Abstract
Background:
Chronic rhinosinusitis (CRS) is an inflammatory disorder of the nose and sinuses. Because fungi were postulated as a potential cause of CRS in the late 1990s, contrasting articles have advocated and refuted the use of antifungal agents in its management. Although good research shows an interaction of the immune system with fungus in CRS, e.g., allergic fungal sinusitis (AFS), this does not imply that fungi are the cause of CRS or that antifungals will be effective in management. This study was designed to assess the potential advantage of either topical or systemic antifungal therapy in the symptomatic treatment of CRS to aid physicians in making informed decisions about treating patients with CRS.
Methods:
A systematic review of the literature was performed with meta-analysis. All studies obtained from searches were reviewed and trials meeting the eligibility criteria were selected. CRS was defined using either the European Position Paper on Rhinosinusitis and Nasal Polyps or American Academy of Otolaryngology–Head and Neck Surgery criteria. Authors were contacted and original data were used for data analysis.
Results:
Five studies investigating topical antifungals and one investigating systemic antifungals met the inclusion criteria. All trials were double blinded and randomized. Pooled meta-analysis showed no statistically significant benefit of topical or systemic antifungals over placebo. Symptoms scores statistically favored the placebo group for this outcome. Adverse event reporting was higher in the antifungal group.
Conclusion:
Reported side-effects of antifungal therapies may outweigh any potential benefits of treatment based on this meta-analysis and the authors therefore do not advocate the use antifungal treatment in the management of CRS.
Keywords: Allergic fungal sinusitis, amphotericin B, chronic rhinosinusitis, meta-analysis, symptoms, systemic antifungal, topical antifungal
Chronic rhinosinusitis (CRS) is an inflammatory disorder of the nose and sinuses, which is clinically defined as persistence of symptoms of nasal blockage, obstruction, congestion, or discharge for at least 12 weeks, combined with endoscopic abnormalities (polyps, mucopurulent discharge, and/or mucosal swelling) or an abnormal sinus computed tomography scan. Other symptoms may include facial pain or reduced sense of smell.1 Allergic fungal sinusitis (AFS) is a well-recognized subgroup of CRS, in which a strong IgE-mediated hypersensitivity to fungal elements exacerbates and may be the dominant inflammatory process. In the past, fungi were thought to be important only in AFS, which was considered to be a less common distinct subset of CRS.2
It has now been proposed that fungal-related sinus disease is extremely common and accounts for the majority of CRS.3 Ponikau et al. from the Mayo Clinic documented fungus as a potential cause of CRS and advocated the use of topical antifungals.3 However, fungal colonization of the nose and paranasal sinuses have been found in both normal patients and in those with CRS.4 Since then, there has been increasing controversy, and contrasting articles have both advocated and refuted the use of both topical and systemic antifungal agents in the management of these patients.5
Although good research indicates an interaction of the immune system with fungus in CRS,6 this does not necessarily imply that antifungals will be effective in managing the disease. Inappropriate immune activation may be the driving pathological mechanism and fungal elements only the innocent target of the process, and it is well known that fungi are ubiquitous in both our environment and sinuses.4
CRS has a significant impact on the quality of life and health burden within the adult population.7 The impact of the disease on quality of life, as measured by short form 36 scores, is comparable with or worse than that of other chronic conditions such as chronic obstructive pulmonary disease, congestive heart failure, and back pain.8 Systemic antifungals have significant side effects, particularly with regard to the hepatic and renal toxicity. Topical amphotericin is expensive and also associated with potential adverse events.9 With the potential for fungus to be a common mediator of CRS, and a patient population of >60 million in the United States and European Union, it is essential that the need for and reported benefit and adverse effects of antifungals are well documented before broadly applying this form of therapy.10
METHODS
Criteria for Considering Studies for This Review
Types of Studies.
Randomized placebo-controlled trials (RCTs), which fulfilled the criteria described previously, were included.
Types of Participants.
Both adults and children with CRS as defined by either the European Position Paper on Rhinosinusitis and Nasal Polyps criteria1,11 or by the American Academy of Otolaryngology–Head and Neck Surgery10,12 were included. Fungus can be shown in almost all diseased and normal sinuses4; thus, associated fungus confirmed either histologically or on culture was not used as an inclusion criteria. The immunologic role of the fungus and the host is still an area of ongoing research. Patients with classic AFS satisfying the Bent-Kuhn criteria13 for the diagnosis of AFS was used for subset analysis.
