group of these triple transgenic mice was treated with 0.5 mg/kg AP20187 daily starting on the day of cuprizone removal (IFN-γ+; AP20187 mice). A second group of these triple-transgenic mice was treated with vehicle (IFN-γ+; vehicle mice). Triple transgenic mice that were never released from doxycycline, but were treated with 0.2% cuprizone chow, followed by daily injections of AP20187 or vehicle starting on the day of cuprizone removal, were used as controls (IFN-γ−; AP20187 mice and IFN-γ−; vehicle mice, respectively). As expected, we found that AP20187 treatment elevated the level of p-eIF2α in remyelinating oligodendrocytes in the corpus callosum of control animals and IFN-γ–expressing mice (data not shown), and that AP20187 treatment did not significantly alter the number of remyelinating oligodendrocytes or the degree of remyelination in the corpus callosum of control animals (Figures 4 and 5).
Consistent with our previous study,14 we found that the presence of IFN-γ in the CNS significantly reduced the number of remyelinating oligodendrocytes and the extent of remyelination in the corpus callosum of IFN-γ+; vehicle mice after 3 weeks of cuprizone removal compared with control IFN-γ−; AP20187 mice and IFN-γ−; vehicle mice (Figures 4 and 5). Interestingly, the number of remyelinating oligodendrocytes in the corpus callosum of IFN-γ+; AP20187 mice was significantly increased compared with IFN-γ+; vehicle mice (Figure 4, C–E). Furthermore, CC1 and TUNEL double labeling showed that AP20187 treatment significantly reduced the number of apoptotic remyelinating oligodendrocytes in the corpus callosum of IFN-γ+; AP20187 mice after 3 weeks of cuprizone removal compared with IFN-γ+; vehicle mice (Figure 4, C, D, and F). MBP immunostaining showed that AP20187 treatment attenuated the degree of remyelination failure in the demyelinated lesions of IFN-γ–expressing mice after 3 weeks of cuprizone removal (Figure 5, C, D, and I). More important, EM analysis confirmed that AP20187 treatment significantly increased the percentage of remyelinated axons in the corpus callosum of IFN-γ+; AP20187 mice after 3 weeks of cuprizone removal compared with IFN-γ+; vehicle mice (Figure 5, G, H, and J). Nevertheless, PDGFαR immunostaining showed that AP20187 treatment did not significantly alter the number of OPCs in the corpus callosum of either control mice or IFN-γ–expressing mice (data not shown). As expected, we also found that AP20187 treatment did not affect T-cell infiltration or microglia/macrophage activation in the corpus callosum of control animals or IFN-γ–expressing mice (data not shown). Collectively, these data demonstrate that PERK activation in remyelinating oligodendrocytes promotes cell survival and remyelination in response to IFN-γ.
Enhancing PERK Activation in Remyelinating Oligodendrocytes Promotes Remyelination in EAE Demyelinated Lesions
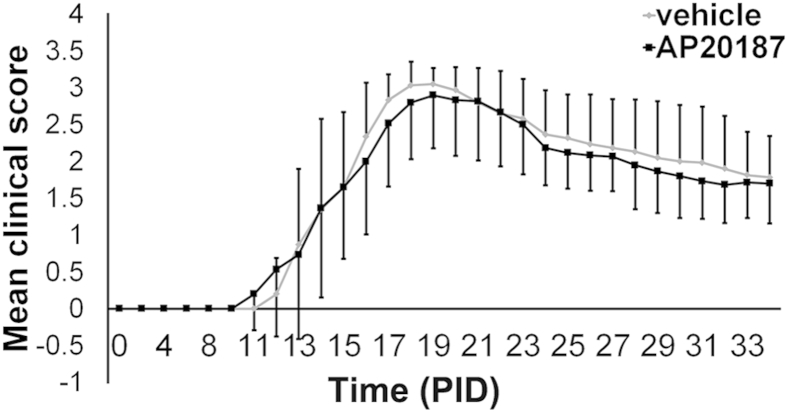
Although several studies have shown that ER stress is activated in multiple cell types in MS and EAE demyelinated lesions, including oligodendrocytes, T cells, and microglia/macrophages,8–10,37 the role that ER stress plays in these cell types during the remyelination process remains unknown. It is believed that inflammatory mediators, such as immune cytokines and reactive oxygen/nitrogen species, contribute significantly to oligodendrocyte death in MS and EAE.12,38 Several lines of evidence have suggested that PERK activation protects cells against these inflammatory mediators and ER stress.11,22 Thus, we further determine the cell-autonomous role of PERK activation in remyelinating oligodendrocytes in the EAE model. Seven-week-old female PLP/Fv2E-PERK mice were immunized with myelin oligodendrocyte glycoprotein 35 to 55 peptide to induce EAE. These mice developed neurological signs of disease starting at approximately postimmunization day (PID) 14, and started recovering from EAE at approximately PID2211 (Figure 6). Remyelination in MS and EAE demyelinated lesions has been reported to initiate during the acute stage of diseases.39,40 To activate PERK signaling in the remyelinating oligodendrocytes within the demyelinated lesions at the recovery stage of EAE, PLP/Fv2E-PERK mice were given i.p. injections of 0.5 mg/kg AP20187 daily starting on PID20; controls were injected with vehicle. CC1 and p-eIF2α double immunostaining showed that a few oligodendrocytes were p-eIF2α positive in the lumbar spinal cord of PLP/Fv2E-PERK mice treated with vehicle. As expected, the level of p-eIF2α was markedly increased in oligodendrocytes in PLP/Fv2E-PERK mice treated with AP20187 (Supplemental Figure S2). Nevertheless, AP20187 treatment did not significantly change the clinical symptoms of mice with EAE (Figure 6).
