Abstract
Background:
There is conflicting evidence whether nasal nitric oxide (NO) is associated with current rhinitis and with other possible predictors. Most studies have been performed in clinical cohorts and there is a lack of studies based on a general population sample. The aim of the present study was to investigate predictors for levels of nasal nitric oxide (NO) in a general population.
Methods:
The population consisted of 357 subjects from Gothenburg participating in the follow-up of the European Respiratory Health Survey in 1999–2001. All subjects completed an extensive respiratory questionnaire. Nasal NO was measured from one nostril at a time with a sampling rate of 50 mL/s for 16 seconds and the nasal NO concentration was determined as the mean value within the plateau phase. Mattress dust samples were collected for cats and mites in a subsample of subjects. Ambient and exhaled NO was also measured. The predictors for nasal NO were analyzed in multiple linear regression models.
Results:
There was no relation between the levels of nasal NO and reporting current rhinitis. Nasal NO was significantly increased among those with high levels of IgE against cats and current smokers had significantly lower nasal NO. There was also a positive association between ambient NO and nasal NO. There were no significant associations between nasal NO and sex, age, or height, or between nasal NO and measured levels of cat antigen.
Conclusion:
In this general population sample we found no relation between current rhinitis and nasal NO levels. There was a clear association between sensitization to cat and nasal NO, but there was no relation to current exposure to cat allergen. Our data support that nasal NO has a limited value in monitoring upper airway inflammation.
Keywords: Allergic rhinitis, epidemiology, FENO, nasal nitric oxide, nNO, rhinitis, sensitization
The role of nitric oxide (NO) in the pathophysiology of the respiratory tract is not fully understood but seems to be related to lower airways inflammation. In general population studies and in occupational cohorts it has been shown that NO from the lower respiratory tract, called the fraction of exhaled NO (FENO), is increased among subjects reporting current asthma symptoms and among atopic subjects.1,2 It has also been shown that FENO correlates with the degree of airway inflammation and asthma3,4; thus, FENO is currently considered as a marker of inflammation in the lower airways.
In healthy subjects, the major contribution of NO to the respiratory tract comes from the upper airways. The levels of NO are several 100–1000 times higher in the nose than in the lower airways, and it would be reasonable to assume that nasal NO could be affected as a marker of nasal inflammation. There have been reports about increased concentrations of nasal NO among subjects with seasonal rhinitis5,6and perennial rhinitis.7 However, there are also studies in which rhinitis does not seem to be associated with increased levels of nasal NO8–10 and thus the role of nasal NO in upper airway inflammation remains unclear. Most studies include selected populations or series of hospitalized patients, and there is an obvious lack of studies of random population samples.
In this study nasal NO and FENO was measured within the European Community Respiratory Health Survey (ECRHS) follow-up of the Gothenburg center in Sweden. This gave us the opportunity to assess the distribution of nasal NO in a general population in relation to different indices of nasal and airways inflammation.
The primary end point of this study was to examine whether there is a relationship between nasal NO and rhinitis, taking asthma, ambient NO, sensitization, and exposure to allergens into account.
MATERIALS AND METHODS
The ECRHS is an international multicenter study of asthma and allergy. The first part, ECRHS I, was conducted in 1991–1993 and the follow-up study, ECRHS II, was conducted in 1999–2001. The design of ECRHS I and II has been described in detail elsewhere.11 In summary (ECRHS I), 29 study centers used a short respiratory questionnaire to study a random sample of at least 3000 subjects per center aged 20–44 years. Among the responders, a random sample and a “symptomatic sample” were invited for further investigations in ECRHS II. The symptomatic sample consisted of participants that reported symptoms of “waking with shortness of breath in the last 12 months,” “asthma attack in the last 12 months,” or “taking asthma medication.”
In the Gothenburg part of the ECRHS II follow-up study, the random sample included 682 subjects and the “symptomatic sample” included 184 subjects. All subjects gave their written informed consent and the national study was approved by the Ethics Committee of Uppsala University.
