Abstract
Background:
Epistaxis is a common otolaryngology emergency and is often controlled with first-line interventions such as cautery, hemostatic agents, or anterior nasal packing. A subset of patients will continue to bleed and require more aggressive therapy.
Methods:
Intractable spontaneous epistaxis was traditionally managed with posterior nasal packing and prolonged hospital admission. In an effort to reduce patient morbidity and shorten hospital stay, surgical and endovascular techniques have gained popularity. A literature review was conducted.
Results:
Transnasal endoscopic sphenopalatine artery ligation and arterial embolization provide excellent control rates but the decision to choose one over the other can be challenging. The role of transnasal endoscopic anterior ethmoid artery ligation is unclear but may be considered in certain cases when bleeding localizes to the ethmoid region.
Conclusion:
This article will focus on the management of intractable spontaneous epistaxis and discuss the role of endoscopic arterial ligation and embolization as it pertains to this challenging clinical scenario.
Keywords: Anterior, artery, embolization, endoscopic, endovascular, epistaxis, ethmoid, packing, posterior, sphenopalatine
Epistaxis is the most common otolaryngology emergency and accounts for ∼1 in 200 emergency room consultations.1 The majority of cases are controlled with common first-line interventions such as chemical or electrical cautery, the application of hemostatic agents, or short-term anterior nasal packs. However, there is a subset of patients with intractable spontaneous epistaxis who continue to bleed and require more aggressive therapy.
Intractable epistaxis is a challenging problem that was traditionally managed with posterior nasal packing and prolonged hospital admission and associated with significant patient morbidity and high health care costs. With improvements in both endoscopic and endovascular techniques, transnasal endoscopic sphenopalatine artery ligation (TESPAL) and arterial embolization have gained popularity over the traditional approach. Both techniques can be successful in controlling intractable epistaxis but the decision to use one versus the other can be challenging.2,3 This article will focus on the management of intractable spontaneous epistaxis and discuss the role of TESPAL and embolization as it pertains to this challenging clinical scenario.
ANATOMY
The vascular supply of the nasal cavity arises from terminal branches derived from both the internal carotid artery (ICA) and the external carotid artery (ECA). The majority of epistaxis occurs on the anterior nasal septum at a region called Little's area, which is supplied by Kiesselbach's plexus. This vascular network of vessels is a confluence of three terminal arteries (sphenopalatine artery [SPA], anterior ethmoid artery [AEA], and superior labial artery) and accounts for ∼90–95% of epistaxis.4 Although there is no clear definition of anterior epistaxis versus posterior epistaxis, ∼5–10% of patients bleed beyond visualization with a nasal speculum and headlight. The two primary anatomic sites of posterior epistaxis include the posterior lateral nasal wall5 and posterior nasal septum.6 Because of inherent challenges with visualization and poor treatment localization, the majority of intractable epistaxis cases would be classified as posterior epistaxis.
