Abstract
Background:
Benign lesions of the nasal cavity represent a diverse group of pathologies. Furthermore, each of these disorders may present differently in any given patient as pain and discomfort, epistaxis, headaches, vision changes, or nasal obstruction. Although these nasal masses are benign, many of them have a significant capacity for local tissue destruction and symptomatology secondary to this destruction. Advances in office-based endoscopic nasendoscopy have equipped the otolaryngologist with a safe, inexpensive, and rapid means of directly visualizing lesions within the nasal cavity and the initiation of appropriate treatment.
Methods:
The purpose of this study is to review the diagnosis, management, and controversies of many of the most common benign lesions of the nasal cavity encountered by the primary care physician or otolaryngologist.
Results:
This includes discussion of inverted papilloma (IP), juvenile angiofibroma, squamous papilloma, pyogenic granuloma, hereditary hemorrhagic telangiectasia, schwannoma, benign fibro-osseous lesions, and other benign lesions of the nasal cavity, with particular emphasis on IP and juvenile angiofibroma.
Conclusion:
A diverse array of benign lesions occur within the nasal cavity and paranasal cavities. Despite their inability to metastasize, many of these lesions have significant capability for local tissue destruction and recurrence.
Keywords: Cocaine rhinitis, endoscopic surgery, hereditary hemorrhagic telangiectasia, pyogenic granuloma, nasal mass, inverted papilloma, rhinolith, squamous papilloma
Benign nonneoplastic lesions or disorders of the nasal cavity represent a diverse group of disorders. Advances in fiberoptic nasal endoscopy over the past couple of decades have made it possible for even the most inexperienced endoscopist to detect the vast majority of these lesions, and initiate early treatment, or refer to a center where this may be provided. This may result in a shorter hospital stay, reduced morbidity, and faster recovery for the patient. This is a brief review of the most common benign lesions of the nasal cavity encountered by the primary care physician or otolaryngologist and a discussion of their management. Although, many of these lesions may extend into or involve the paranasal sinuses, particularly when left undetected, the focus of this review is lesions or disorders having the potential to originate in the nasal cavity. No Institutional Review Board approval was necessary for this study because it did not involve the review or creation of patient-specific data.
INVERTED PAPILLOMAS
Inverted papilloma (IP), or Schneiderian papilloma inverted type, is a locally aggressive, benign nasal lesion remarkable for its tendency for local recurrence and association with carcinoma (Fig. 1). IPs were first described in 1854 by Ward and Billroth and later characterized histologically by Ringertz in 1938. They represent 0.5–4% of all nasal “tumors.” IP is associated with a sinonasal carcinoma in ∼5% of patients. Unlike nasal inflammatory polyps, IPs have historically been considered true neoplasms.1 However, recently, new concepts in the pathogenesis of IPs have been raised by Lanza and others.2 They suggest that IPs may instead be an end stage of a chronic inflammatory condition rather than a true neoplasm.
Figure 1.
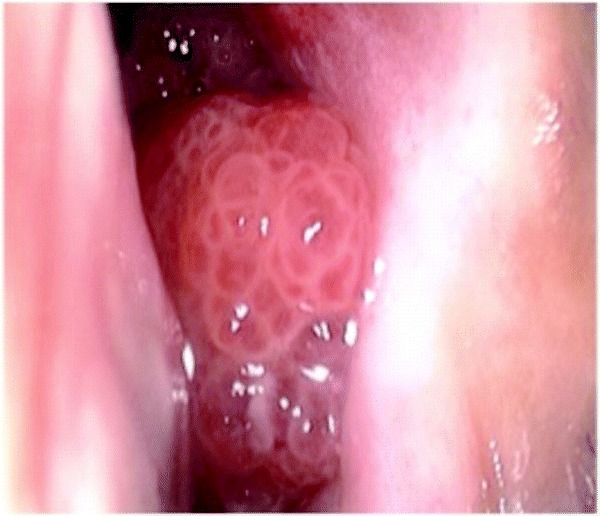
Inverted papilloma characteristically arises from the lateral nasal wall and has a typical granular mulberry-like appearance. Only a small aspect of the mass is evident prior to dissection as the majority of the mass has invaginated into the surrounding structures.
The characteristic microscopic feature of IP is digitiform proliferation of squamous epithelium into the underlying stroma (Fig. 2). Unlike most papillomas of the body, this mucosal lesion inverts, rather than everts, into the underlying connective tissue stroma.3
Figure 2.
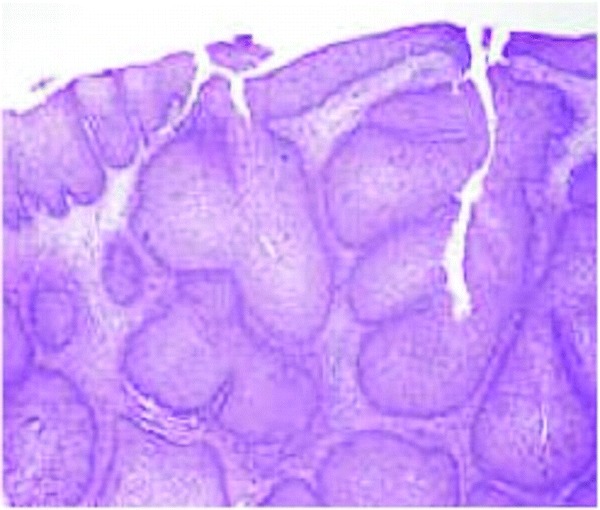
Inverted papillomas are characterized by epithelial proliferation which grows down into preexisting mucosal glands, displacing and replacing normal epithelium. The characteristic fungiform pattern results from this invagination of mucosal epithelium into the underlying stroma.