Because of technical problems with moisture in the sampling tube, the NO analyzer had to be sent to Switzerland to be repaired. In all 284 subjects from the random sample and 73 from the symptomatic sample were measured for nasal NO, in total 357 individuals. Subjects who were not investigated (= dropouts) had a significantly lower prevalence of rhinitis, but they were similar to the study populations regarding age and asthma prevalence. Baseline data on examined subjects and dropouts have been compared with examine any major selection bias. Data on smoking status were categorized into never-smokers and ever-smokers (smoking for >1 year or reported having smoked at least 20 packs of cigarettes in total) in that data set.
All subjects were interviewed face-to-face by a trained interviewer using an extensive questionnaire. Rhinitis was defined as being troubled with sneezing, rhinorrhea, or blocked nose in the last 12 months, without having a common cold (noninfectious rhinitis). In addition, there were questions about “symptoms from the nose in combination with itching in the eyes,” “medication for nasal symptoms,” and “use of topical steroids; any time/for how many years/use latest year.” Rhinitis questions based on specific symptoms have been validated by Sibbald and Rink.12 They compared the questions from a postal questionnaire with in-depth interviews in the same individuals, showing a sensitivity of 95% and a specificity of 96%. The questions used in this study have been used in several larger population-based rhinitis studies.13,14
In the study, asthma was defined as physician-diagnosed asthma.15 A subject was considered a smoker if he/she had been smoking for >1 year or reported having smoked at least 20 packs of cigarettes in total. Smokers were further classified as current smokers or exsmokers, based on the smoking status during the month before the study.
Specific serum IgE was measured against Dermatophagoides pteronyssinus, cat, timothy grass, and Cladosporium herbarum, using the Pharmacia CAP System (Pharmacia Diagnostics, Uppsala, Sweden). A person was defined as atopic if the titers against at least one of the tested allergens were ≥0.35 kU/L as previously described. The specific IgE levels were further subdivided into low, middle, and high class.16
Analyzed blood samples within the ECRHS II study were used to determine if the participants had atopy (defined as total IgE >100) and if they had a specific IgE to house-dust mite, grass, cat, and/or Cladosporium–radioallergosorbents test (RAST). The specific IgE or RAST were also divided into subgroups: low, middle, and high class.
Measurement of NO
Nasal NO was measured by sampling air from one nostril at a constant sampling rate, leaving the other nostril open, according to the 1999 American Thoracic Society guidelines for measurement of nasal NO.17 To ensure velum closure, simultaneous oral exhalation was performed with a positive pressure of >5 cm H2O in the mouth. A nasal probe was fitted to one nostril. Air was sampled at a constant aspiration rate of 50 mL/s under 16 seconds. The NO concentration was determined as the mean value within the plateau phase, increasing from the 9th second until the 15th second under the measuring procedure. The NO concentration was measured at both nostrils, and then the mean value was calculated.
FENO, like nasal NO, was measured with a chemiluminescence analyzer (model CLD 77AM breath analyzer; Ecophysics, Dürnten, Switzerland) and a computer biofeedback system (Exhalation Breath Analyzer TM; Aerocrine A, Stockholm Sweden).18 The subjects were instructed not to use tobacco, eat or drink within 1 hour before the examination and not to eat nitrogen-rich food within 4 hours before the examination.18 Part of the results regarding FENO has previously been published.19 The room (ambient) NO level was recorded and ranged from 0 to 55 ppb.
Assessment of Exposure to Allergen
Mattress dust samples were taken following a standardized protocol, and Fel d 1(cat), Der p 1(mite), and Der f 1(mite) were analyzed using ELISA methods from the home of a subsample of the subjects.20 The result from the whole ECRHS II has previously been presented.21 The lower limit of detection was 0.1 μg/g of dust, with no upper limit. In the Gothenburg center Fel d 1 was detectable in 109 of 140 mattress dust samples and ranged from undetectable to 3615 μg/g (median, 0.31 μg/g; interquartile range, 0.14–2.97 μg/g). The levels of Fel d 1 were clearly associated with the presence of a cat (median, Fel d1 in homes with no cat was 0.21 μg/g and in homes where cats were allowed in the bedroom was 530 μg/g).
The mite allergens Der p1 and Der f1 were below the limit of detection in 123 and 101 of 140 samples, respectively.