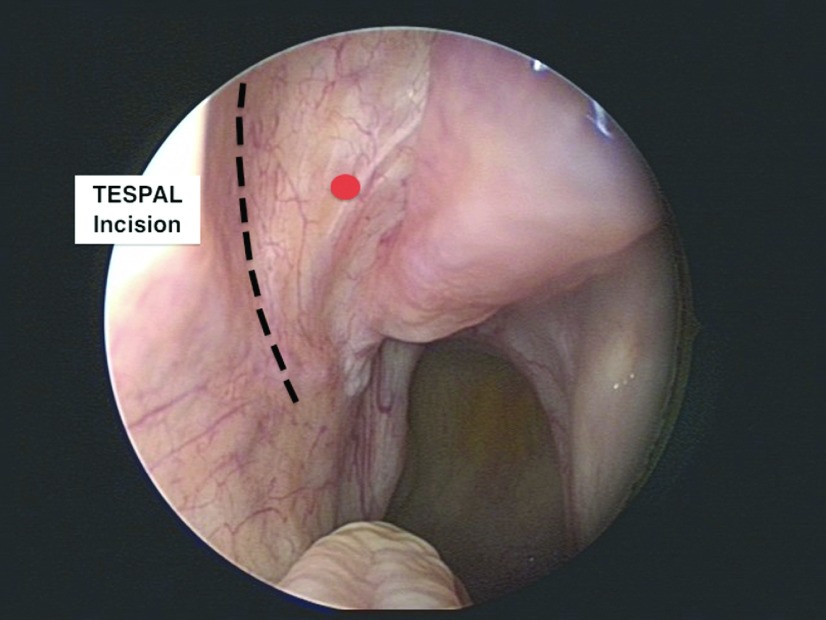
From a clinical perspective, the most common artery involved in posterior intractable epistaxis is the SPA. The SPA is a terminal branch of the internal maxillary artery (IMA) and enters the nasal cavity in the region of the posterior attachment of the middle turbinate through the sphenopalatine foramen (SPF). There have been several studies showing several SPF anatomic variations (Table 1).7–12 Despite some differences in the prevalence of anatomic variations, all studies indicated that the SPF was higher than the posterior attachment of the middle turbinate, usually located at the junction of the middle and superior meatuses. Therefore, during TESPAL, the lower limit of dissection should be the inferior edge of the posterior attachment of the middle turbinate (Fig. 1). The ethmoid crest (also known as crista ethmoidalis) is an important surgical landmark, because it is present in all patients and is positioned anterior to the SPF in 98% of cases.10 A thorough understanding of the SPF and SPA anatomy is required to optimize surgical therapy of intractable epistaxis and minimize treatment failures.
Table 1.
Summary of SPF and SPA anatomic variations
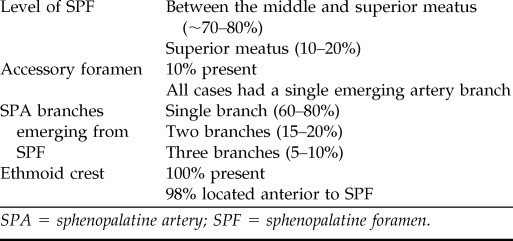
SPA = sphenopalatine artery; SPF = sphenopalatine foramen.
Figure 1.
Incision site for TESPAL. The red dot indicates the approximate locate of the spa.
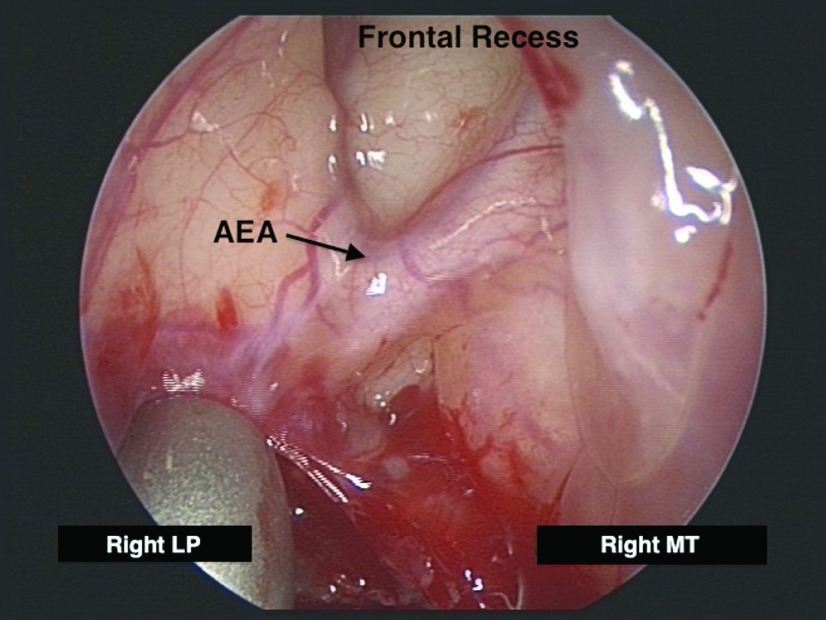
Epistaxis from the AEA is less common and is typically associated with midface trauma or iatrogenic injury during endoscopic sinus surgery. The AEA branches off the ophthalmic artery within the orbital cavity and traverses medially along the ethmoid skull base to supply the superior lateral nasal wall and septum. Externally, in the space between the orbital periosteum and lamina papyracea, the AEA can be identified ∼2 cm posterior to the lacrimal crest.13 Endonasally, the AEA can be located either at the ethmoid skull base attachment of the ethmoid bulla lamella or just posterior to this location, along the posterior aspect of the frontal recess (Fig. 2). Two recent cadaver studies indicated that the AEA runs in a bony arterial mesentery in approximately one-third of cases and is amenable to surgical ligation in ∼20% of cases (Table 2).14,15 However, another cadaver study by Camp et al. showed that all 16 AEAs were successfully clipped endoscopically without any noted skull base injuries.16 Patients with an AEA located in a mesentery can be identified on preoperative sinus CT and they tend to have longer lateral lamella (Keros, type 2 or 3) and a high ethmoid skull base (Fig. 3).