Types of Interventions.
Studies involving both systemic and topical antifungal therapies were considered. Systemic antifungals can be given orally or i.v. Topical therapy may be administered by douching, nebulization, atomization, inhalations, irrigation, spray, drops, or powder insufflations.
Types of Outcome Measures
Primary Outcomes
- Symptom improvement as defined by
- Collated symptom scores (visual analog scales or Likert severity categories)
- Validated disease-specific quality-of-life questionnaires, such as the 31-item Rhinosinusitis Outcome Measure, 20-Item Sino-Nasal Outcome Test,14 Rhinosinusitis Disability Index, or Chronic Sinusitis Survey
Secondary Outcomes
Adverse events associated with treatment
- Surrogate outcoes
- Endoscopic scores
- Radiographic scores (i.e., Lund-Mackay)
Data Collection and Analysis
Electronic systematic searches for RCTs were conducted with no language, publication year, or publication status restrictions. A search strategy was used with a combination of medical subject headings terms and key words in collaboration with the Cochrane Ear, Nose, and Throat Disorders Group. The Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; mRCT; and additional sources were searched for published and unpublished trials.
The reference lists of identified publications for additional trials were scanned and where necessary, authors were contacted. One review author (PS) reviewed and selected trials and evaluated them against the inclusion criteria. In cases where PS was unsure as to whether the trial was relevant, a second review author (RJH) was consulted.
A structured data collection form was used. The review authors (PS and RJH) conducted the data extraction and assessed the quality of the method used in each included trial. If necessary, authors of studies were contacted for clarification.
We considered
Number of participants
Age of participants
Characteristics of trial such, e.g., duration of trial
Method of randomization
Method of blinding
Whether an intention-to-treat analysis was conducted
Exclusion criteria
Diagnostic criteria
Duration of treatment
Outcomes
Duration of illness
Severity of illness
Adverse effects
Other medicines being used
Assessment of risk of bias was conducted in accordance with the Cochrane Collaboration tool for assessing risk of bias.15 This tool deals with sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. In trials lacking details of randomization and other characteristics, authors of the studies were contacted to obtain further information.
Standardized mean differences (SMDs) were obtained from the reported results to compare trials using different scales as outcome scales. Raw data were extracted from graphs and tables. For SD results for the mean changes that were not available from the articles, authors were contacted to provide original data. Where this was not possible, SDs were imputed from studies using similar scales and methods. Dichotomous data were collected for adverse events.
Assessment of Heterogeneity
Clinical Heterogeneity.
All included studies were considered and where issues appeared that might have added to clinical heterogeneity, these were noted and considered in the analysis.
Statistical.
Forest plots were visually inspected to investigate statistical heterogeneity. Heterogeneity between studies was investigated using the I2 statistic,15 which provides an estimate of the percentage of variation observed in results that is unlikely to be caused by chance. A value of ≥50% was taken to indicate heterogeneity.
RESULTS
Description of Studies
Results of the Search.
A total of 374 references (324 from the search conducted in December 2009 and 50 from the search conducted in June 2010) from the searches were received: 269 of these were removed in first-level screening (i.e., removal of duplicates and clearly irrelevant references), leaving 105 references for further consideration. A flowchart of study selection is provided in Fig. 1. There were six studies that met the inclusion criteria. Characteristics of the included studies can be found in Table 1.
Figure 1.