Figure 6.
Enhancing PERK activation specifically in oligodendrocytes at the recovery stage of EAE does not alter the disease severity. Mean clinical scores were recorded daily: 0, healthy; 1, flaccid tail; 2, ataxia and/or paresis of hind limbs; 3, paralysis of hind limbs and/or paresis of forelimbs; 4, tetra paralysis; and 5, moribund or death. N = 12 animals. Error bars represent SD.
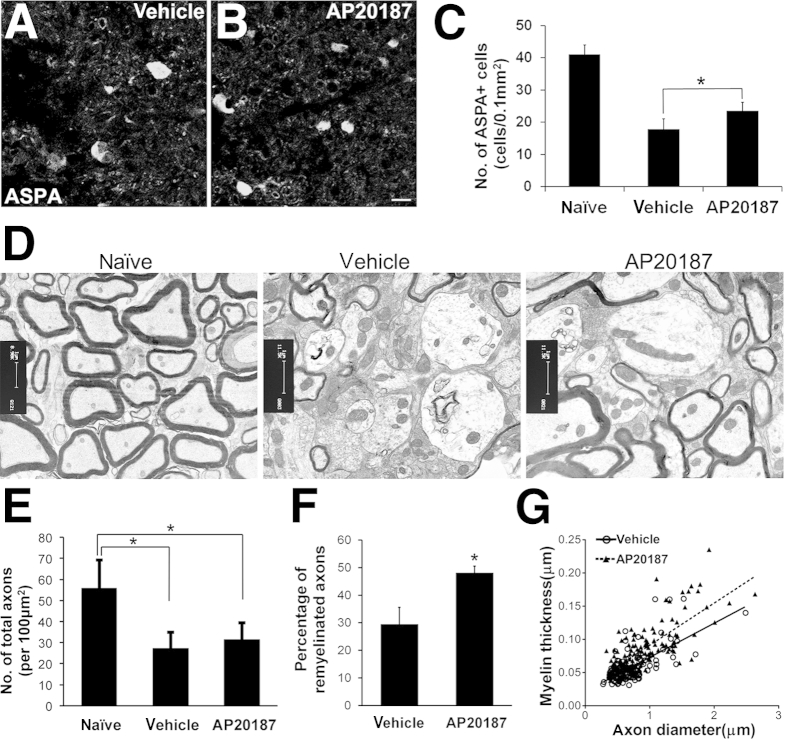
Next, we determined whether AP20187 treatment influences the viability and function of remyelinating oligodendrocytes in the demyelinated lesions of PLP/Fv2E-PERK mice. Our previous studies have shown that oligodendrocytes were largely lost in EAE demyelinated lesions at the peak of disease.8,11 Immunostaining for ASPA, a marker for oligodendrocytes,41,42 showed that at PID34, a few oligodendrocytes were present in the demyelinated lesions in the lumbar spinal cord of PLP/Fv2E-PERK mice treated with vehicle. AP20187 treatment significantly increased the number of oligodendrocytes in the demyelinated lesions of PLP/Fv2E-PERK mice at PID34 (Figure 7, A–C); however, there were significantly fewer oligodendrocytes in the demyelinated lesions of AP20187-treated mice than in the matched regions in adult naïve PLP/Fv2E-PERK mice (Figure 7C and Supplemental Figure S3, A–C). Furthermore, EM analysis was used to observe remyelinated axons, which are recognizable by myelin sheaths that are thin in relation to axon diameter,12 in the demyelinated lesions at PID34. Importantly, there were significantly more remyelinated axons in the demyelinated lesions of AP20187-treated mice than of vehicle-treated mice (Figure 7, D and F). AP20187 treatment also significantly increased the myelin thickness of remyelinated axons in PLP/Fv2E-PERK mice at PID34 (Figure 7G). Nevertheless, AP20187 treatment did not significantly change the total number of axons in the demyelinated lesions of PLP/Fv2E-PERK mice (Figure 7E and Supplemental Figure S3, D–F). As expected, there were significantly fewer total axons in the demyelinated lesions of PLP/Fv2E-PERK mice treated with either AP20187 or vehicle than in the matched regions in adult naïve PLP/Fv2E-PERK mice (Figure 7E and Supplemental Figure S3, D–F). Meanwhile, PDGFαR immunostaining showed that AP20187 treatment did not significantly alter the number of OPCs in the demyelinated lesions of PLP/Fv2E-PERK mice (data not shown). Taken together, these data demonstrate that PERK activation in remyelinating oligodendrocytes promotes cell survival and remyelination in EAE demyelinated lesions.