Clinical presentation is most commonly a male subject who is 50–69 years old. Classically, the “tumor” originates from the lateral nasal wall, particularly in the osteomeatal complex area of the middle meatus, and adjacent inferior and middle turbinates, and subsequently involves the contiguous paranasal sinuses, with the maxillary sinus as the most frequent sinus involved and ethmoid sinus as the second most common. Unilateral nasal obstruction and intermittent epistaxis are the most common presenting symptoms.
Some have suggested inducing and/or promoting agents in the pathogenesis of this disease including human papilloma virus (HPV), alterations in tumor suppressor gene p53, and chronic inflammation. HPV serotypes 6, 11, 16, and 18 are the most commonly associated with IP, and serotypes 16 and 18, similar to cervical cancer, are more commonly associated with malignancy.2,4 The role of exposure to pollutants and toxins in the pathogenesis of IP remains highly disputed; however, there is growing evidence that these factors may be contributory. Animal models of IP have been created by exposure to diethylnitrosamine.5 Deitmer and Weiner performed a case control study on 47 patients with IP and found a higher degree of exposure to smoke, dust, and aerosol among patients with IP.6 A recent study by Sham et al. found that outdoor and industrial occupations were significant risk factors, whereas smoking, drinking alcohol, allergic rhinitis, sinusitis, nasal polyps, nonsinonasal papilloma, and nonsinonasal malignancy were not significant factors.7 The prevalence of malignancy associated with IP is 3.4–9.7%.8,9
Various staging systems have been proposed, with the most commonly cited being the Krause system,10 the Cannady system,11 and the Han system.12 A recent study showed that the Krause system and Cannady system provide a good stratification of patients and correlate well with the risk of recurrence.13 The most significant difference in the Cannady and Krause systems is the inclusion of malignancy in the Krause system:
- Krause stage
- IPs confined to the nasal cavity
- IPs involving the ethmoid sinuses, medial and superior region of maxillary sinus
- IPs involving all paranasal sinuses, but confined to the nose and paranasal sinuses
- IPs not confined to the nose and paranasal sinuses or having malignancy
Most authors advocate complete resection as essential for adequate management and long-term control of IPs. Historically, the approach has been a lateral rhinotomy or sublabial degloving approach and a medial maxillectomy for wide exposure or simple endonasal techniques with visualization using a headlight or microscope. Direct visualization or microscope use has fallen out of favor because of a high recurrence rate. Furthermore, in the era of angled endoscopes, line-of-site endonasal surgery for IPs appears to be essentially obsolete except in rare circumstances. There continue to be proponents of open techniques for all IPs; however, over the past 20 years transnasal endoscopic resection, tailored to the intraoperative endoscopic appearance and extent of the lesion, careful review of any questionable intraoperative margins as well as the final pathological specimen, and close follow-up with periodic nasal endoscopies, is now considered more appropriate.2,14,15 The relatively slow growth of IPs permits early detection in the event of any recurrences and even in-office removal in many cases.16–18 Furthermore, recent studies suggest that there are no significant differences in recurrence rates between open and endoscopic techniques when patients are appropriately selected for either technique.19
In a comprehensive study by Lawson,20 various aspects of IP management, such as treatment concepts (aggressive versus conservative) and surgical approaches (traditional versus endoscopic), were compared in 160 patients, with average follow-up of 5.2 years. This is the largest series of IP cases to date. They suggest that endoscopic removal can be performed on select lesions with a recurrence rate (12%) comparable with those of more aggressive techniques (18%). They also propose case selection criteria, in which endoscopic resection is recommended in primary lesions of the lateral nasal wall and those extending into the ethmoid, sphenoid, and medial wall of maxillary sinuses. In the authors' experience, recurrent IPs are not a contraindication to endoscopic resection, but involvement of the floor and the lateral recess of the maxillary sinus may require additional sublabial exposure.
Today, most would agree that in an experienced endoscopist's hands, endoscopic or endoscopic-assisted resection is possible in the vast majority of lesions and results in decreased morbidity compared with open resection.21 Several large studies have been published with recurrence rates ranging from 0 to 14%, with particularly good results among Krause stages 1–3. More aggressive lesions extending lateral to the maxilla, or lesions where carcinoma is detected, may require a combined approach with external incisions, or even staged with a subsequent open approach. Other authors have suggested that frontal sinus involvement is a relative indication for open resection.22 Both endoscopic and combined approaches have shorter hospital stays and reduced morbidity compared with open techniques.8,13,23–25
Various surgical techniques have been described, ranging from a limited endoscopic sphenoethmoidectomy and wide middle meatal antrostomy to a “traditional” endoscopic medial maxillectomy with or without preservation of lamina papyracea.7–9,26–28 However, more surgeons are accepting a more tailored or sequential segmental endoscopic surgical procedure based on the extent of disease.29 Residual disease and small recurrences frequently may be removed with in-office procedures, with particularly good results in nasoethmoid disease. Frontal disease or extensive maxillary disease frequently results in the need for additional resection under general anesthesia.30 IPs involving the inferomedial wall of frontal sinus can be removed by performing a modified Lothrop procedure, whereas in cases of lateral or more superior involvement of the frontal sinus, an osteoplastic flap may be additionally needed to gain adequate exposure. Where IP is observed intimately associated with bone, bone removal or thinning with a diamond or cutting bur or ultrasonic bone removal device may be prudent. This is typically performed over the lamina papyracea or fovea ethmoidalis, but may also be necessary in other areas of the skull base. Frozen sections are performed if carcinoma is suspected or margins are questionable. This may modify the extent or technique of surgery. These endoscopic techniques are used for both primary and revision cases of IPs.31
Complications of endoscopic surgery for IPs have been reported between 0 and 19.6%.8,16–21 Most commonly reported complications are epistaxis, epiphora, temporary infraorbital hypesthesia, minimal orbital fat exposure, and periorbital ecchymosis, depending on the extent of orbital bone removal or aggressive mucosal removal within the maxillary sinus cavity and surrounding structures (i.e., lacrimal system, infraorbital nerve, etc.). Cerebral spinal fluid leakage was reported in <6%.13 Most of these were planned or in cases with bony dehiscence and IP was removed from adjacent dura mater (Figs. 3 and 4).
