Abstract
Background:
Increasing interest in sublingual immunotherapy (SLIT) among practitioners and patients has resulted in numerous publications and clinical trials in recent years. With the clinical growth of SLIT, discussions of its efficacy, safety, and immunologic effects have intensified, as have comparisons to subcutaneous immunotherapy (SCIT). In the United States, SCIT has been the traditional form of immunotherapy for inhalant allergy and is the only immunotherapy method approved by the U.S. Food and Drug Administration at this time. The similarities and differences between SLIT and SCIT are often discussed, yet clinical studies directly comparing these immunotherapy methods are scarce.
Methods:
A literature review of specific issues and controversies between SLIT and SCIT for allergic rhinitis was conducted.
Results:
Safety, efficacy, and immunologic effects of these two immunotherapy techniques are reviewed.
Conclusion:
Unanswered questions relating to SLIT are examined.
Keywords: allergic rhinitis, allergy, anaphylaxis, antigen, atopy, hypersensitivity, immunoglobulin E, perennial allergic rhinitis, seasonal allergic rhinitis, subcutaneous immunotherapy, sublingual immunotherapy
According to the 2009 National Health Interview Survey, 7.8% of adults and 9.8% of children in the United States had been diagnosed with hay fever in the preceding 12 months.1,2 There were 13.1 ambulatory care visits for allergic rhinitis in 2006.3 Based on these few figures, the public health impact of allergic rhinitis is evident. Although many allergic rhinitis patients have been successfully treated with environmental control measures, pharmacotherapy, and subcutaneous immunotherapy (SCIT), interest in sublingual immunotherapy (SLIT) has grown considerably in recent years. A search of published literature under the topic “sublingual immunotherapy” revealed 21 citations in English in 1999; this has grown to 737 English citations in a 2011 search of the PubMed database (www.ncbi.nlm.nih.gov/pubmed). At the time of this article preparation, there were 57 clinical SLIT trials listed at www.clinicaltrials.gov. Twelve of these trials are registered in the United States and 32 are registered in Europe.
With the rapidly growing interest in SLIT for allergic rhinitis, comparisons with traditional SCIT have markedly increased. In this brief review of SLIT and SCIT, we will highlight specific similarities, differences, and controversies of these two immunotherapy techniques. Recent findings relating to safety, efficacy, future research needs, and unanswered questions regarding SLIT and SCIT will be discussed (Table 1).
Table 1.
Highlights of SCIT and SLIT for allergic rhinitis
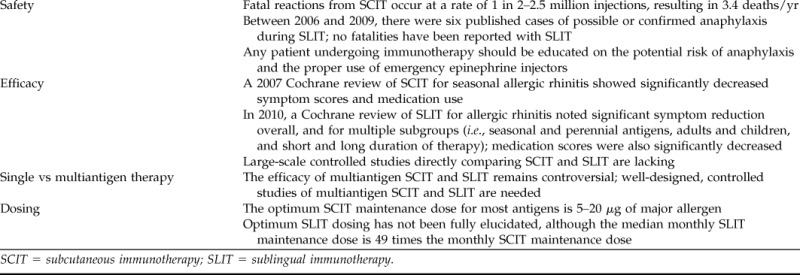
SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy.
IMMUNOTHERAPY SAFETY
Systemic reactions and anaphylaxis are noted complications of immunotherapy. In a 2007 Cochrane meta-analysis of SCIT for seasonal allergic rhinitis, which included 51 randomized placebo-controlled trials, epinephrine was given for adverse reactions in 0.13% of participants on active treatment (19 of 14,085 injections).4 No fatalities were reported in this meta-analysis. Fatal reactions from SCIT in clinical practice are reported at a rate of 1 in 2–2.5 million injections, resulting in 3.4 deaths/year.5,6 Potential contributors to fatal SCIT reactions include delay in epinephrine administration, previous immunotherapy reactions, suboptimal asthma control, administration of injections during peak allergy season, and alterations in antigen extracts.6 In addition to fatal SCIT reactions, there is a systemic reaction rate of 0.05–3.2% of injections (0.84–46.7% of patients) per year and a near-fatal reaction rate of 23 per year (5.4 per 1 million injections).6–9 Serious systemic and fatal reactions due to SCIT are relatively rare. However, the potential for a fatal systemic reaction caused by treatment for a nonfatal condition such as allergic rhinitis gives many practitioners pause.