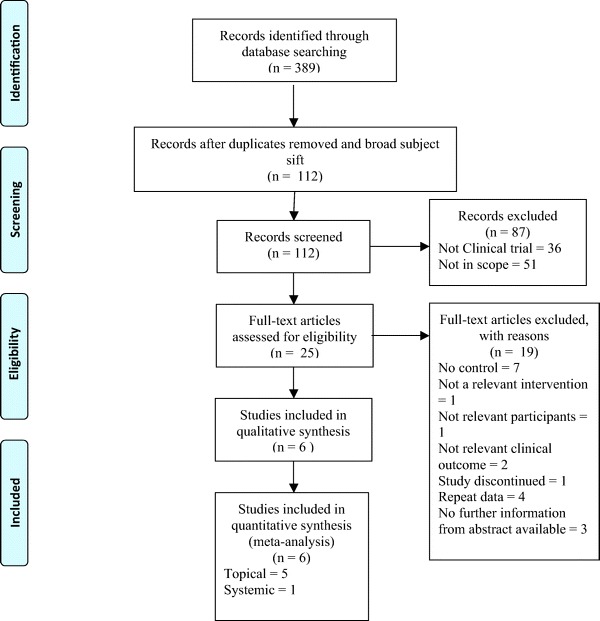
Flowchart illustrating study selection process.
Table 1.
Characteristics of included studies
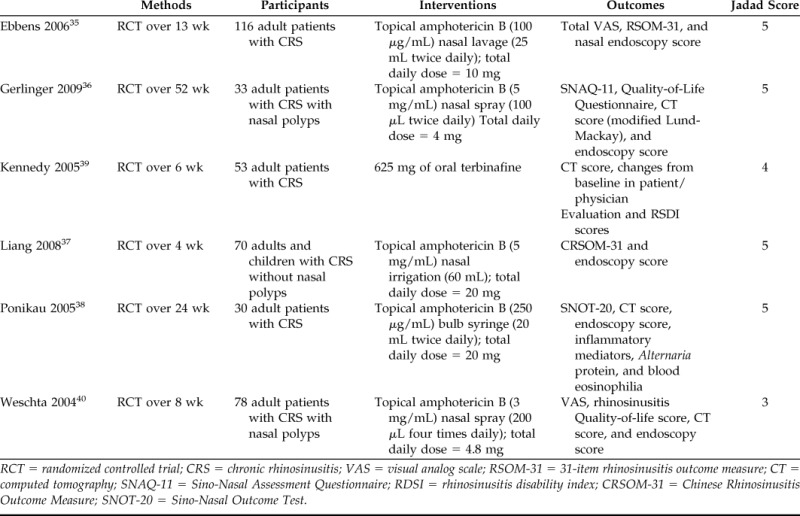
RCT = randomized controlled trial; CRS = chronic rhinosinusitis; VAS = visual analog scale; RSOM-31 = 31-item rhinosinusitis outcome measure; CT = computed tomography; SNAQ-11 = Sino-Nasal Assessment Questionnaire; RDSI = rhinosinusitis disability index; CRSOM-31 = Chinese Rhinosinusitis Outcome Measure; SNOT-20 = Sino-Nasal Outcome Test.
Excluded Studies.
Of the majority of the 374 abstracts retrieved from the searches 302 were not in the scope of our review. Seventy-two trials were identified. Forty-seven of these trials did not focus on the use of topical or systemic antifungal therapy in the treatment of CRS or AFS. We consulted the full-text articles of 25 trials. Four4 were repeat data.9,16–18 Seven7 trials were not randomized or controlled.19–25 One1 study was discontinued and the unpublished data were not made available by the authors.26 One1 trial did not have a relevant intervention, rather considering combination therapy.27 One1 trial did not have relevant participants, focusing on patients with acute rhinosinusitis.28 Two2 trials did not have relevant outcomes.29,30 These trials considered levels of proinflammatory cytokines, chemokines, and growth factors. Three3 studies did not have information available beyond that which was in the abstract; full-text manuscripts were not made available by the authors.31–33
Risk of Bias in Included Studies
Risk of bias was assessed using the Cochrane Collaboration Tool for Assessing Risk of Bias.15 In cases where information regarding methods was not provided, we consulted the authors for further information. The Jadad Composite scale34 was also used. In this system, 1 point was allocated if the study was described as being randomized with an additional point awarded if the method of randomization was described. One point was allocated if the study was described as blinded to patients and assessors with an additional point given if the method of double-blinding was described. The final point was allocated to follow-up regarding patient withdrawal. Studies with 2 points or less are considered to be low-quality studies, whereas studies with at least 3 points are considered to be of high quality. Four trials (66.7%) had a total score of 5.35–38 One (16.7%) trial had a total score of 439 and one (16.7%) trial had a total score of 3.40
Four trials (66.7%) had both adequate sequence generation and allocation concealment as ascertained from the articles or by correspondence.35–38 One trial (16.7%) had adequate sequence generation but no information was given regarding the method of allocation concealment and we received no reply from the author.39 One trial (16.7%) gave no information regarding sequence generation or allocation concealment.40 All trials were reported to be doubled blinded. Four trials (66.7%) explicitly stated the method of blinding either in the article or by correspondence.35–38 All trials addressed dropout and loss to follow-up population. All trials were free of selective reporting. All but one trial39 provided an allocation table or otherwise stated that the two groups were similar at baseline. It was noted that the Mayo Clinic is a collaborative partner with Accentia Biopharmeuticals, a company that holds the worldwide, exclusive commercial rights to SinuNase (topical amphotericin B, Accentia Biopharmaceuticals, Tampa, FL),26 and that Ponikau holds a patent for this product.41
Effects of Interventions
We considered topical and systemic antifungal therapies separately for meta-analysis. There was a considerable range of tools used for outcome assessment, with few trials using the same questionnaires or scales. SMDs were assessed for the different outcome measures.