Figure 7.
Enhancing PERK activation in remyelinating oligodendrocytes promotes cell survival and remyelination in EAE-demyelinated lesions. A–C: ASPA immunostaining shows that AP20187 treatment significantly increases the number of ASPA-positive oligodendrocytes in EAE-demyelinated lesions in the lumbar spinal cord of PLP/Fv2E-PERK mice at PID34. N = 8 animals. Error bars represent SD. D and E: EM analysis shows that AP20187 treatment markedly increases the number of remyelinated axons in demyelinated lesions in the lumbar spinal cord of PLP/Fv2E-PERK mice at PID34. Nevertheless, AP20187 treatment does not significantly alter the number of total axons in demyelinated lesions in PLP/Fv2E-PERK mice. N = 4 animals. Error bars represent SD. F: Quantitative EM analysis shows that AP20187 treatment significantly increases the percentage of axons that are remyelinated in demyelinated lesions in the lumbar spinal cord of PLP/Fv2E-PERK mice at PID34. N = 4 animals. Error bars represent SD. G: Quantitative EM analysis shows that AP20187 treatment significantly increases the thickness of the newly formed myelin sheaths of remyelinated axons in demyelinated lesions in the lumbar spinal cord of PLP/Fv2E-PERK mice at PID34. N = 4 animals. ∗P < 0.05 (C, E, and F). Scale bars: 5 μm (A and B); 1 μm (D).
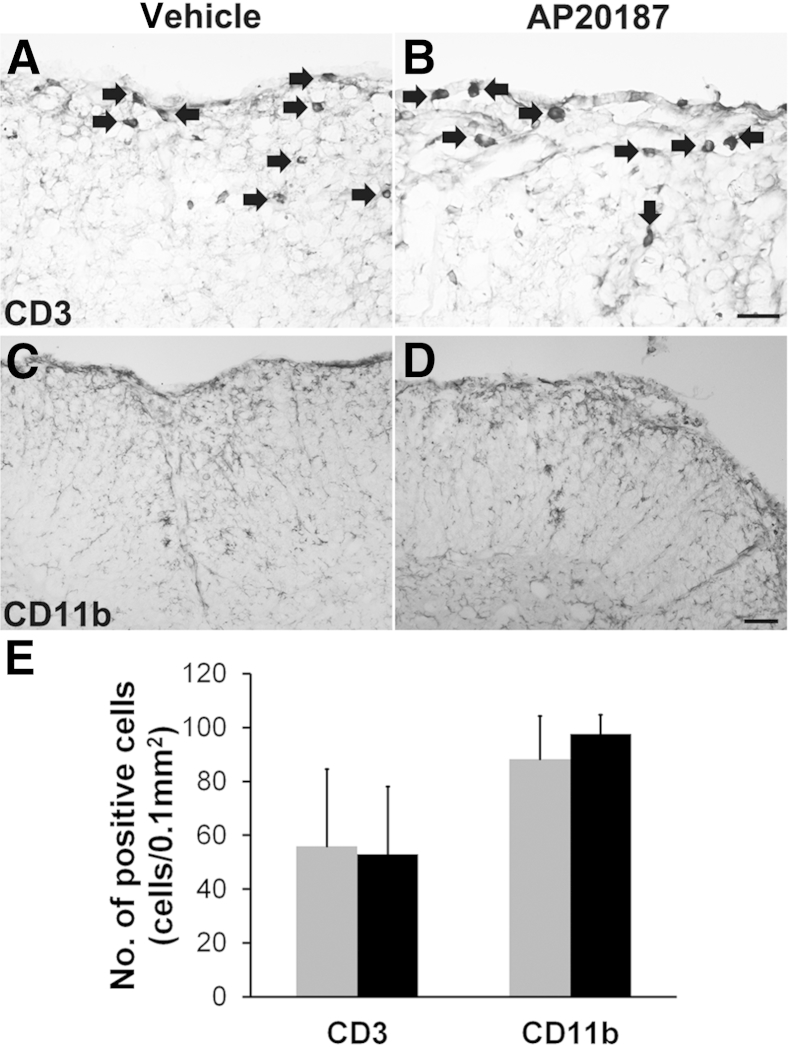
There is no evidence that AP20187 treatment alone has any noticeable effect on mice under physiological or pathological conditions.32,43–45 More important, our previous study demonstrated that AP20187 treatment does not affect the inflammatory response in PLP/Fv2E-PERK mice undergoing EAE.11 Consistently, we found that AP20187 treatment did not significantly alter the number of CD3-positive T cells or the number of CD11b-positive microglia/macrophages in the demyelinated lesions of PLP/Fv2E-PERK mice at PID34 (Figure 8). Thus, it is unlikely that AP20187 treatment promotes remyelination in the EAE demyelinated lesions of PLP/Fv2E-PERK mice by altering the inflammatory response.