Statistical Methods
Statistical analyses were performed using the Statistical Software Pack SAS 8.0 (SAS Institute, Cary, NC). For analysis of the results we used univariate and multivariate analysis and Spearman correlation coefficients. We included covariates in the linear multiple regression model based on prior decisions, but in the final models covariates with a value of p > 0.7 were excluded. We also ran a model with exposure to cat allergen as a covariate and stratified the model according to measured exposure to cat allergens. Nasal NO was regarded as normally distributed. For the whole population the mean level was 114.3 ppb and the median level was 109.8 ppb.
RESULTS
Baseline data of the study population are shown in Table 1. There was no statistical significant difference regarding nasal NO concentration between the random population sample (114 ppb) and the “symptomatic sample” (116 ppb). Hence, in the analysis we have merged these two samples together. The nasal NO concentrations were also similar between never- and exsmokers (120 and 117 ppb, respectively), and thus we have merged never-smokers and exsmokers, defined as nonsmokers. Nasal NO was significantly lower among current smokers compared with nonsmokers (101 ppb versus 119 ppb; p = 0.001). In Table 2 the nasal NO concentrations are presented for the nonsmokers and current smokers according to sex, specific RAST, total IgE, rhinitis, and asthma. In general, current smokers showed lower levels of nasal NO compared with nonsmokers.
Table 1.
Characteristics of the subjects in the study (mean values)
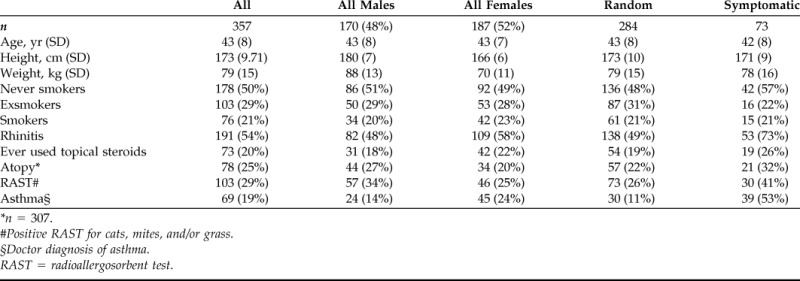
*n = 307.
#Positive RAST for cats, mites, and/or grass.
§Doctor diagnosis of asthma.
RAST = radioallergosorbent test.
Table 2.
Values of nasal NO considering smoking habits, sex, atopy, rhinitis, and asthma
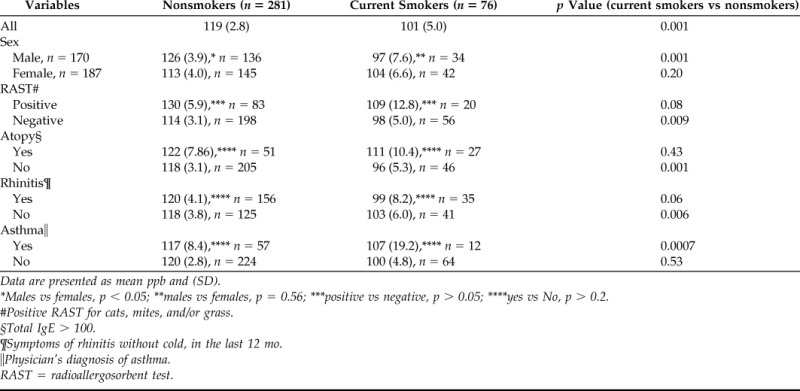
Data are presented as mean ppb and (SD).
*Males vs females, p < 0.05; **males vs females, p = 0.56; ***positive vs negative, p > 0.05; ****yes vs No, p > 0.2.
#Positive RAST for cats, mites, and/or grass.
§Total IgE > 100.
¶Symptoms of rhinitis without cold, in the last 12 mo.
‖Physician's diagnosis of asthma.
RAST = radioallergosorbent test.
There was no difference between the dropouts and the investigated sample regarding height, age, and sex distribution. However, the dropouts had significantly higher prevalence of ever-smokers (61% versus 39%) and lower prevalence of current rhinitis compared with the investigated subjects (39% versus 54%).