Figure 3.
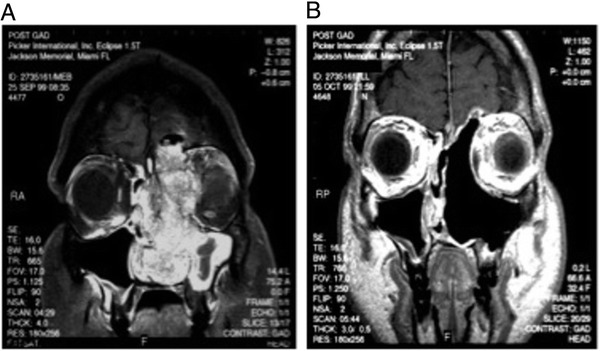
Despite the nonmalignant histopathology of IP, it has a significant capacity for local invasion and destruction as seen in this preoperative MRI. (A) A large mass is seen arising from the left with invasion of the lamina papyracea and cribriform with extension to the dura mater. Transnasal endoscopic resection required a medial maxillectomy, partial superior septectomy, total ethmoidectomy and resection of the left anterior hemi-skull base. (B) MRI was obtained one month postoperatively demonstrating complete resection of the mass.
Figure 4.
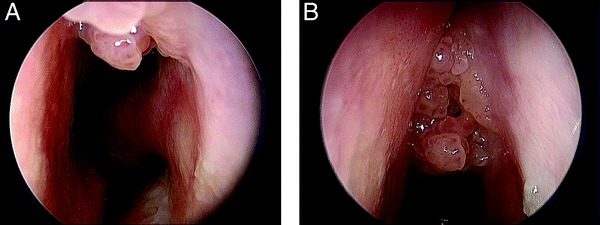
(A and B) Classic granular mulberry-like appearance of an IP seen arising high on the lateral nasal sidewall.
At this time, radiation therapy should not be considered a first-line treatment modality for IPs. Despite this, there is growing evidence for the role of adjuvant radiation therapy in patients with carcinoma associated with IPs. In some centers, radiation therapy is used for local recurrences, but this remains controversial.32 In the authors' experience, careful observation or additional surgical therapy remain the treatments of choice in the vast majority of cases. Finally, we have only a limited understanding of the role of HPV in oncogenesis of head and neck tumors. There is growing evidence that HPV contributes to IP-associated sinonasal carcinomas with the possibility of p53 down-regulation by HPV as an early, critical step in oncogenesis. The clinical impact of HPV prevention or treatment on IPs and IP-associated carcinomas remains to be seen.
SQUAMOUS PAPILLOMA
Squamous papillomas (SPs) are common benign lesions occurring in the entirety of the upper aerodigestive tract. The behavior of these lesions varies depending on site of occurrence and host factors. Unlike IPs, sinonasal SPs are generally solitary and have a low risk of recurrence, malignant transformation, or bony destruction or remodeling. Gross appearance varies from pale to fleshy pink, and they may be sessile or pedunculated. They may have irregular, rough free edges (Fig. 3). Like IPs, they are associated with HPV serotypes 6 and 11. They most commonly arise in the nasal vestibule and caudal septum and present as unilateral nasal obstruction or a visible mass. Histologically, they arise from stratified squamous epithelium and are characterized by the growth of multiple papillary fronds, infrequent mitosis, and rare nuclear atypia. The presence of mitotic figures or nuclear atypia should raise suspicion of an alternative diagnosis, and more aggressive efforts to exclude the possibility of malignancy should be undertaken. The justification for removal largely lies in the need to relieve symptoms and to exclude the possibility of malignancy. Removal of the sinonasal SP is generally recommended, but given the characteristic gross appearance of these lesions and the extremely low likelihood of malignancy, in selected patients careful observation is an acceptable alternative. Surgical resection may be accomplished endoscopically or by direct visualization of lesions in the nasal vestibule.