Summary
Topical Antifungal Therapy versus Placebo
Symptom Scores.
Symptom scores were collected from three trials for meta-analysis.35,36,40 There were a total of 101 patients allocated to the topical amphotericin B group and 105 allocated to the placebo group. Liang et al. and Ponikau et al. did not consider symptom scores in their outcomes.
Pooled results favored the control (SMD = 0.35 [0.07, 0.62]; p =0 .01). The I2 statistic was 45%, which represents acceptable homogeneity (χ2 = 3.64; df = 2; p = 0.16). A forest plot illustrating this outcome is provided in Fig. 2.
Figure 2.
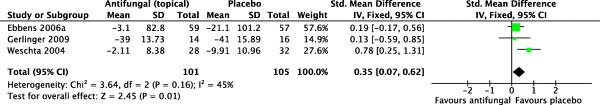
Forest plot illustrating standardized mean differences for symptom scores.
Disease-Specific Quality-of-Life Scores.
Five trials were pooled for meta-analysis regarding the outcome of disease-specific quality-of-life scores,35–38,40 with a total of 143 and 151 patients for the antifungal group and the placebo group, respectively.
Pooled results showed no statistically significant benefit for topical amphotericin B over placebo (SMD = 0.18 [−0.05, 0.42]; p = 0.12). The I2 statistic was 10%, with good homogeneity (χ2 = 4.46; df = 4; p = 0.35). A forest plot illustrating this outcome is provided in Fig. 3.
Figure 3.
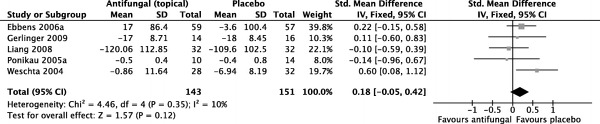
Forest plot illustrating standardized mean differences for disease-specific quality-of-life scores.
Nasal Endoscopy Scores.
For nasal endoscopy scores, data from four trials were pooled for meta-analysis,35–38 with 101 patients allocated to topical antifungals and 103 patients allocated to placebo. Weschta et al. did not consider endoscopy scores in their outcomes.
Pooled results did not show any statistically significant benefit over placebo (SMD = −0.00 [−0.26, 0.26]; p = 0.98). The I2 statistic was 62%, representing substantial heterogeneity (χ2 = 7.93; df = 3; p = 0.05}. A forest plot illustrating this outcome is provided in Fig. 4.
Figure 4.
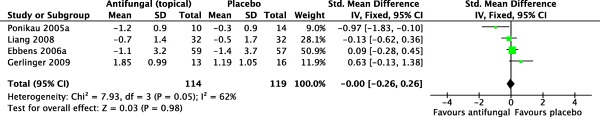
Forest plot illustrating standardized mean differences for nasal endoscopy scores.
Radiographic Scores.
Three trials were pooled for meta-analysis for radiographic scores,36,38,40 with Ebbens et al. and Gerlinger et al. not considering radiographic scores as an outcome in their respective trials. A total of 52 patients were allocated to the intervention group and 62 patients were allocated to placebo.