Figure 8.
AP20187 treatment does not alter the inflammatory response in the CNS of PLP/Fv2E-PERK mice undergoing EAE. A, B, and E: CD3 immunostaining shows that AP20187 treatment does not affect T-cell (arrows) infiltration into the white matter of the lumbar spinal cord in PLP/Fv2E-PERK mice at PID34. N = 4 animals. C–E: CD11b immunostaining shows that AP20187 treatment does not alter the number of microglia/macrophages present in the white matter of the lumbar spinal cord in PLP/Fv2E-PERK mice at PID34. N = 4 animals. E: Error bars represent SD. Black bars, AP20187; gray bars, vehicle. Scale bars: 20 μm (A and B); 50 μm (C and D).
Discussion
MS is a chronic inflammatory demyelinating disease of the CNS.46 Remyelination is necessary to restore neurological function in patients with MS; however, the remyelination is considered to be insufficient.1,2 It is generally believed that the accumulated load of demyelinated lesions that fail to remyelinate leads to the progressive deterioration of neurological function in patients with MS. One of the major challenges in MS research is to understand the causes of remyelination failure and to develop therapeutic strategies that promote oligodendrocyte regeneration and remyelination. Several lines of evidence have suggested that ER stress in oligodendrocytes influences the development of myelin disorders.4,47 PERK signaling activated in response to ER stress is essential to preserve cell function and survival under stressful conditions. Nevertheless, PERK signaling is not exclusively beneficial to cell survival. Persistent activation of PERK signaling can result in cell apoptosis through the induction of CHOP, a pro-apoptotic transcription factor, in ER-stressed cells.6,7 Interestingly, a previous study showed that CHOP induction promotes the survival of ER-stressed myelinating oligodendrocytes in the mouse models of Pelizaeus-Merzbacher disease.48 The unique function of CHOP in myelinating oligodendrocytes raises the possibility that PERK signaling has particularly beneficial effects on (re)myelinating oligodendrocytes.
Activation of PERK signaling has been observed in oligodendrocytes in MS and EAE demyelinated lesions.9,10,37 Nevertheless, the role that it plays in remyelinating oligodendrocytes in these diseases remains ambiguous. We have generated transgenic mice that allow temporally controlled activation of PERK signaling specifically in oligodendrocytes.11 In this study, by using the unique mouse model, we provide direct evidence that PERK activation in remyelinating oligodendrocytes is cytoprotective, promoting remyelination in demyelinated lesions in immune-mediated demyelinating diseases. First, we demonstrated that persistent activation of PERK signaling was not deleterious to myelinating oligodendrocytes in young, developing mice or to remyelinating oligodendrocytes in cuprizone-induced demyelinated lesions. Second, we showed that specifically enhancing PERK activation in myelinating oligodendrocytes protects the cells and myelin against the detrimental effects of the immune cytokine, IFN-γ, during development. Third, we showed that enhanced PERK activation in remyelinating oligodendrocytes attenuated IFN-γ–induced cell apoptosis and remyelination failure in the cuprizone model. Fourth, we showed that enhanced PERK activation in remyelinating oligodendrocytes at the recovery stage of EAE facilitated oligodendrocyte regeneration and remyelination in EAE demyelinated lesions. Our previous work demonstrated that PDGFαR-positive OPCs and inflammatory cells (T cells and microglia/macrophages) do not express the transgene product, Fv2E-PERK, in PLP/Fv2E-PERK mice11 and that AP20187 treatment does not affect the proliferation of OPCs or the activity of inflammatory cells in mice undergoing EAE.11 Consistent with these results, we found that PERK activation in (re)myelinating oligodendrocytes had no noticeable effect on OPCs or inflammatory cells. Although the precise molecular mechanisms by which PERK signaling protects remyelinating oligodendrocytes against inflammatory mediators and ER stress in immune-mediated demyelinating diseases remain to be elucidated, the results presented herein suggest that the manipulation of PERK signaling in remyelinating oligodendrocytes could promote myelin repair in MS demyelinated lesions.