PYOGENIC GRANULOMAS
Pyogenic granulomas (PG) are benign vascular lesions that most commonly occur on the gingiva with a predilection for the maxillary over the mandibular gingiva. They more rarely occur on the lips, tongue, buccal mucosa, and nasal cavity. They are known by many names: eruptive hemangioma, granulation tissue-type hemangioma, granuloma gravidarum, lobular capillary hemangioma, and pregnancy tumor. The commonly used term “pyogenic granuloma” is actually a misnomer because the disease process is neither related to suppurative infection nor does it show granulomatous changes on histology. These lesions are nonneoplastic in nature and arise from endothelial hyperplasia, which gives them a characteristic hue ranging from red to purple. Local irritation, trauma to mucosa, poor oral hygiene, and hormonal stimulation during pregnancy are thought to predispose one to PG.33 PG may occur in all ages but most commonly occurs in women in the second decade of life, which is likely related to the high incidence of pregnancy during this time period. PGs may occur as early as the first trimester of pregnancy, but the incidence increases until the 7th month of pregnancy. They occur in ∼2% of all pregnant women with the most common presentation of a rapidly growing nasal mass and recurrent epistaxis.34 Management of PG depends on the timing and severity of symptoms as they frequently spontaneously resolve after pregnancy ends. Patients who experience severe bouts of epistaxis or whose lesions fail to resolve after pregnancy should be considered for intervention. Traditional treatment has relied on endoscopic surgical resection and cauterization with a high success rate, although the use of lasers and sclerosing agents has gained some popularity in recent years.
HEREDITARY HEMORRHAGIC TELANGIECTASIAS
Hereditary hemorrhagic telangiectasias (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal disorder characterized by telangiectasias and arteriovenous malformations with convoluted venules extending through the dermis. The vascular walls have excessive smooth muscle without appropriate elastic fiber production. The incidence of HHT is 1/10,000, and it arises from mutations in either the endoglin gene on chromosome 9 (HHT1) or activin receptor-like kinase 1 on chromosome 12 (HHT2). There is no specific racial or sexual predilection for the disease.35 Although genetic testing for HHT is becoming more widely used in the United States, this has traditionally been a clinical diagnosis.
The Curacao criteria have been widely used over the past 10 years36; HHT is classified as definite if three or four criteria are present, possible or suspected if two are present, and unlikely if fewer than two are present:
Spontaneous, recurrent epistaxis
Mucocutanous telangiectasias
Internal arteriovenous malformations (gastrointestinal, hepatic, cerebral, and pulmonary)
First-degree relative with HHT
In a review of 73 patients with HHT, >90% of patients with HHT experienced epistaxis before the age of 21 years. The average age of the first episode of epistaxis was 12 years old and the mean frequency was 18 episodes/mo. Mean duration of bleeding was 7.5 minutes.37 The most common sites of involvement include Little's area, quadrangular plate, and the heads of the middle and inferior turbinates.
There is currently no cure for HHT and treatment strategy relies on reducing bleeding episodes, both intranasally and elsewhere. The problem of new lesions or incompletely resected lesions leads to recurrent epistaxis. Simple chemical or electrical cauterization has been widely used in the treatment of intranasal telangiectasias but it leads to tissue destruction as well as leaves the remaining vascular malformation behind. Medical therapy has gained considerable attention both with the use of hormonal therapy and the use of vascular endothelial growth factor antagonist bevacizumab. Progesterone–estrogen in combination or tamoxifen alone have been shown to reduce epistaxis in HHT. Each of these still requires more extensive testing and experience before being widely used.38–40 Additionally, use of female hormones on male patients can lead to a host of secondary side effects. The use of lasers (Nd:YAG and argon), embolization, septodermoplasty, modified Young's procedure, and radiofrequency ablation are frequently used.
In our institution, we commonly use a 30° telescope to identify telangiectasias and the microdebrider to selectively remove individual lesions (Fig. 5). Electrocautery is used minimally to preserve adjacent normal nasal mucosa because these patients will likely require multiple operations. In a 10-year review of this technique at the University of Miami, there were no episodes of bleeding or regrowth of telangiectasias from previous surgical sites. Patients with very large telangiectasias underwent planned staged procedures and those with new telangiectasias had additional surgical resections. The average operations per patient were 1.6 during the study period. Intraoperative and postoperative bleeding was minimal and easily managed. Patients tolerated the procedures well and all were discharged on the same day of surgery.41 Ablation may be performed with radiofrequency devices as well, in the office setting (for more limited lesions) or the operating room, to achieve the same results.
Figure 5.
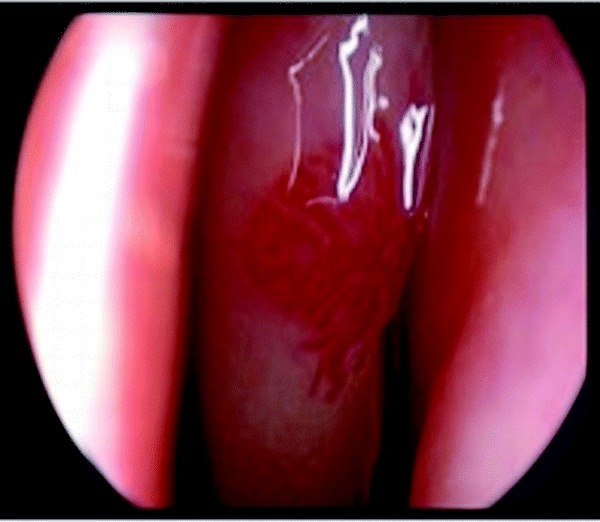
Endoscopic view of a telangiectasia on the middle turbinate of a patient with HHT. Telangiectasias and arteriovenous malformations with convoluted venules extending through the dermis arise because the vascular walls have excessive smooth muscle without appropriate elastic fiber production.