The potential for fatal systemic reactions from SCIT was highlighted in the 1986 report of the British Committee on the Safety of Medicines.10 Based on 26 anaphylaxis-related deaths in this report, the safety of SCIT was questioned and strict criteria for SCIT administration in the United Kingdom were initiated. These new regulations included a postinjection observation period of 2 hours and the requirement that injections be given in a facility with full CPR capabilities. Subsequently, interest increased in noninjection routes of immunotherapy administration, including oral (swallow), sublingual, bronchial, and intranasal. Of these, sublingual administration was the most promising with regard to its clinical efficacy, tolerability, and safety.
The safety profile of SLIT is one of the least controversial aspects in its overall comparison with SCIT. Before 2006, there were no literature reports of anaphylaxis due to SLIT. Between 2006 and 2009, there were six published cases of anaphylaxis or possible anaphylaxis related to SLIT.11–15 Certain factors have been hypothesized as contributors to SLIT anaphylaxis, including rush escalation or no escalation, use of latex antigen, multiple antigen therapy, treatment during peak pollen season, previous intolerance to SCIT, and noncompliance with treatment regimens.
In the 2010 Cochrane systematic review of SLIT for allergic rhinitis, Radulovic et al. report that there were no cases of anaphylaxis and no requirement for the use of adrenaline in 60 randomized, placebo-controlled trials.16 This Cochrane review did note mild-to-moderate systemic reactions in both treatment and placebo groups. Treatment discontinuation was attributed to adverse events in 41 of 824 SLIT participants and 12 of 861 placebo participants; this included both local and systemic reactions, although discontinuation for local reactions was more common. Because SLIT is a potentially attractive option for the treatment of respiratory allergy in children, it is also important to evaluate adverse events in this patient group. Two meta-analyses have been dedicated to SLIT in the pediatric population. In 2006, Penagos et al. noted no severe systemic or lethal events in a meta-analysis of 10 SLIT trials for allergic rhinitis in children, although 1 of the included studies reported 3 patients with severe asthma attributed to SLIT overdose.17 Similarly, a 2008 meta-analysis of SLIT for allergic asthma in children reported no fatal or severe systemic reactions in nine included studies.18 In a postmarketing survey of single and multiple antigen SLIT in 433 children receiving 40,169 SLIT doses, 13 events were judged to be of moderate severity and required medical advice.19 There was no emergency treatment required, and no difference was seen in adverse events between single and multiple antigen regimens. SLIT has also been reported safe in children ≤5 years of age.20,21
Although the safety profile of SLIT is often quoted as being superior to SCIT, the practitioner must remain aware of the risks of immunotherapy in general. Regardless of the route of immunotherapy selected, patients should be educated on expected side effects versus worrisome systemic reactions. SCIT doses are routinely given in the physician's office, especially during escalation. It has also been suggested that the first dose of SLIT be given in the physician's office; after this, SLIT doses are routinely administered at home. Because the risk of anaphylaxis exists for both SCIT and SLIT, many also advocate that any patient receiving immunotherapy should carry an emergency epinephrine injector and be fully educated on its appropriate use.