Figure 2.
Endoscopic anatomy of the right anterior ethmoid artery. AEA = anterior ethmoid artery.
Table 2.
Summary of endoscopic AEA anatomy

AEA = anterior ethmoid artery.
Figure 3.
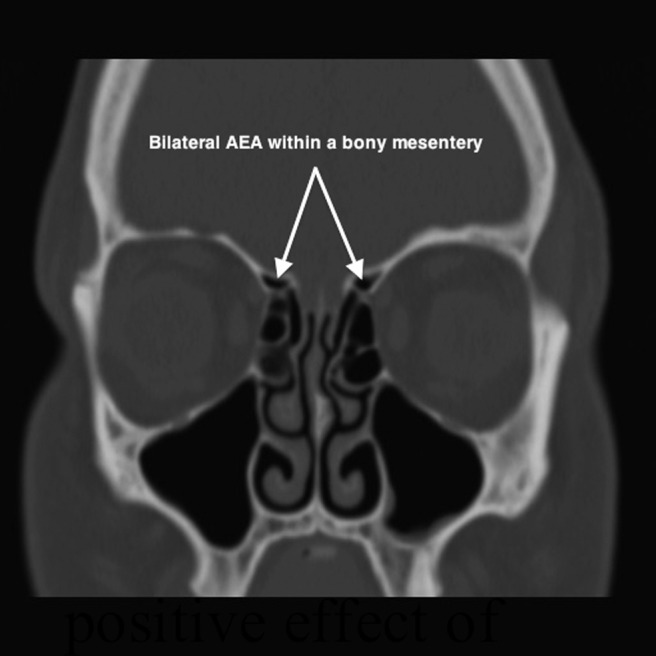
Computed tomography view of the anterior ethmoid artery. AEA = anterior ethmoid artery.
GENERAL MANAGEMENT OF EPISTAXIS
Before the definitive management of intractable epistaxis, several important interventions should be used to optimize treatment success. The first priority is to control or slow the active hemorrhage with some form of temporizing packing. This can be achieved with commercially available anterior–posterior balloon combo packs or a Foley catheter combined with anterior packing.17 The goal of both methods is to slow the rate of blood loss and occlude the posterior choanae to prevent blood from traveling into the upper airway.
Once the active hemorrhage is controlled, any precipitating medical factors should be identified and corrected if clinically safe to do so. The anticoagulated patient can pose a challenging problem, because they tend to have more severe epistaxis and bleed from several sites. Because of the risk for serious medical complications, reversal or discontinuation of the anticoagulation medication should not be performed unless it is deemed safe by the initiating medical specialty. During warfarin-related epistaxis, ∼80% of patients were outside their disease-specific international normalized ratio (INR) range; therefore, in addition to a complete blood count, all patients should have an INR evaluated at the time of presentation.18 In 2008, Walker et al. proposed a protocol to manage anticoagulation-related epistaxis19 (Table 3). Therapy often requires a multidisciplinary team to optimize safe therapy for the control of epistaxis.
Table 3.
Management protocol for anticoagulation-related epistaxis

CBC = complete blood count; INR = international normalized ratio.
SURGERY FOR INTRACTABLE EPISTAXIS
Surgical intervention for intractable epistaxis provides targeted therapy and can prevent prolonged posterior nasal packing with consequent hospital admission. A randomized trial by Moshaver et al. evaluated patients with intractable epistaxis and compared early surgical intervention (using TESPAL) to posterior nasal packing. The results showed that TESPAL resulted in a significantly shorter hospital stay and reduced health care costs. The overall success rate of TESPAL was 89% and there was no difference in complication rates between the two treatment modalities. The following section will describe two endoscopic surgical techniques used to manage intractable epistaxis: (1) TESPAL and (2) transnasal endoscopic anterior ethmoid artery ligation (TEAEAL).