Pooled data did not show any statistically significant results (SMD = 0.02 [−0.36, 0.41]; p = 0.90}. The I2 statistic was 88%, representing considerable heterogeneity (χ2 = 17.03; df = 2; p = 0.0002). A forest plot illustrating this outcome is provided in Fig. 5.
Figure 5.
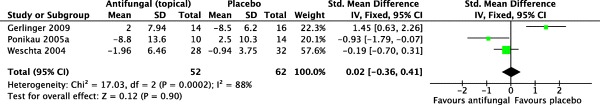
Forest plot illustrating standardized mean differences for computed tomography scores.
Systemic Antifungal Therapy versus Placebo.
Only one trial was identified with available data that investigated the efficacy of a systemic antifungal therapy versus a placebo.39 This trial reported radiographic scores and symptom scores as outcomes. There were a total of 23 patients allocated to the antifungal group and 26 patients allocated to the placebo group.
For symptom scores, there was no significant benefit of terbinafine over placebo (SMD = −0.07 [−0.64, 0.51]; p = 0.82). Similarly, for radiographic, there was no significant benefit of terbinafine over placebo (SMD = −0.14 [−19.22, 18.94]; p = 0.99).
Adverse Events.
Adverse events are described in Tables 2 and 3. A meta-analysis of adverse events was performed and found no statistically significant difference between the amphotericin and placebo groups (risk ratio, 3.36; 95% CI, 0.86–13.0; p = 0.08). Adverse events were reported inconsistently throughout the various trials. Weschta et al.40 reported a significant difference between placebo and antifungal groups with the antifungal group reporting more adverse events. The main side effect reported in trials investigating topical antifungals was local irritation, which was not deemed by the authors to be a serious adverse event.
Table 2.
Adverse events with topical therapy
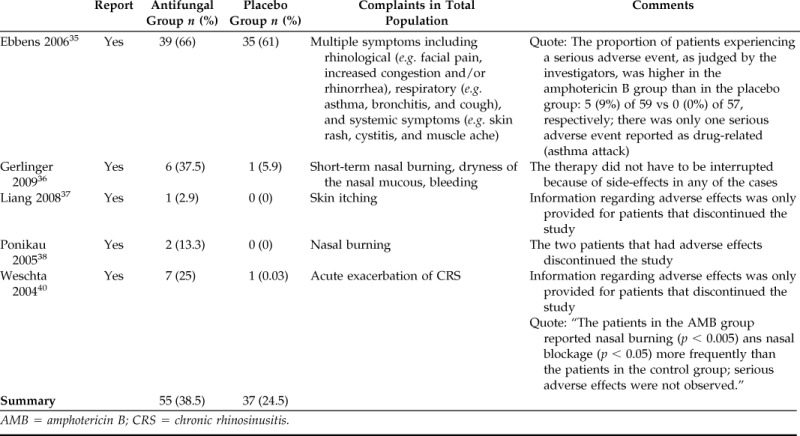
AMB = amphotericin B; CRS = chronic rhinosinusitis.
Table 3.
Adverse events with systemic therapy

ADR = adverse drug reaction.
DISCUSSION
Proponents of antifungals for the treatment of CRS and AFS argue that in CRS, fungi in sinonasal mucosa cause the activation of sensitized patients' immune systems, thereby driving the eosinophilic inflammation. Consequently, eliminating fungus in the sinus and nasal cavity through the use of antifungals would potentially reduce this inflammatory response.3
There is no evidence of any benefit of topical antifungals from the included studies. Topical antifungal therapy reported beneficial effects in only one of five trials38 for radiographic and endoscopic scores, but not for symptoms. There was substantial heterogeneity in these two outcomes, possibly because of differences in patient populations and disease factors. The control groups were favored in one of five trials40,42–44 for symptom scores and disease-specific quality-of-life scores. The pooled results showed significant symptom improvement in the placebo group across those studies reporting this outcome.