IMMUNOTHERAPY EFFICACY
The efficacy of SLIT for allergic rhinitis, when compared with SCIT, incites greater controversy. A Cochrane systematic review of injection immunotherapy for seasonal allergic rhinitis was published in 2007 by Calderon et al.4 This meta-analysis included 51 double-blind, placebo-controlled, randomized trials of specific immunotherapy for seasonal allergic rhinitis to tree, grass, or weed pollens. Fifteen trials were assessed for standard mean difference (SMD) of symptom scores and showed a significant reduction of symptoms in the immunotherapy group (SMD, −0.73; 95% CI, −0.97 to −0.50; p < 0.00001). Data from 13 trials showed significant medication reduction in the immunotherapy group (SMD, −0.57; 95% CI, −0.82 to −0.33; p < 0.00001). Demonstration of increased SCIT efficacy in symptom control has also been shown for longer durations of maintenance therapy (up to 3 years), although the increased efficacy evidence from this study is weak versus SCIT therapy duration of 1 year.22
Recent large meta-analyses of SCIT efficacy for perennial allergic rhinitis have not been performed. In a 2011 Cochrane systematic review of 88 randomized controlled SCIT trials for allergic asthma, however, therapy with mite antigen was shown to have a marginal benefit in asthma symptoms (SMD, −0.48; 95% CI, −0.96–0.00).23 SCIT for cat and dog allergens did not show improvement in asthma symptoms in this meta-analysis. In contrast, objective measures of bronchial hyperreactivity improved with SCIT in this Cochrane review for mite immunotherapy (SMD, −0.98; 95% CI, −1.39 to −0.58), pollen (SMD, −0.55; 95% CI, −0.84 to −0.27), and animal dander (SMD, −0.61; 95% CI, −0.95 to −0.27).23 Bronchial hyperreactivity was not significantly improved with SCIT for other allergens.
As clinical interest in SLIT has grown over the last 10 years, large randomized controlled trials and meta-analyses have been reported with increasing frequency. The first randomized clinical trial of low-dose SLIT with dust-mite antigen included 20 patients and was reported in 1986 by Scadding and Brostoff.24 Multiple large-scale randomized, double-blind, placebo-controlled SLIT efficacy trials have been published in the last 5 years, beginning with the 2006 studies of Durham et al.25 and Dahl et al.26 Both the Durham (855 patients randomized) and Dahl (634 patients randomized) studies were multicenter multinational trials of pre- and coseasonal administration of Timothy grass tablets in patients with symptomatic seasonal allergic rhinitis, and both showed statistically significant reduction is allergic rhinitis symptoms and medication use versus placebo groups.25,26
The most recent meta-analysis of SLIT for allergic rhinitis was published in 2010 by Radulovic et al.16 For symptom assessment, 49 randomized placebo-controlled trials were included with 2333 total participants receiving SLIT and 2256 receiving placebo. The SMD for symptom scores favored SLIT at −0.49 (95% CI, −0.64 to −0.34; <0.00001). Subgroup analysis revealed significant symptom improvement with SLIT for seasonal and perennial allergens, adults and children, treatment durations ranging from <6 months to >12 months, and major allergen content of 5–20 μg and >20 μg. With regard to individual antigens, there was significant symptom improvement for house-dust mites, grass pollen, ragweed, Parietaria, and trees. Medication scores were assessed in 38 studies and revealed an SMD of −0.32 (95% CI, −0.43 to −0.21; p < 0.00001). A meta-analysis of SLIT for allergic rhinitis in pediatric patients was published in 2006 by Penagos et al.17 Ten pediatric studies, including 484 patients, were evaluated and revealed significant reduction in symptoms (SMD, −0.56; 95% CI, 1.01–0.10; p = 0.02) and medication use (SMD, −0.76; 95% CI, 1.46–0.06; p = 0.03) with SLIT. Subgroup analyses indicated that treatment duration of >18 months and SLIT with pollen extracts were beneficial over shorter treatment durations and dust-mite antigens in children.
Meta-analyses of SLIT have also been performed with regard to specific antigens. A meta-analysis specific for seasonal grass pollen SLIT treatment was also performed by Di Bona et al.27 This meta-analysis included 19 randomized, placebo-controlled SLIT trials, with 2971 total patients. It was found that grass allergen SLIT significantly reduced symptoms (SMD, −0.32; 95% CI, −0.44 to −0.21) and medication use (SMD, −0.33; 95% CI, −0.50 to −0.16) versus placebo. Similarly, a meta-analysis of SLIT for house-dust mite allergic rhinitis showed significant symptom reduction in 194 active SLIT participants versus 188 placebo participants (SMD, −0.95; 95% CI, −1.77 to −0.14; p = 0.02).28 Significant medication reduction was also seen with SLIT for house-dust mite allergic rhinitis (SMD, −1.88; 95% CI, −3.65 to −0.12; p = 0.04).