IFN-γ is thought to be a major contributing factor to poor remyelination in MS lesions.1,13 Our previous studies have shown that the deleterious effects of IFN-γ on developmental myelination and remyelination are mediated, at least in part, by PERK signaling.14,15,18 The data concerning the effects of PERK signaling, however, appear contradictory. We showed that PERK haploinsufficiency exacerbated IFN-γ–induced myelinating oligodendrocyte loss and hypomyelination in young, developing mice and IFN-γ–induced remyelinating oligodendrocyte apoptosis and remyelination failure in the cuprizone model.14,15 In contrast, although our previous publication showed that global inactivation of the GADD34 gene, which encodes the stress-inducible regulatory subunit of a phosphatase complex that dephosphorylates eIF2α, increased the activity of the PERK-eIF2α pathway and ameliorated IFN-γ–induced myelinating oligodendrocyte loss and hypomyelination in young, developing mice,18 we found that GADD34 inactivation exacerbated IFN-γ–induced remyelinating oligodendrocyte death and remyelination failure in cuprizone-induced demyelinated lesions (unpublished data, W. Lin). It is known that IFN-γ influences the viability of oligodendrocytes in the CNS by regulating the functions of T cells and microglia/macrophages.28,29 Moreover, recent studies have shown that PERK signaling is a potent regulator of the functions of T cells and macrophages in inflammatory diseases.30,31,49 Clearly, the mouse models used in our previous studies did not allow us to determine the specific contribution of oligodendrocytes or inflammatory cells (T cells and microglia/macrophages) to the contradictory effects of PERK signaling on developmental myelination and remyelination in response to IFN-γ. By using a transgenic mouse model that allows for the temporally controlled activation of PERK signaling specifically in oligodendrocytes, we showed herein that PERK activation in both myelinating oligodendrocytes and remyelinating oligodendrocytes is beneficial in response to IFN-γ. Therefore, we demonstrated that PERK activation in (re)myelinating oligodendrocytes protects these cells cell-autonomously against the cytotoxicity of IFN-γ, resulting in enhanced (re)myelination. In addition, these results suggest that the antagonistic effects of PERK signaling on developmental myelination and remyelination in response to IFN-γ are likely the result of PERK’s effects on inflammatory cells (T cells or microglia/macrophages).
The principle function of oligodendrocytes is to produce myelin, the unique multilamellar sheath that wraps around axons, in the CNS.25 Because of the enormous surface area of myelin, each (re)myelinating oligodendrocyte must synthesize a large quantity of myelin proteins during the active phase of myelination.50 Recent studies have suggested that oligodendrocytes are highly sensitive to the disruption of protein translation.4,51 There is evidence that loss-of-function mutations of translation initiation factor, eIF2B, exclusively affect the CNS white matter in most cases of vanishing white matter disease, a fatal autosomal-recessive hypomyelinating disease.52,53 Although PERK activation protects cells against cytotoxic effects of inflammatory mediators and ER stress by phosphorylating eIF2α,11,22 p-eIF2α inhibits global protein biosynthesis by suppressing the activity of eIF2B, with the two proteins forming a non-productive p-eIF2α–eIF2B complex.54 Therefore, it was necessary to exclude the possibility that PERK activation promotes (re)myelinating oligodendrocyte survival but impairs their myelinating function. Importantly, we showed herein that PERK activation in (re)myelinating oligodendrocytes did not suppress the (re)myelination process under normal or pathological conditions. A previous study had shown that AP20187 can penetrate the blood-brain barrier; however, its blood-brain barrier permeability is moderate.55 Thus, our data likely suggest that the treatment with a low dosage of AP20187 (0.5 or 2 mg/kg) moderately activates PERK signaling in oligodendrocytes in PLP/Fv2E-PERK mice and that moderate activation of PERK signaling has no significant effect on the function of remyelinating oligodendrocytes in immune-mediated demyelinating diseases. On the other hand, it could be interesting to determine whether strong activation of PERK signaling in myelinating oligodendrocytes would lead to oligodendrocyte dysfunction, reproducing vanishing white matter disease in mice.
Although we showed herein that enhanced PERK activation in remyelinating oligodendrocytes promoted remyelination in EAE demyelinated lesions, we found that starting AP20187 treatment on PID20 did not significantly affect the severity of the clinical symptoms in PLP/Fv2E-PERK mice during the recovery stage of disease. It is believed that inflammation contributes significantly to the clinical symptoms of EAE.56 Recent studies also suggest that axon degeneration contributes to the clinical symptoms of MS and EAE and is the principal cause of chronic disability in MS.57,58 Axon degeneration largely occurs during the acute stage of EAE. It is not surprising that enhanced PERK activation in oligodendrocytes at the recovery stage of disease did not alter the degree of axon loss in EAE demyelinated lesions (Figure 7E and Supplemental Figure S3, D–F). Moreover, we showed that starting AP20187 treatment on PID20 did not alter the degree of inflammation in PLP/Fv2E-PERK mice at the recovery stage of EAE. Therefore, it is possible that the lack of effect of oligodendrocyte-specific enhancement of PERK activation on inflammation and axon loss in EAE mice is responsible for the minimal change in the clinical symptoms at the recovery stage of the disease. Clearly, the inability of enhanced PERK activation in oligodendrocytes to ameliorate EAE clinical symptoms, despite its beneficial effects on remyelination, is deserving of additional study.