Nasal NO was measured in one nostril at a time and the concentration differed largely between the nostrils in some subjects. For the left nostril the mean nasal NO was 114 ppb (SE, 2.4) and for the right nostril it was 117 ppb (SE, 3.0). The difference was, however, not statistically different. The mean difference between the nostrils was 14 ppb (SE, 1.8) and ∼95% of the subjects (n = 331) had a difference of ≤45 ppb. There were only seven subjects with a difference of 100 ppb or more. In a multiple linear regression model, atopy was significantly associated with a high difference of nasal NO levels between the nostrils.
The mean FENO level was 14.8 ppb (SD, 13.2 ppb). The mean ambient NO level was 3.1 ppb (SD, 6.0 ppb), ranging from 0 to 55 ppb. There was a positive correlation between nasal NO and FENO in the whole population (rs = 0.33; p < 0.001), both in nonsmokers (rs = 0.29; p < 0.001) and in current smokers (rs = 0.33; p = 0.004).
In the final multiple linear regression model, current rhinitis was excluded due to low explanatory power (Table 3). High levels of specific IgE to cat was associated with (29.0 ppb; 95% CI, 6.9–51.0) increased levels of nasal NO. Current smoking was associated with lower levels of nasal NO (−14.7 ppb; 95% CI, −26.4 to −3.1).
Table 3.
Multiple linear regression models with nasal NO as the dependent variable in relation to different independent variables
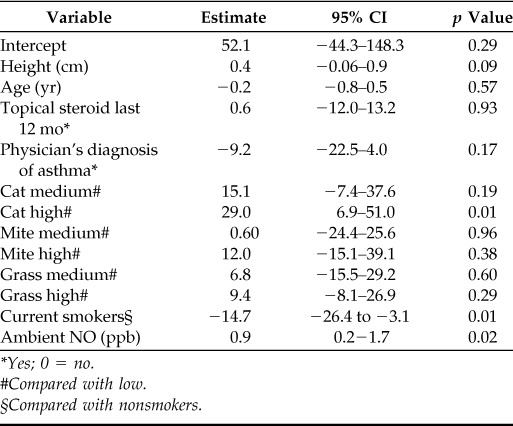
*Yes; 0 = no.
#Compared with low.
§Compared with nonsmokers.
There was no correlation between exposure to Fel d 1 and nasal NO (rs = 0.05; p = 0.57) in the subsample of 140 subjects included in the measurements. Because only 16 of the 140 subjects were sensitized to cat, no statistical analysis was made within the sensitized group.
When exposure to cat allergen (“yes” or “no”) was included in the final regression model, the results remained unchanged. There were still significant associations between nasal NO and high levels of specific IgE to cat (38.8 ppb; 95% CI, 3.6–74.0) and current smoking (−26.6 ppb; 95% CI, −47.5 to −5.7). No significant association between exposure to cat allergen and nasal NO was found.
There was a high level of statistical significance in the multiple linear regression model between nasal NO and ambient NO, with a correlation coefficient of 0.9.
The questions about rhinitis and in combination with data about sensitization to different allergens and atopy (IgE > 100), gave us the opportunity to make different models for subgroups. The analyses did not show any differences.
DISCUSSION
To our knowledge, this is the first study evaluating the usefulness of nasal NO in monitoring upper airway inflammation in a larger population sample.
The main finding from this population-based study was that nasal NO was increased in subjects sensitized to cats and high levels of specific IgE to cats. Nasal NO was significantly lower among current smokers. Nasal NO was also significantly and positively associated with ambient NO.
The aim of the current study was to see if there are any predictors for levels of nasal NO in the general population and if nasal NO can be used as a tool to identify inflammation in the nasal mucosa. The combination of questions about rhinitis and data about sensitization to different allergens and atopy (IgE >100) gave us the opportunity to make different models for subgroups. The analyses did not show any differences.
The strength of the general population design is that it allows us to control for confounders in a rather unbiased way; however, the study design has some limitations. Only 52% of the subjects from the original sample were participating in the study, and the high number of dropouts was mainly because of technical difficulties with the NO analyzer. Among the included subjects there was an accumulation of subjects with current rhinitis and of subjects who had never smoked. This may cause a slightly higher mean nasal NO level among the investigated subjects; however, in view of our results we consider our study group as being reasonably representative for the source population.