JUVENILE NASOPHARYNGEAL ANGIOFIBROMA
Juvenile nasopharyngeal angiofibromas (JNA) are histologically benign, slow growing, locally invasive vascular neoplasms that arise from myofibroblasts. They occur almost exclusively in male adolescent subjects and represent only 0.5% of all head and neck tumors. They originate in the superior aspect of the sphenopalatine foramen at the junction of the pterygoid process of the sphenoid bone and the sphenoid process of the palatine bone. Patients most often present with unilateral nasal obstruction and recurrent epistaxis with variable associated symptoms based on the expansion of tumor into adjacent structures. Despite their histologically benign nature, these neoplasms have the potential for life-threatening complications secondary to bleeding and intracranial extension. The characteristic submucosal centrifugal growth makes these lesions obligatorily extradural, even in cases with intracranial extension.1 On exam, a smooth, lobulated mass is most often noted in the nasopharynx or lateral nasal wall that varies in color from pale, purple, to beefy red.
Diagnostic imaging techniques such as CT, MRI, MRA, and conventional angiography have contributed to improved preoperative assessment of tumor extent, intracranial extension, and localization of potentially embolizable feeding vessels. The classic finding on CT scan is anterior bowing of the posterior maxillary wall known as a Holman-Miller sign. Angiography with preoperative embolization has shown to be a significant adjunct in the management of these lesions by reducing intraoperative blood loss and improve intraoperative visualization.1,42,43
Several staging systems have been proposed for JNAs. Among the most commonly used was proposed by Radkowski et al.44 (Table 1). Surgical management with complete tumor resection is the mainstay treatment of JNAs but this is challenging because of adjacent neurological structures and the highly vascular nature of the tumor itself. Also, because of continuing growth of the male craniofacial skeleton in the first and second decade of life, soft tissue elevation, facial osteotomies, and the use of metal plate fixations may potentially lead to subsequent asymmetric facial growth.35,45 Open surgical approaches involve transpalatine, transfacial via lateral rhinotomy or facial degloving and anterior craniofacial or frontotemporal craniotomy; however, advances in endoscopic techniques over the past 20 years have led to capabilities for complete excision, decreased surgical morbidity, and similar long-term disease control with endoscopic and open operations.46 Current relative indications for open resection are internal carotid artery, cavernous sinus, or optic nerve involvement after previous resection.47 Most authors performing endoscopic surgery use a wide middle antrostomy with early control of the sphenopalatine and/or internal maxillary arteries feeding vessels.48–51 Septal and nasopharyngeal vessels are similarly controlled before definitive tumor removal transnasally, or transorally, depending on its size. Larger tumors required sublabial or buccolabial incisions to gain lateral access. Some authors have reported techniques using the Nd:YAG or KTP laser.52–54 No major complications were found from any of these recent studies.37–40,55 Most complications reported were minor, such as intranasal synechia and a sphenoid mucocele.
Table 1.
Staging system of JNA

Ssource: Ref. 45.
JNA = juvenile nasopharyngeal angiofibroma.
Because they are rarely encountered in adults, it is believed that JNAs may exhibit spontaneous regression in certain cases. In addition, documented cases exist in which subtotal resection or incomplete radiotherapy have achieved tumor regression.1
Therefore, we believe that incomplete resection or small recurrences per se should not indicate further management and agree that currently available imaging modalities may allow these tumors to be closely monitored. Delayed therapy until symptom recurrence such as bleeding, obstruction, or visual disturbances may be a good option in many cases.
NASAL AND SINONASAL SCHWANNOMAS
Schwannomas are benign nerve sheath tumors that may occur throughout the body. Although one-third of these tumors arise in the head and neck, their occurrence in the nasal cavity is rare, representing only 4% of all head and neck schwannomas. They commonly arise along cranial nerve trigeminal and vestibulocochlear cranial nerves and other nerves coursing through the parapharyngeal space.56 Nasal and sinonasal schwannomas (SNS) arising along the septum may occur along the course of the anterior ethmoid nerve at the anterior aspect of the nasal septum as a part of the first division of the trigeminal nerve or along the nasopalatine nerve, part of the second division of the trigeminal nerve.57 SNS occurring in the paranasal sinuses most commonly occur in the ethmoid and maxillary sinuses. Given the relative radioresistance and chemoresistance of these lesions, complete surgical excision is currently considered the preferred treatment.58 There have been fewer than 100 cases of SNS reported with variable and nonspecific presenting symptoms. Direct visualization reveals a tough mass that is a generally unremarkable lesion and is only diagnosed on histology.59 These lesions may be removed by standard endoscopic techniques, although more advanced techniques may be required where intracranial extension is present.60–62 Lesions occurring in close relationship to vital neurovascular structures may be considered for γ-knife radiosurgery based on experience in acoustic neuromas and vestibular schwannomas, although experience is lacking in this area on lesions occurring within the nasal cavity and paranasal sinuses.49,63
BENIGN FIBRO-OSSEOUS LESIONS
Benign fibro-osseus lesions (BFOLs) represent a diverse group of bony lesions of the craniofacial complex that most commonly occur in the jaw. BFOL classification schemes are varied and controversial, but there is general agreement that osseus dysplasias represent a single disease process. The common feature of all BFOL is replacement of normal bone with a tissue composed of collagen fibers and fibroblasts that contain varying amounts of mineralized substance, which may be bony or cementum-like in appearance.64
Brannon and Fowler54 have suggested a modification of earlier classification schemes with decreasing bony content in the following order:
- Osseous dysplasia
- Nonhereditary—periapical, focal, and florid
- Hereditary—familial gigantiform cementoma
- Fibro-osseous neoplasms
- Conventional ossifying fibroma
- “Juvenile,” “active,” and “aggressive” osseofibroma
- Fibrous dysplasia (FD)
- Polyostotic FD
- Polyostotic with endocrinopathy (McCune-Albright form)
- Craniofacial FD
Of BFOL, osteomas are by far the most common, and, in fact, they are the most common tumor of the paranasal sinuses. Ninety-five percent of sinonasal osteomas occur in the frontoethmoidal region. Three competing etiologic theories exist for osteomas. The developmental theory suggests that embryonic cells are trapped at the junction of the endochondrally ossified ethmoid bone with the membranously ossified frontal bone, and they reactivate with unregulated osseus proliferation. The traumatic theory and the infectious theory rely on inflammation to result on excessive bony production. None of these theories satisfactorily resolves all cases of sinonasal osteomas.65
Surgical management of BFOL should be undertaken before skull base erosion or intracranial extension arises. This may be performed in by an endoscopic approach, endoscopic-assisted approach, or (rarely) an external only approach. For endoscopic-assisted approaches, the external incisions may be small and use a brow, forehead, or Lynch incision. Surgical goal is removal of the entirety of the tumor, but en bloc removal is unnecessary. Resection should begin by removal of the central aspect of the tumor, leaving behind only a “shell.” Removal of the shell component from the orbital wall and skull base is then accomplished in a piecemeal fashion.