Often-discussed benefits of immunotherapy are the long-lasting and preventative effects that can be seen after treatment. Recent studies have shown such effects with SLIT. In an open, randomized study of 216 children with allergic rhinitis with or without asthma, Marogna et al. showed a decrease in new sensitizations in children receiving SLIT (3.1%) versus controls (34.8%; odds ratio, 16.85; 95% CI, 5.73–49.13).29 In addition, after 3 years, there was a decrease in positive methacholine challenge results in the SLIT group. A large-scale (257 patients) double-blind, placebo-controlled trial of SLIT in patients with grass pollen allergy by Durham et al. showed sustained reduction in rhinoconjunctivitis symptoms and medication scores in the SLIT group at the 1-year time point after cessation of a 3-year SLIT program.30 Finally, Marogna et al. have noted that clinical benefit persists for 8 years after SLIT treatment is given for a 4- to 5-year duration; new sensitizations were also reduced in SLIT groups.31
Controlled studies involving both SCIT and SLIT treatment groups for direct comparison are relatively lacking.32–36 A summary of the characteristics of five SCIT versus SLIT comparison studies is shown in Table 2. In 2010, a prospective, randomized, open-label three-parallel-group trial was conducted in 48 children with allergic rhinitis or allergic asthma who were monosensitized to house-dust mites.33 Both SLIT and SCIT showed significant reduction in rhinitis symptom score and medication score, as well as significant reduction of serum-specific dust-mite IgE, compared with pharmacotherapy. A placebo-controlled double-blind double-dummy study in 71 adult patients (all patients received both sublingual medication and subcutaneous injections) was reported by Khinchi et al. in 2004.34 In this study, both SLIT and SCIT showed efficacy versus placebo. There was no statistically significant difference between SLIT and SCIT groups; however, the study was not powered to detect a difference in the immunotherapy groups if one truly existed. Finally, a comparison of the magnitude of effects seen in SLIT and SCIT Cochrane reviews was performed by Cox in 2008, noting that the magnitude of effects seen with SCIT may be larger than that with SLIT.7 Although large clinical studies directly comparing the efficacy of SCIT and SLIT have not been performed, certain patients and practitioners may be willing to accept slightly reduced efficacy of SLIT in the face of a significantly higher safety profile and convenience.
Table 2.
SLIT vs SCIT comparison studies
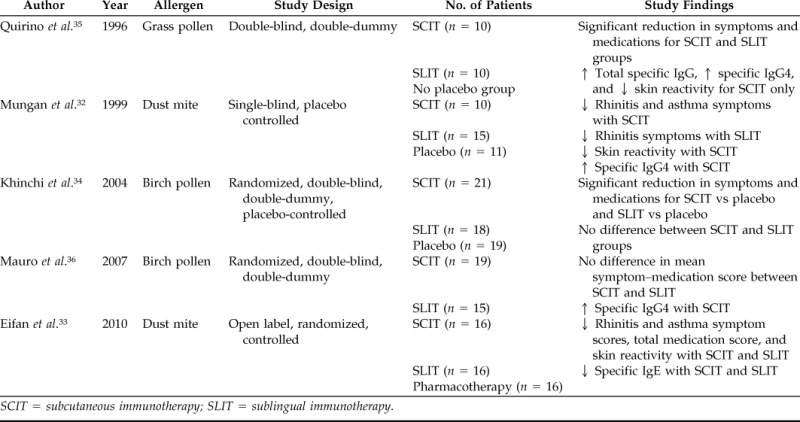
SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy.
In 2009, the World Allergy Organization Position Paper on Sublingual Immunotherapy discussed a number of important points regarding the current status of SLIT efficacy.37 Among these points, although SLIT meta-analyses have shown benefit in the treatment of allergic rhinitis in adults and allergic rhinitis and asthma in children, there are limitations in the overall conclusions of these meta-analyses imposed by the significant heterogeneity of the studies included in them. Second, the efficacy and dose dependence of SLIT for grass pollen allergy in adults and children has been well demonstrated in large, sufficiently powered, double-blind, randomized, controlled trials.