The five studies differed in methodology. Delivery volume and surgical state are established factors influencing the effectiveness to topical delivery to the sinuses.42–44 Three trials used nasal irrigation35,37,38 and two trials used nasal sprays36,40 to administer the antifungal or placebo. Patients who had endoscopic sinus surgery (ESS) were reported heterogeneously. Some trials required patients to have had previous ESS before administration of the antifungal or placebo35,36 and other trials had some patients who had not had previous ESSs.38,40 In one trial, previous ESS was part of the exclusion criteria and therefore no patients had previous ESSs.37 Although traditional concepts of ESS is aimed at relieving obstruction and improving ventilation, ESS has been shown to allow effective delivery of topical therapies to the mucosa of the sinuses compared with the preoperative state.42,43
The concentrations of the antifungal differed among studies. This may influence the proposed action because fungal growth may not be impeded at a concentration of 100 μg/mL in vitro compared with convincing inhibition at 200 and 300 μg/mL.45 Two trials used amphotericin B at concentrations of 100 μg/mL.35,36 There is currently some controversy surrounding both the optimum dosage and the preparation of the antifungal treatment, which may influence the ultimate outcome of treatment.
Systemic antifungal therapy reported no benefits over placebo for symptom scores or radiographic scores. Because there was only one trial that fit our inclusion criteria for systemic antifungals, there is no heterogeneity of approach.
Although it is well known that fungi are both ubiquitous in the sinuses and the environment and can therefore be found in normal sinuses, there are certain phenotypes of the disease process that may more readily yield positive culture or behave differently with regard to antifungal therapy. These situations might, in fact, represent a process where the fungi are causative and these specific situations may call for antifungal therapy to be used.
Although there was incomplete reporting of data in the published literature of the included studies, authors of four of the five topical antifungal RCTs provided original data to allow a meta-analysis.35,37,38,40 Some imputation and transformation was performed but original data provided limited this to only one study.36
The results of this meta-analysis confirms the conclusion from a previous nonsystematic review conducted by Lim et al.,46 which states that “no definite conclusions could be made regarding the use of antifungals.” Lim et al. found 14 studies that fulfilled their inclusion criteria; however, only 7 studies were controlled trials and only 5 were double-blind randomized trials. Two of their RCTs were excluded in this review because they did not deal with antifungals as an intervention.47,48 Three more trials were included in this review.36,37,39 No meta-analysis was performed in the study by Lim et al.46 Rather, it was purely qualitative.
CONCLUSION
Based on this meta-analysis, the authors do not advocate the use of either topical or systemic antifungal treatment in the routine management of CRS. Although there appears to be considerable evidence against the use of topical and systemic antifungals in the treatment of CRS, clinical diversity in the surgical state of patients, delivery volume, and concentrations of antifungals in included studies may bring about heterogeneity of treatment effect and are factors that should be considered for any topical therapy trial in CRS. It is therefore advised that antifungal therapy should only be considered in specific instances or situations where clinical features may suggest a possible benefit from treatment.
Footnotes
Will be presented as part of the independent learning programme of the University of New South Wales Medical School Programme
RJ Harvey has served on an advisory board for Schering-Plough and GlaxoSmithKline, was a previous consultant with Medtronic, Speakers Bureau for Merek Sharp Dolme, and Arthrocare and has received grant support from NeilMed. R Sacks is consultant for Medtronic and Speakers Bureau for Merek Sharp Dolme. The remaining authors have no conflicts to declare pertaining to this article
REFERENCES
- 1. Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl 20:1–136, 2007. [PubMed] [Google Scholar]
- 2. Hutcheson PS, Schubert MS, Slavin RG. Distinctions between allergic fungal rhinosinusitis and chronic rhinosinusitis. Am J Rhinol Allergy 24:405–408. [DOI] [PubMed] [Google Scholar]
- 3. Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc 74:877–884, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Lackner A, Stammberger H, Buzina W, et al. Fungi: A normal content of human nasal mucus. Am J Rhinol 19:125–129, 2005. [PubMed] [Google Scholar]
- 5. Ebbens FA, Georgalas C, Rinia AB, et al. The fungal debate: Where do we stand today? Rhinology 45:178–189, 2007. [PubMed] [Google Scholar]
- 6. Ponikau JU, Sherris DA, Kita H. The role of ubiquitous airborne fungi in chronic rhinosinusitis. Clin Allergy Immunol 20:177–184, 2007. [PubMed] [Google Scholar]
- 7. Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 113:104–109, 1995. [DOI] [PubMed] [Google Scholar]
- 8. Metson RB, Gliklich RE. Clinical outcomes in patients with chronic sinusitis. Laryngoscope 110:24–28, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Ebbens F, Bachert C, Mullol J, et al. Amphotericin B nasal lavages equally as effective as placebo. Clin Otolaryngol Allied Sci 31:169, 2006. [Google Scholar]
- 10. Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: Developing guidance for clinical trials. J Allergy Clin Immunol 118(suppl):S17–S61, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Fokkens W, Lund V, Mullol J. EP3OS 2007: European position paper on rhinosinusitis and nasal polyps 2007. A summary for otorhinolaryngologists. Rhinology 45:97–101, 2007. [PubMed] [Google Scholar]
- 12. Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 129(suppl):S1–S32, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Morpeth JF, Rupp NT, Dolen WK, et al. Fungal sinusitis: An update. Ann Allergy Asthma Immunol 76:128–139, 1996. [DOI] [PubMed] [Google Scholar]
- 14. Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 126:41–47, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2. The Cochrane Collaboration, 2008. (updated September 2009; cited 2010). Available online at www.cochrane-handbook.org; accessed November 2010.