In the present study we found no relation between current rhinitis and the concentration of nasal NO. (This study is one of few general population studies in this field.) A Norwegian general population study showed that nasal NO levels were similar in subjects with allergic or perennial rhinitis compared with controls.9 Other studies, based on clinical materials, confirm our results,8,10 and some studies have shown increased nasal NO among subjects with rhinitis.5–7
There was no significant relation between nasal NO and sex (p ∼ 0.9), weight, or age. However, a positive nonsignificant association was found between height and nasal NO (p = 0.09). This association has previously been reported in a study by Struben et al., where nasal NO was associated with height but not with age, sex, or weight among 100 healthy nonsmoking volunteers.22In another study in adolescents (>12 years), there was no relation between nasal NO and height.23 To summarize, all these studies, including ours, are too small to draw more definite conclusions from; to do so would require much larger population samples.
We found a clear association between nasal NO and sensitization to cats but not to grass or mites. Because the study was performed outside the grass pollen season the lack of association with sensitization to grass pollen is not surprising. The absence of an association between nasal NO and sensitization to mites could be explained by the exposure to mite allergen, which was substantially lower than that to cat allergen in the subgroup where allergens were measured at home (Fel d1, 109 positive; Der pl, 17 positive; and Der fl, 39 positive of 140 dust samples).
There was, however, no association between nasal NO and measured levels of cat allergens. Interestingly, in a recent study from Moody et al. in New Zealand a similar observation was made, i.e., subjects with cat sensitization had increased nasal NO levels.10 Concordantly, they did not find a relation between current nasal symptoms and nasal NO, which supports the observation that current exposure to allergens is not related to nasal NO.
In the present study the lack of association between cat allergen and nasal NO may be explained by the fact that the number of sensitized subjects was low in the homes where the measurements took place.
In contrast to measured exhaled NO (FENO)24 we found a clear relationship between nasal NO and ambient NO. The observed relationship was quite strong, with a correlation coefficient of 0.9, meaning that 1-ppb increase of the ambient NO will increase the nasal NO with 0.9 ppb. Struben et al. made similar observations in their study on children, but their correlation coefficient was lower, 0.5.23 Hence, we suggest that all analyses of nasal NO with the present method have to be adjusted for ambient NO.
The nasal NO concentrations in this study were calculated as mean values, after measuring one nostril at a time. In some subjects there were large differences in nasal NO between the nostrils, where atopy was found to predict the nostril difference. Two previous studies found no difference.7,25 Our findings indicate that both nostrils should be measured and that the highest observed value should be used because low value could be an expression of congestion.
Nasal mucosa congestion and nasal polyposis have been shown to have an impact on the measured nasal NO concentrations.26One limitation with the present study is that no nasal endoscopy was performed before measuring nasal NO, and we can therefore not exclude presence of these known factors.
There are two main approaches to the measurement of nasal NO. One is infusion of NO-free air into one nostril, at a certain aspiration flow, while extracting air from the other nostril where it is analyzed, and the other is direct sampling from the nose with an airstream generated by the analyzer.5 Both methods are performed during mouth breathing or breathholding. To what extent the present results are influenced by the method of measuring nasal NO is difficult to judge.
CONCLUSION
In this general population-based study we found no association between current rhinitis and nasal NO. We found, however, a clear association between sensitization to cat and nasal NO.
The results do not support the use of nasal NO as a tool to identify inflammation in the nasal mucosa.
There was a strong relationship between ambient NO and nasal NO; therefore, all analyses of nasal NO should be adjusted for ambient NO with this method.