UNANSWERED QUESTIONS
Single Antigen Therapy versus Multiantigen Therapy
One of the biggest sources of discussion in the SCIT versus SCIT debate is the clinical use of single antigens versus multiple antigens in immunotherapy prescriptions. Although most clinical trials of specific immunotherapy (SCIT and SLIT) have tested the effects of only 1 antigen, the average SCIT preparation in the United States includes 10 antigens.38 In the 2010 Cochrane systematic review of SCIT for allergic asthma, only 6 of 88 trials tested multiple antigens.23 In the 2010 Cochrane review of SLIT for allergic rhinitis, only one trial involved multiple antigens.16
Some difficulties in treating with multiple-antigen immunotherapy in a controlled clinical trial setting include identifying potential subjects with a similar multiantigen allergy profile in the absence of other positive reactions, as well as accurately assessing symptoms related to specific allergy triggers when multiple environmental triggers may be present. Although efficacy has been shown with multiantigen SLIT (over single-antigen SLIT and placebo) in some studies,39 a recent multiantigen SLIT study in the United States failed to establish significant differences in symptoms versus single-antigen SLIT or placebo.40 The belief that immunotherapy is more effective in patients who are sensitized to only a single antigen tends to be more prevalent outside the United States, whereas practitioners treating allergy in the United States are more inclined to treat with multiple antigens in an immunotherapy prescription.17,38 It is interesting to note, however, that a recent open study in 51 Italian children with allergic polysensitization found that allergic sensitization to multiple allergens should not be considered a barrier to treatment with SLIT. In this study by Ciprandi et al., treatment groups included single-antigen, dual-antigen, and multiple-antigen therapies, with significant improvements noted in symptoms, medication use, and number of sensitizations after 12 months of therapy.41 The efficacy of multiantigen SLIT requires further clarification, especially in light of the suggestion that multiantigen treatment may have contributed to the few cases of SLIT anaphylaxis.11,13
Optimum Immunotherapy Dosing
Although standardization of antigens and regulation of antigen maintenance dose brings about some controversy with regard to SCIT, the recommended optimal maintenance dose for most SCIT published was published in a 1998 World Health Organization Position Paper.42,43 An optimal dose for SCIT has been defined as “the dose of an allergen vaccine inducing a clinically relevant effect in the majority of patients without causing unacceptable side effects” and is typically 5–20 μg of major allergen per dose (50–250 of major allergen per year).
A recommended treatment dose for SLIT is less clear. Because of different antigen production and standardization techniques worldwide, translation of clinical trial antigen doses to daily clinical practice may be difficult. At this time, there is no universally accepted SLIT dosing schedule. However, published SLIT doses are notably higher than SCIT doses. Furthermore, maintenance schedules differ between SLIT (typically given daily) and SCIT (typically given monthly). An individual SLIT dose may range from 0.0006 to 21 times an individual SCIT dose, but the median monthly SLIT dose is ∼49 times higher than the median SCIT dose (range, 0.017 to >500 times higher).44 Many studies have indicated that improvement in clinical response occurs more frequently with moderate-to-high SLIT doses, but the optimal SLIT dose still has not been fully elucidated for most antigens.45–47 A notable exception is the optimal SLIT maintenance dose for grass pollen antigen. Dose-finding studies by Durham et al. and Didier et al. have identified that the most advantageous maintenance dose for SLIT with grass pollen is between 15 and 25 μg of major allergen daily.25,45 Finally, because of the safety and tolerability of SLIT, maintenance treatment has often been given preseasonally or coseasonally in clinical trials treating for a single seasonal antigen.25,26 This is in contrast to year-round monthly SCIT maintenance injections. The effect of these seasonal SLIT dosing schedules has not been extensively studied with regard to the potential for recurrence of symptoms long term.
CONCLUSIONS
SCIT has long been an accepted form of treatment for allergic rhinitis, but interest in SLIT has grown considerably in recent years. This has sparked debate regarding the benefits and shortcomings of each of these immunotherapy methods. The safety of SLIT is not routinely questioned, although a few cases of nonfatal anaphylaxis have been reported. Recent meta-analyses of SLIT for allergic rhinitis have shown overall efficacy, as well as efficacy in multiple subgroup analyses. However, questions have been raised regarding the magnitude of SLIT efficacy versus SCIT, and few controlled studies have been performed to directly compare SLIT and SCIT. Many unanswered questions remain regarding SLIT and its comparison with SCIT, including the clinical practice of multiantigen therapy, which is not routinely tested in randomized clinical trials.