- 16. Gerlinger I, Fittler A, Mayer A, et al. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis. Can recidive polyposis be prevented? Orv Hetil 149:1737–1746, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Riechelmann H. Fungus and nasal polyps. International Consensus Conference on Nasal Polyposis, Brussels, Belgium, April 23–25 2004. [Google Scholar]
- 18. Sherris DA, Ponikau JU, Weaver A, et al. Treatment of chronic rhinosinusitis with intranasal amphotericin B: A prospective, randomized, placebo-controlled trial. Program and Abstracts of papers presented at the Scientific Sessions of the American Academy of Allergy, Asthma, and Immunology (AAAAI) 60th Annual Meeting, San Francisco, CA, pS331, 2004. [Google Scholar]
- 19. Chan KO, Genoway KA, Javer AR. Effectiveness of itraconazole in the management of refractory allergic fungal rhinosinusitis. J Otolaryngol Head Neck Surg 37:870–874, 2008. [PubMed] [Google Scholar]
- 20. Gupta R, Bahadur S, Thakar A, et al. Management protocols of allergic fungal sinusitis. Ind J Otolaryngol Head Neck Surg 59:35–40, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helbling A, Baumann A, Hanni C, Caversaccio M. Amphotericin B nasal spray has no effect on nasal polyps. J Laryngol Otol 120:1023–1025, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Hofman T, Skrobisz W, Hofman A, Hofman M. The estimation of efficacy of fluconazole treatment in patients with chronic sinusitis. Mikologia Lekarska 11:297–301, 2004. [Google Scholar]
- 23. Jen A, Kacker A, Huang C, Anand V. Fluconazole nasal spray in the treatment of allergic fungal sinusitis: A pilot study. Ear Nose Throat J 83:692, 4–5, 2004. [PubMed] [Google Scholar]
- 24. Ponikau JU, Sherris DA, Kita H, Kern EB. Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. J Allergy Clin Immunol 110:862–866, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Shin SH, Ye MK. Effects of topical amphotericin B on expression of cytokines in nasal polyps. Acta Otolaryngol 124:1174–1177, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Accentia-Biopharmaceuticals. A prospective, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of intranasal amphotericin B suspension in patients with refractory, postsurgical chronic sinusitis (CS) 2008 (cited 2009). Available online at www.clinicaltrials.gov/ct2/show/NCT00425620; accessed January 2011.