OTHER BENIGN TUMORS OF THE NASAL CAVITY
There are numerous reports of various benign tumors that are fairly common, occurring in other parts of the body presenting atypically within the nasal cavity and paranasal sinuses. Generally, these tumors will require excision and histopathological examination to arrive at a diagnosis. Chondromas typically occur in the long bones of the body, but they rarely occur in the head and neck. When they do occur in the head and neck, they arise in the maxillofacial region, the larynx, and nasal septum. They are usually benign but may present with aggressive features of bone erosion and should be surgically excised.66 There are reported cases of benign fibrous histiocytoma occurring both in the pediatric population and in adults, although these lesions are extremely rare. Approximately 10% of teratomas occur in the head and neck, with the majority of these occurring in the cervical region or nasopharynx. However, they have been reported in the nasal vestibule and on the nasal septum67,68 Rare lesions, both malignant and benign, of the nasal cavity underscore the need for excision of lesions and careful pathological review.
CONCLUSIONS
A diverse array of benign lesions occurs within the nasal cavity and paranasal sinuses. Despite their inability to metastasize, many of these lesions have significant capability for local tissue destruction and recurrence, particularly in the cases of IPs and JNAs. Because of this, these lesions had been universally treated with open resection, but endoscopic instrumentation and techniques have dramatically advanced over the past 20 years. Today, even the most inexperienced otolaryngologist is capable of performing nasendoscopy to identify the presence of a mass. Early diagnosis and expedient initiation of treatment or referral to an appropriate center is crucial to minimize therapy-related morbidity and provide the greatest opportunity for curative therapy.
Footnotes
Presented at the North American Rhinology and Allergy Conference, Puerto Rico, February 5, 2011
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Cody DT, II, DeSanto LW. Neoplasm of the nasal cavity. In Otolaryngology Head and Neck Surgery, Vol. 2, ed. 3 Cumming CW FJ, Harker LA, Krause CJ, et al. (Eds). St. Louis, MO: Mosby-Year Book, Inc., 883–901, 1998. [Google Scholar]
- 2. Caruana SM, Zwiebel N, Cocker R, McCormick SA, et al. p53 Alteration and human papilloma virus in paranasal sinus cancer. Cancer 79:1320–1328, 1997. [PubMed] [Google Scholar]
- 3. Ringert N. Pathology of malignant tumors arising in the nasal and paranasal cavities and maxilla. Acta Otolaryngol (Stockh) 27(suppl):31–42, 1938. [Google Scholar]
- 4. Furata YST, Nagashima K, Inoue K, et al. Molecular pathological study of human papillomavirus infection in inverted papilloma and squamous cell carcinoma of the nasal cavities and paranasal sinuses. Laryngoscope 101:79–85, 1991. [DOI] [PubMed] [Google Scholar]
- 5. Herrold KM. Epithelial papillomas of the nasal cavity. Arch Pathol 78:189–195, 1964. [PubMed] [Google Scholar]
- 6. Dietmer T, Wiener C. Is there an occupational etiology of inverted papilloma of the nose and sinuses. Acta Otolaryngol (Stockh) 116:762–765, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Sham CL, Lee DL, van Hasselt CA, et al. A case-control study of the risk factors associated with sinonasal inverted papilloma. Am J Rhinol Allergy 24:e37–e40, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Pasquini ESV, Farneti G, Modugno GC, Ceroni AR. Inverted papilloma: Report of 89 cases. Am J Otolaryngol 25:178–185, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Yoskovitch AY, Braverman I, Nachtigal D, et al. Sinonasal schneiderian papilloma. J Otolaryngol 27:122–126, 1998. [PubMed] [Google Scholar]
- 10. Krause JH. Development of a staging system for inverted papilloma. Laryngoscope 110:965–968, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Cannady SB, Batra PS, Sautter NB, et al. New staging system for sinonasal inverted papilloma in the endoscopic era. Laryngoscope 117:1283–1297, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Han JK, Smith TL, Loehrl T, et al. An evolution in the management of sinonasal inverting papilloma. Laryngoscope 111:1395–1400, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Gras-Cabrerizo JR, Montserrat-Gili JR, Massegur-Solench H, et al. Management of sinonasal inverted papillomas and comparison of classification staging systems. Am J Rhinol Allergy 24:66–69, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Tufano RP, Thaler ER, Lanza DC, et al. Endoscopic management of sinonasal inverted papilloma. Am J Rhinol 13:423–426, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Lawson W, Patel Z. The evolution of management for inverted papilloma: An analysis of 200 cases. Otolaryngol Head Neck Surg 140:330–335, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Kaza S, Capasso R, Casiano RR. Endoscopic resection of inverted papilloma: University of Miami experience. Am J Rhinol 17:185–190, 2003. [PubMed] [Google Scholar]