In summary, the results presented herein demonstrate that activation of PERK signaling in remyelinating oligodendrocytes cell-autonomously promotes cell survival, and subsequently results in enhanced remyelination in immune-mediated demyelinating diseases. Currently, there is no effective therapy for MS that promotes oligodendrocyte regeneration and myelin repair. This study suggests that therapeutic strategies that activate PERK signaling in remyelinating oligodendrocytes may have beneficial effects on the restoration of neurological function and may delay disability progression in patients with MS.
Footnotes
Supported by National Multiple Sclerosis Society grants TA3026-A-1 and RG4813-A-2 (W.L.) and NIH grant NS073132 (W.L.). D.R. is a Principal Research Fellow of the Wellcome Trust.
Supplemental Data
Persistent activation of PERK signaling in remyelinating oligodendrocytes does not affect remyelination in cuprizone-induced demyelinated lesions. A: CC1 and p-eIF2α double immunostaining shows that the immunoreactivity of p-eIF2α is undetectable in oligodendrocytes in the corpus callosum of vehicle-treated mice after three weeks of cuprizone removal, but is detectable in oligodendrocytes in AP20187-treated mice. B: MBP immunostaining shows that AP20187 treatment does not significantly affect the degree of remyelination in the corpus callosum of PLP/Fv2E-PERK mice after three weeks of cuprizone removal. N = 3 animals. Scale bars: 10 μm (A); 23 μm (B).
AP20187 treatment enhances PERK activation in remyelinating oligodendrocytes in PLP/Fv2E-PERK mice undergoing EAE. CC1 and p-eIF2α double immunostaining shows that a small number of oligodendrocytes are p-eIF2α positive in the lumbar spinal cord of PLP/Fv2E-PERK mice treated with vehicle at PID34. Interestingly, the level of p-eIF2α is markedly increased in oligodendrocytes in PLP/Fv2E-PERK mice treated with AP20187. N = 3 animals. Scale bar = 10 μm.
AP20187 treatment enhances oligodendrocyte regeneration and remyelination in EAE demyelinated lesions in PLP/Fv2E-PERK mice. A–C: ASPA immunostaining shows that AP20187 treatment significantly increases the number of oligodendrocytes in the demyelinated lesions of PLP/Fv2E-PERK mice at PID 34. Nevertheless, oligodendrocytes numbers in the demyelinated lesions of AP20187-treated mice are markedly lower than in the matched regions in adult naïve PLP/Fv2E-PERK mice. N = 8 animals. D–F: Toluidine blue staining shows that there are markedly fewer total axons in the demyelinated lesions of PLP/Fv2E-PERK mice treated with either AP20187 or vehicle at PID34 than in the matched regions in age-matched naïve PLP/Fv2E-PERK mice. N = 4 animals. Scale bars: 10 μm (A–F).
References
- 1.Franklin R.J., Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 2.Kotter M.R., Stadelmann C., Hartung H.P. Enhancing remyelination in disease–can we wrap it up? Brain. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin W., Popko B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci. 2009;12:379–385. doi: 10.1038/nn.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 8.Lin W., Bailey S.L., Ho H., Harding H.P., Ron D., Miller S.D., Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mháille A.N., McQuaid S., Windebank A., Cunnea P., McMahon J., Samali A., FitzGerald U. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2008;67:200–211. doi: 10.1097/NEN.0b013e318165b239. [DOI] [PubMed] [Google Scholar]
- 10.Cunnea P., Mháille A.N., McQuaid S., Farrell M., McMahon J., Fitzgerald U. Expression profiles of endoplasmic reticulum stress-related molecules in demyelinating lesions and multiple sclerosis. Mult Scler. 2011;17:808–818. doi: 10.1177/1352458511399114. [DOI] [PubMed] [Google Scholar]
- 11.Lin W., Lin Y., Li J., Fenstermaker A.G., Way W.S., Clayton B., Jamison S., Harding H.P., Ron D., Popko B. Oligodendrocyte-specific activation of PERK signaling protects mice against experimental autoimmune encephalomyelitis. J Neurosci. 2013;33:5980–5991. doi: 10.1523/JNEUROSCI.1636-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradl M., Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lees J.R., Cross A.H. A little stress is good: IFN-gamma, demyelination, and multiple sclerosis. J Clin Invest. 2007;117:297–299. doi: 10.1172/JCI31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin W., Kemper A., Dupree J.L., Harding H.P., Ron D., Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 15.Lin W., Harding H.P., Ron D., Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J Cell Biol. 2005;169:603–612. doi: 10.1083/jcb.200502086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin W., Kemper A., McCarthy K.D., Pytel P., Wang J.P., Campbell I.L., Utset M.F., Popko B. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24:10074–10083. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin W., Lin Y. Interferon-γ inhibits central nervous system myelination through both STAT1-dependent and STAT1-independent pathways. J Neurosci Res. 2010;88:2569–2577. doi: 10.1002/jnr.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin W., Kunkler P.E., Harding H.P., Ron D., Kraig R.P., Popko B. Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-gamma. Am J Pathol. 2008;173:1508–1517. doi: 10.2353/ajpath.2008.080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidman R.L., Abervine J.B., Pierce E.T. Harvard University Press; Cambridge: 1971. Atlas of the Mouse Brain and Spinal Cord. [Google Scholar]