- 27. Deka RC, Chokkalingam V, Vishnoi RK, Kumar R. Topical Amphotericin B and steroid in AFS. Otolaryngol Head and Neck Surg 137(suppl 1):P40, 2007. [Google Scholar]
- 28. Mosges R, Spaeth J, Berger K, Dubois F. Topical treatment of rhinosinusitis with fusafungine nasal spray. A double-blind, placebo-controlled, parallel-group study in 20 patients. Arzneimittelforschung 52:877–883, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Ebbens FA, Georgalas C, Luiten S, et al. The effect of topical amphotericin B on inflammatory markers in patients with chronic rhinosinusitis: A multicenter randomized controlled study. Laryngoscope 119:401–408, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Weschta M, Rimek D, Formanek M, et al. Effect of nasal antifungal therapy on nasal cell activation markers in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg 132:743–747, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Frigas E. A pilot, prospective, double blind, placebo-controlled treatment trial with itraconazole orally in patients with chronic rhinosinusitis and asthma. J Allergy Clin Immunol 119:S142, 2007. [Google Scholar]
- 32. Lopatin AS, Akulich II, Kochetkov PA. Systemic antifungals in the treatment of nasal polyposis: Do they work? International Consensus Conference on Nasal Polyposis, Brussels, Belgium, April 23–25, 2004. [Google Scholar]
- 33. Stergiou A, Casiano R, Katz L, et al., editors. Intranasal amphotericin B in patients with refractory, postsurgical chronic sinusitis: A phase III pivotal clinical trial design. The XXVI Congress of the European Academy of Allergology and Clinical Immunology (EAACI), Goteborg, Sweden, June 9–13, 2007. [Google Scholar]
- 34. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 17:1–12, 1996. [DOI] [PubMed] [Google Scholar]
- 35. Ebbens FA, Scadding GK, Badia L, et al. Amphotericin B nasal lavages: Not a solution for patients with chronic rhinosinusitis. J Allergy Clin Immunol 118:1149–1156, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Gerlinger I, Fittler A, Fonai F, et al. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis, with a review of the antifungal therapy. Eur Arch Otorhinolaryngol 266:847–855, 2009. [DOI] [PubMed] [Google Scholar]
- 37. Liang KL, Su MC, Shiao JY, et al. Amphotericin B irrigation for the treatment of chronic rhinosinusitis without nasal polyps: A randomized, placebo-controlled, double-blind study. Am J Rhinol 22:52–58, 2008. [DOI] [PubMed] [Google Scholar]
- 38. Ponikau JU, Sherris DA, Weaver A, Kita H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: A randomized, placebo-controlled, double-blind pilot trial. J Allergy Clin Immunol 115:125–131, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Kennedy DW, Kuhn FA, Hamilos DL, et al. Treatment of chronic rhinosinusitis with high-dose oral terbinafine: A double blind, placebo-controlled study. Laryngoscope 115:1793–1799, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Weschta M, Rimek D, Formanek M, et al. Topical antifungal treatment of chronic rhinosinusitis with nasal polyps: A randomized, double-blind clinical trial. J Allergy Clin Immunol 113:1122–1128, 2004. [DOI] [PubMed] [Google Scholar]
- 41. Ponikau J. Inventor methods and materials for treating and preventing inflammation of mucosal tissue. United States of America, April 29, 2003. Patent number 6207703.
- 42. Harvey RJ, Goddard JC, Wise SK, Schlosser RJ. Effects of endoscopic sinus surgery and delivery device on cadaver sinus irrigation. Otolaryngol Head Neck Surg 139:137–142, 2008. [DOI] [PubMed] [Google Scholar]
- 43. Grobler A, Weitzel EK, Buele A, et al. Pre- and postoperative sinus penetration of nasal irrigation. Laryngoscope 118:2078–2081, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Beule A, Athanasiadis T, Athanasiadis E, et al. Efficacy of different techniques of sinonasal irrigation after modified Lothrop procedure. Am J Rhinol Allergy 23:85–90, 2009. [DOI] [PubMed] [Google Scholar]
- 45. Shirazi MA, Stankiewicz JA, Kammeyer P. Activity of nasal amphotericin B irrigation against fungal organisms in vitro. Am J Rhinol 21:145–148, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Lim M, Citardi MJ, Leong JL. Topical antimicrobials in the management of chronic rhinosinusitis: A systematic review. Am J Rhinol 22:381–389, 2008. [DOI] [PubMed] [Google Scholar]
- 47. Desrosiers MY, Salas-Prato M. Treatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large-particle nebulizer: Results of a controlled trial. Otolaryngol Head Neck Surg 125:265–269, 2001. [DOI] [PubMed] [Google Scholar]
- 48. Sykes DA, Wilson R, Chan KL, et al. Relative importance of antibiotic and improved clearance in topical treatment of chronic mucopurulent rhinosinusitis. A controlled study. Lancet 2:359–360, 1986. [DOI] [PubMed] [Google Scholar]