- 17. Levine HL. The office diagnosis of nasal and sinus disorders using rigid nasal endoscopy. Otolarygol Head Neck Surg 102:370–373, 1990. [DOI] [PubMed] [Google Scholar]
- 18. Bolger WE, Kennedy DW. Nasal endoscopy in the outpatient clinic. Otolaryngol Clin North Am 25:791–802, 1992. [PubMed] [Google Scholar]
- 19. Sautter NB, Cannady SB, Citardi MJ. Comparison of open versus endoscopic resection of inverted papilloma. Am J Rhinol 21:320–323, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Lawson WKM, Biller HF. Treatment outcomes in the management of inverted papilloma: An analysis of 160 cases. Laryngoscope 113:1548–1556, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Lawson W, Patel ZM. The evolution of management for inverted papilloma: An analysis of 200 cases. Otolaryngol Head Neck Surg 140:330–335, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Dubin MG, Sonnenburg RE, Melroy CT. Staged endoscopic and combined open/endoscopic approach in the management of inverted papilloma of the frontal sinus 19:442–445, 2005. [PubMed] [Google Scholar]
- 23. Tomenzoli DCP, Pagella F, Berlucchi M, et al. Different endoscopic surgical strategies in the management of inverted papilloma of the sinonasal tract: Experience with 47 patients. Laryngoscope 114:193–200, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Llorente JL, Deleyiannis F, Rodrigo JP, et al. Minimally invasive treatment of the nasal inverted papilloma. Am J Rhinol 17:335–341, 2003. [PubMed] [Google Scholar]
- 25. Kraft MSD, Kaufmann T, Holzmann D. Long-term results of endonasal sinus surgery in sinonasal papillomas. Laryngoscope 113:1541–1547, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Sadeghi N, Al-Dhahri S, Manoukian JJ. Transnasal endoscopic medial maxillectomy for inverting papilloma. Laryngoscope 113:749–753, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Wormald PJ, Ooi E, van Hasselt CA, Nair S. Endoscopic removal of sinonasal inverted papilloma including endoscopic medial maxillectomy. Laryngoscope 113:867–873, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Kamel RH. Transnasal endoscopic medial maxillectomy in inverted papilloma. Laryngoscope 105:847–853, 1995. [DOI] [PubMed] [Google Scholar]
- 29. Lee TJ, Huang SF, Huang CC. Tailored endoscopic surgery for the treatment of sinonasal inverted papilloma. Head Neck 26:145–153, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Sham CL, Woo JK, van Hasselt CA, et al. Treatment results of sinonasal inverted papilloma: An 18-year study. Am J Rhinol Allergy 23:203–211, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Lee TJ, Huang SF, Lee LA, Huang CC. Endoscopic surgery for recurrent inverted papilloma. Laryngoscope 114:106–112, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Mendenhall WM, Hinerman RW, Malyapa RS. Inverted papilloma of the nasal cavity and paranasal sinuses. Am J Clin Oncol 30:560–563, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Jafarzedeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: A review. J Oral Sci 48:167–175, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Zarrinneshan A, Zapanta P, Wall S. Nasal pyogenic granuloma. Otolaryngol Head Neck Surg 136:130–131, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Bayrak-Toydemir P, Mao R, Lewin S, et al. Hereditary hemorrhagic telangiectasia: An overview of diagnosis and management in the molecular era for clinicians. Genet Med 6:175–191, 2004. [DOI] [PubMed] [Google Scholar]
- 36. Shovlin C, Guttmacher A, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 91:66–67, 2000. [DOI] [PubMed] [Google Scholar]
- 37. Assar A, Friedman C, White R. The natural history of epistaxis in hereditary hemorrhagic telangiectasias. Laryngoscope 101:977–980, 1991. [DOI] [PubMed] [Google Scholar]
- 38. Jameson JJ, Cave DR. Hormonal and antihormonal therapy for epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 114:705–709, 2004. [DOI] [PubMed] [Google Scholar]
- 39. Bose P, Holter JL, Selby GB. Becacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med 360:2143–2144, 2009. [DOI] [PubMed] [Google Scholar]
- 40. Oosting S, Nagengast W, de Vries E. More on bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med 361:931–932, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Bublik M, Sargi Z, Casiano RR. Use of the microdebrider in selective excision of hereditary hemorrhagic telangiectasia: A new approach. Otolaryngol Head Neck Surg 137:157–158, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Ungkanont K, Byers RM, Weber RS, Callender DL, et al. Juvenile nasopharyngeal angiofibroma: An update of therapeutic management. Head Neck 18:60–66, 1996. [DOI] [PubMed] [Google Scholar]