- 20.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 21.Marciniak S.J., Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 22.Lu P.D., Jousse C., Marciniak S.J., Zhang Y., Novoa I., Scheuner D., Kaufman R.J., Ron D., Harding H.P. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuss B., Mallon B., Phan T., Ohlemeyer C., Kirchhoff F., Nishiyama A., Macklin W.B. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev Biol. 2000;218:259–274. doi: 10.1006/dbio.1999.9574. [DOI] [PubMed] [Google Scholar]
- 24.Fuss B., Afshari F.S., Colello R.J., Macklin W.B. Normal CNS myelination in transgenic mice overexpressing MHC class I H-2L(d) in oligodendrocytes. Mol Cell Neurosci. 2001;18:221–234. doi: 10.1006/mcne.2001.1011. [DOI] [PubMed] [Google Scholar]
- 25.Baumann N., Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 26.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 27.Codarri L., Fontana A., Becher B. Cytokine networks in multiple sclerosis: lost in translation. Curr Opin Neurol. 2010;23:205–211. doi: 10.1097/WCO.0b013e3283391feb. [DOI] [PubMed] [Google Scholar]
- 28.Popko B., Baerwald K.D. Oligodendroglial response to the immune cytokine interferon gamma. Neurochem Res. 1999;24:331–338. doi: 10.1023/a:1022586726510. [DOI] [PubMed] [Google Scholar]
- 29.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo C.W., Cui D., Arellano J., Dorweiler B., Harding H., Fitzgerald K.A., Ron D., Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J.S., Ocvirk S., Berger E., Kisling S., Binder U., Skerra A., Lee A.S., Haller D. Endoplasmic reticulum stress response promotes cytotoxic phenotype of CD8αβ+ intraepithelial lymphocytes in a mouse model for Crohn’s disease-like ileitis. J Immunol. 2012;189:1510–1520. doi: 10.4049/jimmunol.1200166. [DOI] [PubMed] [Google Scholar]
- 32.Alfa R.W., Tuszynski M.H., Blesch A. A novel inducible tyrosine kinase receptor to regulate signal transduction and neurite outgrowth. J Neurosci Res. 2009;87:2624–2631. doi: 10.1002/jnr.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fancy S.P., Chan J.R., Baranzini S.E., Franklin R.J., Rowitch D.H. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- 34.Matsushima G.K., Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denic A., Johnson A.J., Bieber A.J., Warrington A.E., Rodriguez M., Pirko I. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–29. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Star B.J., Vogel D.Y., Kipp M., Puentes F., Baker D., Amor S. In vitro and in vivo models of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11:570–588. doi: 10.2174/187152712801661284. [DOI] [PubMed] [Google Scholar]
- 37.Chakrabarty A., Danley M.M., LeVine S.M. Immunohistochemical localization of phosphorylated protein kinase R and phosphorylated eukaryotic initiation factor-2 alpha in the central nervous system of SJL mice with experimental allergic encephalomyelitis. J Neurosci Res. 2004;76:822–833. doi: 10.1002/jnr.20125. [DOI] [PubMed] [Google Scholar]
- 38.Buntinx M., Stinissen P., Steels P., Ameloot M., Raus J. Immune-mediated oligodendrocyte injury in multiple sclerosis: molecular mechanisms and therapeutic interventions. Crit Rev Immunol. 2002;22:391–424. [PubMed] [Google Scholar]
- 39.Prineas J.W., Barnard R.O., Kwon E.E., Sharer L.R., Cho E.S. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- 40.Cannella B., Pitt D., Capello E., Raine C.S. Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination. Am J Pathol. 2000;157:933–943. doi: 10.1016/S0002-9440(10)64606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madhavarao C.N., Moffett J.R., Moore R.A., Viola R.E., Namboodiri M.A., Jacobowitz D.M. Immunohistochemical localization of aspartoacylase in the rat central nervous system. J Comp Neurol. 2004;472:318–329. doi: 10.1002/cne.20080. [DOI] [PubMed] [Google Scholar]
- 42.Locatelli G., Wörtge S., Buch T., Ingold B., Frommer F., Sobottka B., Krüger M., Karram K., Bühlmann C., Bechmann I., Heppner F.L., Waisman A., Becher B. Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat Neurosci. 2012;15:543–550. doi: 10.1038/nn.3062. [DOI] [PubMed] [Google Scholar]