Transnasal Endoscopic Sphenopalatine Artery Ligation
Historically, surgical control of posterior epistaxis involved a Caldwell-Luc with transantral ligation of the IMA.20 This external approach was associated with high patient morbidity and often failed to control the epistaxis because of collateral circulation supplying the SPA distal to the IMA ligation site. In an attempt to reduce patient morbidity and improve success rates, therapies evolved into transnasal techniques with focal ligation of the SPA. In 1985, Stamm et al. described the transnasal microscope approach to SPA ligation and showed a 94% success rate for controlling intractable posterior epistaxis with reduced patient morbidity.21 In 1992, Budrovich reported on the endoscopic approach for SPA ligation, which has increased in popularity because of improved visualization and reduced patient morbidity.22
Technique for TESPAL.
After administration of a general anesthetic and airway protection, TESPAL begins with thorough nasal preparation to optimize visualization and improve treatment success. First, the temporizing nasal packing material is removed and the nasal cavity is cleaned of all blood clots. A detailed endoscopic examination should be performed before decongesting the nose to clearly define the site of bleeding. Next, decongesting the nose for 5–10 minutes (we use pledgets soaked with 1:1000 epinephrine) will reduce active bleeding and significantly improve visualization during the procedure. To further optimize intraoperative hemostasis, the sphenopalatine region or greater palatine canal23 may be injected with local anesthetic containing epinephrine (we use 1% Xylocaine with 1:100,000 epinephrine). The importance of this nasal preparation can not be overemphasized.
After preparation, the TESPAL procedure can proceed in a well-described fashion (Table 4). Some surgeons routinely perform maxillary antrostomy to improve SPA identification; however, a recent study by Shires et al. showed that 90% of TESPALs can be successfully performed with an isolated lateral nasal wall incision.24 After SPA identification, studies have indicated that cauterization, rather than clipping, provides a higher success rate.3,25
Table 4.
Surgical steps for TESPAL
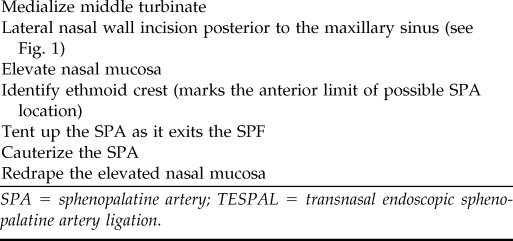
SPA = sphenopalatine artery; TESPAL = transnasal endoscopic sphenopalatine artery ligation.
Success Rate for TESPAL.
Most studies evaluating TESPAL are small and retrospective; however, the reported overall success rate is >85%.3,25–28 In 2003, Kumar et al. reviewed the literature and reported a pooled success rate of 98% with mean hospital admission of 1.6 days after TESPAL. A recent article by Nouraei et al. defined surgical failure as recurrent epistaxis occurring within 2 weeks after TESPAL. They reported a 12% failure rate and identified the following risk factors for early rebleeding requiring nasal packing: (1) warfarin administration, (2) low platelet count on admission, and (3) not using cauterization for SPA ligation.25
Complications in TESPAL.
Minor rebleeding requiring nasal packing occurs in ∼15–20% of cases.25 Major rebleeding requiring a second surgical procedure is rare and would be <1% of cases. Other reported complications of TESPAL include nasal crusting (34%), palatal numbness (12%), and acute sinusitis (3%).29 Starting nasal saline irrigation 48 hours after TESPAL may be considered to reduce crusting and potentially avoiding long-term complications such as synechiae and sinus ostial obstruction.
Transnasal Endoscopic Anterior Ethmoid Artery Ligation
Epistaxis related to the AEA is less common than the SPA and was traditionally ligated using an external Lynch incision with AEA clipping between the lamina papyracea and periorbita. Two recent cadaver studies showed that TEAEAL may be possible in ∼20% of patients, and all cases required that the AEA run in a bony mesentery across the ethmoid skull base.14,15 Although there is limited research on TEAEAL, two small case series have reported success in controlling intractable epistaxis, which localized to the ethmoid region during the endoscopic exam.30,31 Before choosing to perform TEAEAL, it is important for the surgeon to weigh the potential benefits of avoiding an external scar from the traditional external approach, with the potential for serious complications such as cerebral spinal fluid leak or orbital injury related to TEAEAL.