- 43. Fagan JJ, Snyderman CH, Carrau RL, Janecka IP. Nasopharyngeal angiofibromas: Selecting a surgical approach. Head Neck 19:391–399, 1997. [DOI] [PubMed] [Google Scholar]
- 44. Radkowski D, McGill T, Healy GB, et al. Angiofibroma. Changes in staging and treatment. Arch Otolaryngol Head Neck Surg 122:122–129 1996. [DOI] [PubMed] [Google Scholar]
- 45. Andrews JC, Fisch U, Valavanis A, et al. The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. Laryngoscope 99:429–437, 1989. [DOI] [PubMed] [Google Scholar]
- 46. Bleier BS, Kennedy DW, Palmer JN, et al. Current management of juvenile nasopharyngeal angiofibroma: A tertiary center experience 1999–2007. Am J Rhinol Allergy 23:328–330, 2009. [DOI] [PubMed] [Google Scholar]
- 47. Nicolai P, Villaret AB, Farina D, et al. Endoscopic surgery for juvenile angiofibroma: A critical review of indications after 46 cases. Am J Rhinol Allergy 24:e67–e72, 2010. [DOI] [PubMed] [Google Scholar]
- 48. Nicolai P, Berlucchi M, Tomenzoli D, Cappiello J, et al. Endoscopic surgery for juvenile angiofibroma: When and how. Laryngoscope 113:775–782, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Wormald PJ, Van Hasselt A. Endoscopic removal of juvenile angiofibromas. Otolaryngol Head Neck Surg 129:684–691 2003. [DOI] [PubMed] [Google Scholar]
- 50. Roger G, Tran BA, Huy P, et al. Exclusively endoscopic removal of juvenile nasopharyngeal angiofibroma: Trends and limits. Arch Otolaryngol Head Neck Surg 128:928–935, 2002. [DOI] [PubMed] [Google Scholar]
- 51. Onerci TM, Yucel OT, Ogretmenoglu O. Endoscopic surgery in treatment of juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol 67:1219–1225, 2003. [DOI] [PubMed] [Google Scholar]
- 52. Mair EA, Battiata A, Casler JD. Endoscopic laser-assisted excision of juvenile nasopharyngeal angiofibromas. Arch Otolaryngol Head Neck Surg 129:454–459, 2003. [DOI] [PubMed] [Google Scholar]
- 53. Hazarika P, Nayak DR, Balakrishnan R, et al. Endoscopic and KTP laser-assisted surgery for juvenile nasopharyngeal angiofibroma. Am J Otolaryngol 23:282–286, 2002. [DOI] [PubMed] [Google Scholar]
- 54. Nakamura H, Kawasaki M, Higuchi Y, et al. Transnasal endoscopic resection of juvenile nasopharyngeal angiofibroma with KTP laser. Eur Arch Otorhinolaryngol 256:212–214, 1999. [DOI] [PubMed] [Google Scholar]
- 55. Lim IR, Pang YT, Soh K. Juvenile angiofibroma: Case report and the role of endoscopic resection. Singapore Med J 43:208–210, 2002. [PubMed] [Google Scholar]
- 56. Das Gupta TK, Brasfield RD, Strong EW, Hajdu SI. Benign solitary Schwannomas (neurilemomas). Cancer 24:355–366, 1969. [DOI] [PubMed] [Google Scholar]
- 57. Shinohara K, Hashimoto K, Yamashita M., et al. Schwannoma, of the nasal septum removed with endoscopic surgery. Otolaryngol Head Neck Surg 132:963–964, 2005. [DOI] [PubMed] [Google Scholar]
- 58. Shugar MA, Montgomery WW, Reardon EJ. Management of paranasal sinus schwannomas. Ann Otol Rhinol Laryngol 91:65–69, 1982. [DOI] [PubMed] [Google Scholar]
- 59. Dharia A, Karmody C, Rebeiz E. Schwannoma of the nasal cavity. Ear Nose Throat J 86:230–243 2007. [PubMed] [Google Scholar]
- 60. Fujiyoshi F, Kajiya Y, Nakajo M. CT and MR imaging of nasoethmoid schwannoma with intracranial extension. Am J Roentgenol 169:1754–1755, 1997. [DOI] [PubMed] [Google Scholar]
- 61. Zovickian J, Barba D, Alksne JF. Intranasal schwannoma with extension into the intracranial compartment: Case report. Neurosurgery 19:813–815, 1986. [DOI] [PubMed] [Google Scholar]
- 62. Ling L, Chen H, Zhou S, et al. Neurillomas of the nasal vestibule: Report of two cases. Chin Med J 119:1053–1055, 2006. [PubMed] [Google Scholar]
- 63. Wang EM, Pan L, Wang BJ, et al. Clinical experience with Leksell gamma knife in the treatment of trigeminal schwannomas. Chin Med J 118:436–440, 2005. [PubMed] [Google Scholar]
- 64. Brannon RB, Fowler CB. Benign fibro-osseous lesions: A review of current concepts. Adv Anat Pathol 8:126–143, 2001. [DOI] [PubMed] [Google Scholar]
- 65. Eller R, Sillers M. Common fibro-osseus lesions of the paranasal sinuses. Otolarygol Clin North Am 39:585–600, 2006. [DOI] [PubMed] [Google Scholar]
- 66. Sreedharan S, Kamath MP, Hegde MC. Chondroma of the nasal bone: a case report. Ear Nose Throat J 85:44–46, 2006. [PubMed] [Google Scholar]
- 67. Subhash CS, Sanjeev G, Mahil C, et al. Mature teratoma of the nasal vestibule: A case report. Ear Nose Throat J 79:620, 2000. [PubMed] [Google Scholar]
- 68. Ibekwe TS, Kokong DD, Ngwu BA, et al. Nasal septal teratoma in a child. World J Surg Oncol 31:58, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