Footnotes
Funded by the Swedish Council for Worklife Research (FAS); Herman Kreftings Fund for Asthma Research, Torsten and Ragnar Söderberghs Medical Foundation, the Swedish Heart and Lung Foundation, the Norwegian Asthma and Allergy Research Fund, and the Icelandic Research Council
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Olin AC, Alving K, Toren K. Exhaled nitric oxide: Relation to sensitization and respiratory symptoms. Clin Exp Allergy 34:221–226, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Olin AC, Rosengren A, Thelle DS, et al. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest 130:1319–1325, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Jatakanon A, Lim S, Kharitonov SA, et al. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 53:91–95, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aronsson D, Tufvesson E, Bjermer L. Allergic rhinitis with or without concomitant asthma: Difference in perception of dyspnoea and levels of fractional exhaled nitric oxide. Clin Exp Allergy 35:1457–2461, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Kharitonov SA, Rajakulasingam K, O'Connor B, et al. Nasal nitric oxide is increased in patients with asthma and allergic rhinitis and may be modulated by nasal glucocorticoids. J Allergy Clin Immunol 99:58–64, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Martin U, Bryden K, Devoy M, Howarth P. Increased levels of exhaled nitric oxide during nasal and oral breathing in subjects with seasonal rhinitis. J Allergy Clin Immunol 97:768–772, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Arnal JF, Didier A, Rami J, et al. Nasal nitric oxide is increased in allergic rhinitis. Clin Exp Allergy 27:358–362, 1997. [PubMed] [Google Scholar]
- 8. Olin AC, Hellgren J, Karlsson G, et al. Nasal nitric oxide and its relationship to nasal symptoms, smoking and nasal nitrate. Rhinology 36:117–121, 1998. [PubMed] [Google Scholar]
- 9. Henriksen AH, Sue-Chu M, Holmen TL, et al. Exhaled and nasal NO levels in allergic rhinitis: Relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur Respir J 13:301–306, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Moody A, Fergusson W, Wells A, et al. Nasal levels of nitric oxide as an outcome variable in allergic upper respiratory tract disease: Influence of atopy and hayfever on nNO. Am J Rhinol 20:425–429, 2006. [DOI] [PubMed] [Google Scholar]
- 11. European Community Respiratory Health Survey IISC. The European Community Respiratory Health Survey II. Eur Respir J 20:1071–1079, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Sibbald B, Rink E. Epidemiology of seasonal and perennial rhinitis: Clinical presentation and medical history. Thorax 46:895–901, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur Respir J 8:483–491, 1995. [DOI] [PubMed] [Google Scholar]
- 14. Radon K, Gerhardinger U, Schulze A, et al. Occupation and adult onset of rhinitis in the general population. Occup Environ Med 65:38–43, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Toren K, Brisman J, Jarvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest 104:600–608, 1993. [DOI] [PubMed] [Google Scholar]
- 16. Sunyer J, Jarvis D, Pekkanen J, et al. Geographic variations in the effect of atopy on asthma in the European Community Respiratory Health Study. J Allergy Clin Immunol 114:1033–1039, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 160:2104–2117, 1999. [DOI] [PubMed] [Google Scholar]
- 18. Olin AC, Aldenbratt A, Ekman A, et al. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir Med 95:153–158, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Malinovschi A, Janson C, Hogman M, et al. Both allergic and nonallergic asthma are associated with increased FE(NO) levels, but only in never-smokers. Allergy 64:55–61, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Luczynska CM, Arruda LK, Platts-Mills TA, et al. A two-site monoclonal antibody ELISA for the quantification of the major Dermatophagoides spp. allergens, Der p I and Der f I. J Immunol Methods 118:227–235, 1989. [DOI] [PubMed] [Google Scholar]
- 21. Jarvis D, Zock JP, Heinrich J, et al. Cat and dust mite allergen levels, specific IgG and IgG4, and respiratory symptoms in adults. J Allergy Clin Immunol 119:697–704, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Struben VM, Wieringa MH, Mantingh CJ, et al. Silent and humming nasal NO measurements in adults aged 18–70 years. Eur J Clin Invest 35:653–657, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Struben VM, Wieringa MH, Mantingh CJ, et al. Nasal NO: Normal values in children age 6 through to 17 years. Eur Respir J 26:453–457, 2005. [DOI] [PubMed] [Google Scholar]
- 24. American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 171:912–930, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Struben VM, Wieringa MH, Mantingh CJ, et al. Nasal NO measurement by direct sampling from the nose during breathhold: Aspiration flow, nasal resistance and reproducibility. Eur Arch Otorhinolaryngol 263:723–728, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Colantonio D, Brouillette L, Parikh A, Scadding GK. Paradoxical low nasal nitric oxide in nasal polyposis. Clin Exp Allergy 32:698–701, 2002. [DOI] [PubMed] [Google Scholar]