Technique of TEAEAL.
Preoperative CT scan of the sinuses should be performed to review the AEA anatomy to determine whether it is amenable to endoscopic ligation (see foregoing Anatomy section). As with the TESPAL, a general anesthetic is typically required and thorough nasal preparation is recommended to optimize surgical success. In 2007, Pletcher and Metson reported the surgical steps for TEAEAL (Table 5).30 The role of TEAEAL still has to be entirely defined.
Table 5.
Surgical steps for TEAEAL
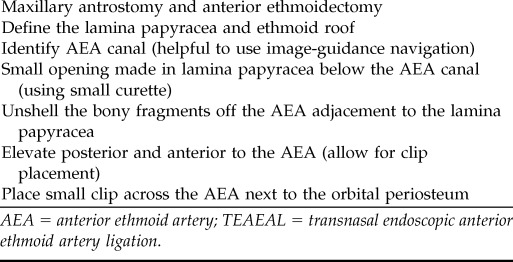
AEA = anterior ethmoid artery; TEAEAL = transnasal endoscopic anterior ethmoid artery ligation.
Success Rate of TEAEAL.
There is limited evidence for the success rate of TEAEAL. Furthermore, AEA ligation is typically combined with SPA ligation, making it more challenging to determine the isolated effect of TEAEAL.
Complications of TEAEAL.
Potential serious complications that must be considered when choosing to perform TEAEAL include cerebral spinal fluid leak, orbital injury, and failure to control epistaxis.
ARTERIAL EMBOLIZATION
Endovascular control of intractable epistaxis was first described in 197432 and has since been evaluated in several large studies to become a well-accepted therapeutic option.33–37 The following section will outline the basic technique, success rates, and potential complications of embolization for intractable epistaxis.
Technique of Embolization.
The first step involves a preembolization angiogram of the ICA and ECA systems. The purpose of this angiogram is to evaluate for contrast blush, which can localize the site of bleeding, and to identify rare etiologies of epistaxis, such as tumors, vascular malformations, or pseudoaneurysm. Additionally, it can identify dangerous anastomoses between the ECA and ICA that can predispose to stroke or blindness. A recent review article by Willems et al. outlines a treatment protocol for embolization of idiopathic intractable epistaxis (Table 6).2
Table 6.
Endovascular treatment protocol for intractable idiopathic epistaxis
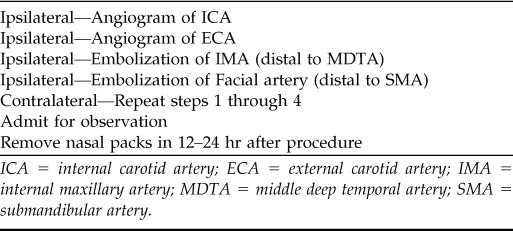
ICA = internal carotid artery; ECA = external carotid artery; IMA = internal maxillary artery; MDTA = middle deep temporal artery; SMA = submandibular artery.
The nose has significant anastomoses from the contralateral arterial system; therefore, it is common to embolize the contralateral IMA and sometimes the contralateral facial artery to optimize treatment success. However, this decision is controversial and may increase the risk of soft tissue ischemia-related complications.38 To reduce the risk of intranasal ischemia during cases of bilateral arterial embolization, it may be beneficial to remove nasal packing early to prevent soft-tissue compression of nasal tissue that further compromises vascularity.
Success of Embolization.
Arterial embolization appears comparable with TESPAL for success in controlling intractable epistaxis, as several recent studies have shown success rates between 80 and 90%.33–36 A study by Christensen et al. pooled the results from 23 studies and indicated a mean success rate of 88%.35
Complications of Embolization.
A recent article by Willem et al. categorizes complications associated with arterial embolization into three categories: minor transient, major transient, and persistent (Table 7). Minor transient complications occur between 25 and 50% of cases and can be managed with conservative therapy. Although, major transient and persistent complications are rare, they must be discussed with the patient because the sequelae can be severe and result in substantial morbidity.