Footnotes
Presented at the North American Rhinology & Allergy Conference, February 5, 2011, Puerto Rico
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Pleis J, Ward B, Lucas J. Summary health statistics for U.S. Adults: National Health Interview Survey 2009. Vital Health Statistics Series 10, No. 249, 2010. [PubMed] [Google Scholar]
- 2. Bloom B, Cohen R, Freeman G. Summary health statistics for U.S. Children: National Health Interview Survey 2009. Vital Health Statistics Series 10, No. 244, 2010. [PubMed] [Google Scholar]
- 3. Schappert S, Rechtsteiner E. Ambulatory medical care utilization estimates for 2006. National Health Statistics Rep 8:1–32, 2008. [PubMed] [Google Scholar]
- 4. Calderon M, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database of Syst Rev 1:CD001936, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox L, Larenas-Linneman D, Lockey R, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol 125:569–574, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Bernstein D, Wanner M, Borish L, Liss G. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol 113:1129–1136, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Cox L. Sublingual immunotherapy and allergic rhinitis. Curr Allergy Asthma Rep 8:102–110, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Amin H, Liss G, Bernstein D. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol 117:169–175, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Stewart G, Lockey R. Systemic reactions from allergen immunotherapy. J Allergy Clin Immunol 90:567–578, 1992. [DOI] [PubMed] [Google Scholar]
- 10. Committee on the Safety of Medicines update: Desensitizing vaccines. BMJ 293:948, 1986.20742706 [Google Scholar]
- 11. Dunsky E, Goldstein M, Dvorin D, Belecanech G. Anaphylaxis to sublingual immunotherapy. Allergy 61:1235, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Antico A, Pagani M, Crema A. Anaphylaxis by latex sublingual immunotherapy. Allergy 61:1236–1237, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Eifan A, Keles S, Bahceciler N, Barlan I. Anaphylaxis to multiple pollen allergen sublingual immunotherapy. Allergy 62:567–568, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Blazowski L. Anaphylactic shock because of sublingual immunotherapy overdose during third year of maintenance dose. Allergy 63:374, 2008. [DOI] [PubMed] [Google Scholar]
- 15. de Groot H, Bijl A. Anaphylactic reaction after the first dose of sublingual immunotherapy with grass pollen tablet. Allergy 64:963–964, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Radulovic S, Calderon M, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 12:CD002893, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penagos M, Compalati E, Tarantini F, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: A meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol 97:141–148, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Penagos M, Passalacqua G, Compalati E, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest 133:599–609, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Agostinis F, Foglia C, Landi M, et al. The safety of sublingual immunotherapy with one or multiple pollen allergens in children. Allergy 63:1637–1639, 2008. [DOI] [PubMed] [Google Scholar]
- 20. di Rienzo V, Minelli M, Musarra A, et al. Post-marketing survey on the safety of sublingual immunotherapy in children below the age of 5 years. Clin Exp Allergy 35:560–564, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Fiocchi A, Paino G, La Grutta S, et al. Safety of sublingual-swallow immunotherapy in children aged 3 to 7 years. Ann Allergy Asthma Immunol 95:254–258, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Giovannini M, Braccioni F, Sella G, et al. Comparison of allergen immunotherapy and drug treatment in seasonal rhinoconjunctivitis: A 3-years study. Eur Ann Allergy Clin Immunol 37:69–71, 2005. [PubMed] [Google Scholar]
- 23. Abramson M, Puy R, Weiner J. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev 8:CD00118, 20106. [DOI] [PubMed] [Google Scholar]
- 24. Scadding G, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to dust mite. Clin Allergy 16:483–491, 1986. [DOI] [PubMed] [Google Scholar]