- 43.Mallet V.O., Mitchell C., Guidotti J.E., Jaffray P., Fabre M., Spencer D., Arnoult D., Kahn A., Gilgenkrantz H. Conditional cell ablation by tight control of caspase-3 dimerization in transgenic mice. Nat Biotechnol. 2002;20:1234–1239. doi: 10.1038/nbt762. [DOI] [PubMed] [Google Scholar]
- 44.Neff T., Horn P.A., Valli V.E., Gown A.M., Wardwell S., Wood B.L., von Kalle C., Schmidt M., Peterson L.J., Morris J.C., Richard R.E., Clackson T., Kiem H.P., Blau C.A. Pharmacologically regulated in vivo selection in a large animal. Blood. 2002;100:2026–2031. doi: 10.1182/blood-2002-03-0792. [DOI] [PubMed] [Google Scholar]
- 45.Steel C.D., Kim W.K., Sanford L.D., Wellman L.L., Burnett S., Van Rooijen N., Ciavarra R.P. Distinct macrophage subpopulations regulate viral encephalitis but not viral clearance in the CNS. J Neuroimmunol. 2010;226:81–92. doi: 10.1016/j.jneuroim.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frohman E.M., Racke M.K., Raine C.S. Multiple sclerosis–the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 47.Gow A., Wrabetz L. CHOP and the endoplasmic reticulum stress response in myelinating glia. Curr Opin Neurobiol. 2009;19:505–510. doi: 10.1016/j.conb.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Southwood C.M., Garbern J., Jiang W., Gow A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron. 2002;36:585–596. doi: 10.1016/s0896-6273(02)01045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasnain S.Z., Lourie R., Das I., Chen A.C., McGuckin M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90:260–270. doi: 10.1038/icb.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeiffer S.E., Warrington A.E., Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 51.Scheper G.C., Proud C.G., van der Knaap M.S. Defective translation initiation causes vanishing of cerebral white matter. Trends Mol Med. 2006;12:159–166. doi: 10.1016/j.molmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 52.van der Knaap M.S., Pronk J.C., Scheper G.C. Vanishing white matter disease. Lancet Neurol. 2006;5:413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- 53.Bugiani M., Boor I., Powers J.M., Scheper G.C., van der Knaap M.S. Leukoencephalopathy with vanishing white matter: a review. J Neuropathol Exp Neurol. 2010;69:987–996. doi: 10.1097/NEN.0b013e3181f2eafa. [DOI] [PubMed] [Google Scholar]
- 54.Proud C.G. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16:3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Burnett S.H., Kershen E.J., Zhang J., Zeng L., Straley S.C., Kaplan A.M., Cohen D.A. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 56.Batoulis H., Recks M.S., Addicks K., Kuerten S. Experimental autoimmune encephalomyelitis–achievements and prospective advances. APMIS. 2011;119:819–830. doi: 10.1111/j.1600-0463.2011.02794.x. [DOI] [PubMed] [Google Scholar]
- 57.Trapp B.D., Nave K.A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 58.Herz J., Zipp F., Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp Neurol. 2010;225:9–17. doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Persistent activation of PERK signaling in remyelinating oligodendrocytes does not affect remyelination in cuprizone-induced demyelinated lesions. A: CC1 and p-eIF2α double immunostaining shows that the immunoreactivity of p-eIF2α is undetectable in oligodendrocytes in the corpus callosum of vehicle-treated mice after three weeks of cuprizone removal, but is detectable in oligodendrocytes in AP20187-treated mice. B: MBP immunostaining shows that AP20187 treatment does not significantly affect the degree of remyelination in the corpus callosum of PLP/Fv2E-PERK mice after three weeks of cuprizone removal. N = 3 animals. Scale bars: 10 μm (A); 23 μm (B).
AP20187 treatment enhances PERK activation in remyelinating oligodendrocytes in PLP/Fv2E-PERK mice undergoing EAE. CC1 and p-eIF2α double immunostaining shows that a small number of oligodendrocytes are p-eIF2α positive in the lumbar spinal cord of PLP/Fv2E-PERK mice treated with vehicle at PID34. Interestingly, the level of p-eIF2α is markedly increased in oligodendrocytes in PLP/Fv2E-PERK mice treated with AP20187. N = 3 animals. Scale bar = 10 μm.
AP20187 treatment enhances oligodendrocyte regeneration and remyelination in EAE demyelinated lesions in PLP/Fv2E-PERK mice. A–C: ASPA immunostaining shows that AP20187 treatment significantly increases the number of oligodendrocytes in the demyelinated lesions of PLP/Fv2E-PERK mice at PID 34. Nevertheless, oligodendrocytes numbers in the demyelinated lesions of AP20187-treated mice are markedly lower than in the matched regions in adult naïve PLP/Fv2E-PERK mice. N = 8 animals. D–F: Toluidine blue staining shows that there are markedly fewer total axons in the demyelinated lesions of PLP/Fv2E-PERK mice treated with either AP20187 or vehicle at PID34 than in the matched regions in age-matched naïve PLP/Fv2E-PERK mice. N = 4 animals. Scale bars: 10 μm (A–F).