Table 7.
Summary of potential complications from arterial embolization for intractable epistaxis

SURGERY VERSUS EMBOLIZATION FOR INTRACTABLE EPISTAXIS
In cases of intractable epistaxis, the decision to choose surgery or embolization is challenging because both modalities have similar high success rates. Thus, therapeutic decision making is commonly influenced by several factors, such as patient comorbidity, presence of anticoagulation, institution surgical and endovascular expertise, patient preference, and health care costs. Although there is no definitive evidence indicating when one treatment modality is superior to the other, this section will discuss the advantages of both options and attempt to clarify this challenging dilemma.
The primary advantage of TESPAL over embolization is a lower risk for major complications such as stroke, blindness, and soft tissue ischemia. Other potential advantages of TESPAL include improved bleeding site localization, improved ability to diagnose less common etiologies of epistaxis (e.g., tumors), the option to perform immediate AEA ligation, and generally lower health care costs. The primary advantage of embolization over TESPAL is the ability to perform the procedure under local anesthesia ± light sedation and avoiding general anesthesia in a patient with pertinent comorbidities. Other advantages of embolization include improved diagnosis of vascular abnormalities (such as vascular malformations or pseudoaneurysm) and potentially less trauma to nasal mucosa (Table 8).
Table 8.
Potential advantages of TESPAL and embolization
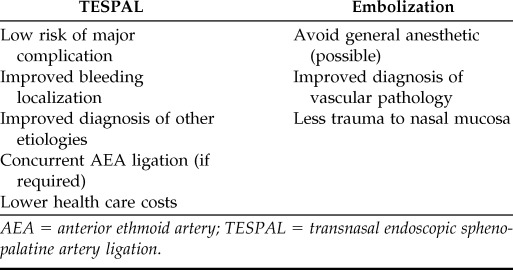
AEA = anterior ethmoid artery; TESPAL = transnasal endoscopic sphenopalatine artery ligation.
In a time of finite health care resources and budgets, it is important to consider the economic impact of medical interventions. The best available evidence has reported TESPAL costs approximately between $6000 and $7500 compared with approximate costs of $12,000 for arterial embolization in 2005 U.S. dollars.26,35,39
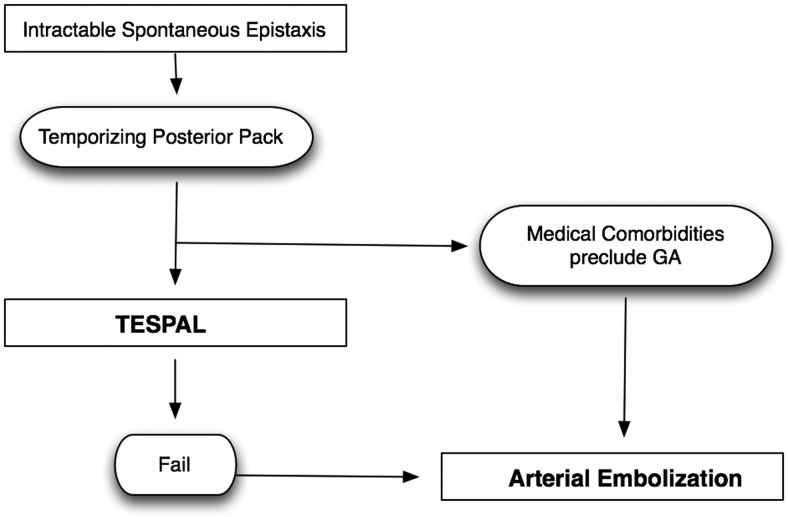
When weighing the advantages of TESPAL and embolization, one might argue that TESPAL may be the better first-line option for intractable epistaxis while reserving embolization for cases of surgical failure. However, there may be clinical situations when embolization would be preferable to TESPAL such as in patients with significant medical comorbidities who would be at considerable risk for general anesthetic. Additionally, surgery in an anticoagulated patient may result in increased bleeding and additional bleeding sites; therefore, embolization in this scenario may be less traumatic to the nasal mucosa and optimize treatment success. Figure 4 outlines a proposed treatment algorithm for the management of intractable epistaxis.
Figure 4.