- 25. Durham S, Yang W, Pedersen M, et al. Sublingual immunotherapy with once-daily grass allergen tablets: A randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 117:802–809, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Dahl R, Kapp A, Colombo G, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 118:434–440, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Di Bona D, Plaia A, Scafidi V, et al. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: A systematic review and meta-analysis. J Allergy Clin Immunol 126:558–566, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Compalati E, Passalacqua G, Bonini M, Canonica G. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: Results of a GA2LEN meta-analysis. Allergy 64:1570–1579, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Marogna M, Thomassetti G, Bernasconi A, et al. Preventative effects of sublingual immunotherapy in childhood: An open randomized controlled study. Ann Allergy Asthma Immunol 101:206–211, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Durham S, Emminger W, Capp A, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol 125:121–128, 2010. [DOI] [PubMed] [Google Scholar]
- 31. Marogna M, Spadolini I, Massolo A, et al. Long-lasting effects of sublingual immunotherapy according to its duration: A 15-year prospective study. J Allergy Clin Immunol 126:969–975, 2010. [DOI] [PubMed] [Google Scholar]
- 32. Mungan D, Misirligil Z, Gurbuz L. Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite-sensitive patients with rhinitis and asthma—A placebo controlled study. Ann Allergy Asthma Immunol 82:485–490, 1999. [DOI] [PubMed] [Google Scholar]
- 33. Eifan A, Akkoc T, Yildiz A, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: An open randomized controlled trial. Clin Exp Allergy 40:922–932, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Khinchi M, Poulsen L, Carat F, et al. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: A randomized, placebo-controlled, double-blind, double-dummy study. Allergy 59:45–53, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Quirino T, Iemoli E, Siciliani E. Sublingual versus injective immunotherapy in grass pollen allergic patients: A double blind (double dummy) study. Clin Exp Allergy 26:1253–1261, 1996. [PubMed] [Google Scholar]
- 36. Mauro M, Russelo M, Incorvaia C, et al. Comparison of efficacy, safety and immunologic effects of subcutaneous and sublingual immunotherapy in birch pollinosis: A randomized study. Eur Ann Allergy Clin Immunol 39:119–122, 2007. [PubMed] [Google Scholar]
- 37. Canonica G, Bosquet J, Casale T, et al. Sub-lingual Immunotherapy: World Allergy Organization Position Paper 2009. Allergy 64(suppl 91):1–59, 2009. [DOI] [PubMed] [Google Scholar]
- 38. Nelson H. Multiantigen immunotherapy for allergic rhinitis and asthma. J Allergy Clin Immunol 123:763–769, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Marogna M, Spadolini I, Massolo A, et al. Effects of sublingual immunotherapy for multiple or single allergens in polysensitized patients. Ann Allergy Asthma Immunol 98:274–280, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Amar S, Harbeck R, Sills M, et al. Response to sublingual immunotherapy with grass pollen extract: Monotherapy versus combination in a multiallergen extract. J Allergy Clin Immunol 124:150–156, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Ciprandi G, Cadario G, Di Gioacchino G, et al. Sublingual immunotherapy in children with allergic polysensitization. Allergy Asthma Proc 31:227–231, 2010. [DOI] [PubMed] [Google Scholar]
- 42. Bosquet J, Lockey R, Malling H. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 102:558–562, 1998. [DOI] [PubMed] [Google Scholar]
- 43. van Rhee R. Indoor allergens: Relevance of major allergen measurements and standardization. J Allergy Clin Immunol 119:270–277, 2007. [DOI] [PubMed] [Google Scholar]
- 44. Cox L, Linnemann D, Nolte H, et al. Sublingual immunotherapy: A comprehensive review. J Allergy Clin Immunol 117:1021–1035, 2006. [DOI] [PubMed] [Google Scholar]
- 45. Didier A, Malling H, Worm M, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol 120:1338–1345, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Horak F, Jaeger S, Worm M, et al. Implementation of pre-seasonal sublingual immunotherapy with a five-grass pollen tablet during optimal dosage assessment. Clin Exp Allergy 39:394–400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malling H, Montagut A, Melac M, et al. Efficacy and safety of 5-grass pollen sublingual immunotherapy tablets in patients with different clinical profiles of allergic rhinoconjunctivitis. Clin Exp Allergy 39:387–393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