Proposed treatment protocol for intractable spontaneous epistaxis.
CONCLUSION
Intractable spontaneous epistaxis is a challenging problem and was traditionally managed with posterior nasal packing and prolonged hospital admission. Because of reduced patient morbidity and excellent success rates, surgical and endovascular techniques to control intractable epistaxis have become standard practice. However, the decision to pursue surgical arterial ligation versus arterial embolization is complex. When considering the lower risk of major complications and reduced economic impact, surgical ligation (TESPAL ± AEA ligation) may be the preferable option before attempting arterial embolization for intractable epistaxis. Embolization may be a better initial option in patients unfit for general anesthesia, in those who are anticoagulated, and in surgical failures. Future research will need to further elucidate the clinical indications for both TESPAL and arterial embolization.
Footnotes
Presented at the Summer Sinus Course, July 22–23, 2011, Chicago, IL
TL Smith is funded by the National Institute on Deafness and Other Communication Disorders and is a consultant for Intersect ENT (Palo Alto, CA) and ENTrigue, Inc. (San Antonio, TX), neither of which provided financial or material support for this study. The remaining author had no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Pallin DJ, Chng YM, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med 46:77–81, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Willems PW, Farb RI, Agid R. Endovascular treatment of epistaxis. AJNR Am J Neuroradiol 30:1637–1645, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar S, Shetty A, Rockey J, et al. Contemporary surgical treatment of epistaxis. What is the evidence for sphenopalatine artery ligation? Clin Otolaryngol Allied Sci 28:360–363, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Chiu T, Dunn JS. An anatomical study of the arteries of the anterior nasal septum. Otolaryngol Head Neck Surg 134:33–36, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Thornton MA, Mahesh BN, Lang J. Posterior epistaxis: Identification of common bleeding sites. Laryngoscope 115:588–590, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Chiu TW, McGarry GW. Prospective clinical study of bleeding sites in idiopathic adult posterior epistaxis. Otolaryngol Head Neck Surg 137:390–393, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Herrera Tolosana S, Fernandez Liesa R, et al. Sphenopalatinum foramen: An anatomical study. Acta Otorrinolaringol Esp 62:274–278, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Antunes Scanavini AB, Navarro JA, Megale SR, et al. Morphometric evaluation of the sphenopalatine foramen for endonasal surgery. Rhinology 48:441–445, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Scanavine AB, Navarro JA, Megale SR, et al. Anatomical study of the sphenopalatine foramen. Braz J Otorhinolaryngol 75:37–41, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padua FG, Voegels RL. Severe posterior epistaxis-endoscopic surgical anatomy. Laryngoscope 118:156–161, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Wareing MJ, Padgham ND. Osteologic classification of the sphenopalatine foramen. Laryngoscope 108:125–127, 1998. [DOI] [PubMed] [Google Scholar]
- 12. Midilli R, Orhan M, Saylam CY, et al. Anatomic variations of sphenopalatine artery and minimally invasive surgical cauterization procedure. Am J Rhinol Allergy 23:e38–e41, 2009. [DOI] [PubMed] [Google Scholar]
- 13. McDonald SE, Robinson PJ, Nunez DA. Radiological anatomy of the anterior ethmoidal artery for functional endoscopic sinus surgery. J Laryngol Otol 122:264–267, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Floreani SR, Nair SB, Switajewski MC, et al. Endoscopic anterior ethmoidal artery ligation: A cadaver study. Laryngoscope 116:1263–1267, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Solares CA, Luong A, Batra PS. Technical feasibility of transnasal endoscopic anterior ethmoid artery ligation: Assessment with intraoperative CT imaging. Am J Rhinol Allergy 23:619–621, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Camp AA, Dutton JM, Caldarelli DD. Endoscopic transnasal transethmoid ligation of the anterior ethmoid artery. Am J Rhinol Allergy 23:200–202, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Ho EC, Mansell NJ. How we do it: A practical approach to Foley catheter posterior nasal packing. Clin Otolaryngol Allied Sci 29:754–757, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Smith J, Siddiq S, Dyer C, et al. Epistaxis in patients taking oral anticoagulant and antiplatelet medication: Prospective cohort study. J Laryngol Otol 125:38–42, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Melia L, McGarry GW. Epistaxis: Update on management. Curr Opin Otolaryngol Head Neck Surg 19:30–35, 2011. [DOI] [PubMed] [Google Scholar]
- 20. Chandler JR, Serrins AJ. Transantral ligation of the internal maxillary artery for epistaxis. Laryngoscope 75:1151–1159, 1965. [DOI] [PubMed] [Google Scholar]
- 21. Stamm AC, Pinto JA, Neto AF, et al. Microsurgery in severe posterior epistaxis. Rhinology 23:321–325, 1985. [PubMed] [Google Scholar]
- 22. Budrovich R, Saetti R. Microscopic and endoscopic ligature of the sphenopalatine artery. Laryngoscope 102:1391–1394, 1992. [DOI] [PubMed] [Google Scholar]
- 23. Douglas R, Wormald PJ. Pterygopalatine fossa infiltration through the greater palatine foramen: Where to bend the needle. Laryngoscope 116:1255–1257, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Shires CB, Boughter JD, Sebelik ME. Sphenopalatine artery ligation: A cadaver anatomic study. Otolaryngol Head Neck Surg 145:494–497, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Nouraei SA, Maani T, Hajioff D, et al. Outcome of endoscopic sphenopalatine artery occlusion for intractable epistaxis: A 10-year experience. Laryngoscope 117:1452–1456, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Moshaver A, Harris JR, Liu R, et al. Early operative intervention versus conventional treatment in epistaxis: Randomized prospective trial. J Otolaryngol 33:185–188, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Seno S, Arikata M, Sakurai H, et al. Endoscopic ligation of the sphenopalatine artery and the maxillary artery for the treatment of intractable posterior epistaxis. Am J Rhinol Allergy 23:197–199, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Minni A, Dragonetti A, Gera R, et al. Endoscopic management of recurrent epistaxis: The experience of two metropolitan hospitals in Italy. Acta Otolaryngol 130:1048–1052, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Snyderman CH, Goldman SA, Carrau RL, et al. Endoscopic sphenopalatine artery ligation is an effective method of treatment for posterior epistaxis. Am J Rhinol 13:137–140, 1999. [DOI] [PubMed] [Google Scholar]
- 30. Pletcher SD, Metson R. Endoscopic ligation of the anterior ethmoid artery. Laryngoscope 117:378–381, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Woolford TJ, Jones NS. Endoscopic ligation of anterior ethmoidal artery in treatment of epistaxis. J Laryngol Otol 114:858–860, 2000. [DOI] [PubMed] [Google Scholar]
- 32. Sokoloff J, Wickbom I, McDonald D, et al. Therapeutic percutaneous embolization in intractable epistaxis. Radiology 111:285–287, 1974. [DOI] [PubMed] [Google Scholar]
- 33. Elden L, Montanera W, Terbrugge K, et al. Angiographic embolization for the treatment of epistaxis: A review of 108 cases. Otolaryngol Head Neck Surg 111:44–50, 1994. [DOI] [PubMed] [Google Scholar]
- 34. Tseng EY, Narducci CA, Willing SJ, et al. Angiographic embolization for epistaxis: A review of 114 cases. Laryngoscope 108:615–619, 1998. [DOI] [PubMed] [Google Scholar]
- 35. Christensen NP, Smith DS, Barnwell SL, et al. Arterial embolization in the management of posterior epistaxis. Otolaryngol Head Neck Surg 133:748–753, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Sadri M, Midwinter K, Ahmed A, et al. Assessment of safety and efficacy of arterial embolisation in the management of intractable epistaxis. Eur Arch Otorhinolaryngol 263:560–566, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Strach K, Schrock A, Wilhelm K, et al. Endovascular treatment of epistaxis: Indications, management, and outcome. Cardiovasc Intervent Radiol 34:1190–1198, 2011. [DOI] [PubMed] [Google Scholar]
- 38. Guss J, Cohen MA, Mirza N. Hard palate necrosis after bilateral internal maxillary artery embolization for epistaxis. Laryngoscope 117:1683–1684, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Miller TR, Stevens ES, Orlandi RR. Economic analysis of the treatment of posterior epistaxis. Am J Rhinol 19:79–82, 2005. [PubMed] [Google Scholar]