Abstract
Objective
Schizophrenia is associated with atopy and increased inflammatory markers. We report a population-based longitudinal study of the associations between childhood atopic disorders, subsequent serum inflammatory markers, interleukin 6 (IL-6) and C-reactive protein (CRP), and the risk of psychotic experiences (PEs).
Method
PEs were assessed at age 13 years (n = 6785). Presence of clinician-diagnosed atopic disorders (asthma and eczema) was determined from parent-completed questionnaires at age 10 years (n = 7814). Serum IL-6 and CRP were measured at age 9 years (n = 5076). Logistic regression examined the association between (1) atopy and PEs, (2) inflammatory markers and PEs, and (3) mediating effects of inflammatory markers on the atopy–PEs association. Linear regression examined the association between atopy and inflammatory markers. Age, gender, social class, ethnicity and body mass index were included as potential confounders.
Results
At age 10 years, about 14% of the sample was reported to have asthma, 12% eczema, and 7% both asthma and eczema. Compared with children with no atopy, risk of PEs at age 13 years was increased for all of these groups; adjusted odds ratios (95% CI) were, respectively, 1.39 (1.10–1.77), 1.33 (1.04–1.69), and 1.44 (1.06–1.94). Atopy was associated with increased serum IL-6 and CRP; however, this did not mediate association between atopy and PEs. Inflammatory markers were not associated with later PEs.
Conclusion
Childhood atopic disorders increase the risk of psychotic experiences in adolescence. Follow-up of these individuals will be useful to determine the effect of atopy and inflammation on different trajectories of early-life PEs.
Abbreviations: PEs, psychotic experiences; IL-6, interleukin 6; CRP, C-reactive protein; OR, odds ratio, 95%; CI, 95% confidence interval
Keywords: Atopic disorders, Asthma, Eczema, Childhood, Adolescence, Psychotic experiences, Psychotic symptoms, Schizophrenia, Inflammatory markers, IL-6, CRP, Cytokine, Immunity, Birth cohort, Prospective study, ALSPAC
1. Introduction
Several lines of evidence link adult psychotic illness with infection and abnormalities in various components of the immune system. Increased prevalence of antibodies to Toxoplasma gondii has been reported in schizophrenia (Torrey et al., 2012). Maternal infection (Buka et al., 2001a, Brown et al., 2004a, Brown et al., 2005, Mortensen et al., 2007, Brown and Derkits, 2010, Mortensen et al., 2010, Khandaker et al., 2012b), raised serum inflammatory markers during pregnancy (Buka et al., 2001b, Brown et al., 2004b), and childhood infection (Rantakallio et al., 1997, Dalman et al., 2008, Khandaker et al., 2012a) have been reported to be associated with the increased risk of schizophrenia in adult life. Studies of individuals with first episode psychosis or acute psychotic relapse suggest an increase in proinflammatory cytokines in serum (Miller et al., 2011), a marker of activation of the innate immune response (Weizman and Bessler, 1999). A recent meta-analysis has reported increased serum C-reactive protein (CRP) in schizophrenia (Miller et al., 2013). However, longitudinal studies of circulating markers of inflammation in childhood and subsequent risk of psychotic outcomes are lacking.
Increased autoantibodies against various brain regions and ion channels have been reported in adult psychotic illness (Rothermundt et al., 2001, Zandi et al., 2011), a marker of activation of the adaptive immune response. Epidemiological studies have also reported increased prevalence of autoimmune conditions in schizophrenia, and family members of patients with schizophrenia (Eaton et al., 2006, Chen et al., 2012). Adaptive immune response elicited by non-infectious antigens underlies atopic disorders such as asthma, atopic dermatitis, urticaria and allergic rhinitis (Janeway et al., 2001). Therefore, it has been suggested that examination of possible links between immune responses and the development of schizophrenia should include atopic conditions as possible contributing factors (Rottem and Shoenfeld, 2003). The evidence base on this subject is growing. So far, two cross-sectional studies have reported increased prevalence of asthma in schizophrenia (Chen et al., 2009, Weber et al., 2009). Recently, a large population-based longitudinal study has reported increased risk of adult schizophrenia in individuals with atopic disorders (asthma, atopic dermatitis, urticaria and allergic rhinitis) earlier in life (Pedersen et al., 2012). Effects of inflammatory response on the developing brain have been proposed as one mechanism that may underlie this association (Pedersen et al., 2012); also, other epidemiological observations of associations between early life infection and adult schizophrenia (Brown and Derkits, 2010, Khandaker et al., 2012a, Khandaker et al., 2012b). However, empirical data on this topic involving human subjects is limited (Buka et al., 2001b, Brown et al., 2004b).
Population-based birth cohort studies have reported increased risk of adult psychotic illness among individuals reporting psychotic experiences (PEs) during childhood and adolescence (Poulton et al., 2000, Zammit et al., 2013). Early-life PEs also have been linked with a number of risk factors for schizophrenia (Horwood et al., 2008, Thomas et al., 2009, Zammit et al., 2009). Therefore, it has been suggested that studies of early-life PEs may be helpful to elucidate the pathophysiology of adult psychotic disorders (Kelleher and Cannon, 2011, Murray and Jones, 2012).
Using data from the population-based Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, we report associations between early-life atopic disorders, serum inflammatory markers (interleukin 6 or IL-6 and CRP) at age 9 years, and the risk of PEs at age 13 years. We predicted that atopic disorders will be associated with both increased levels of inflammatory markers and the risk of PEs. We also predicted that inflammatory markers will be associated with the risk of PEs, and finally, that they will mediate the association between atopic disorders and PEs. Fig. 1 illustrates the timing of data collection and the hypotheses tested.
Fig. 1.
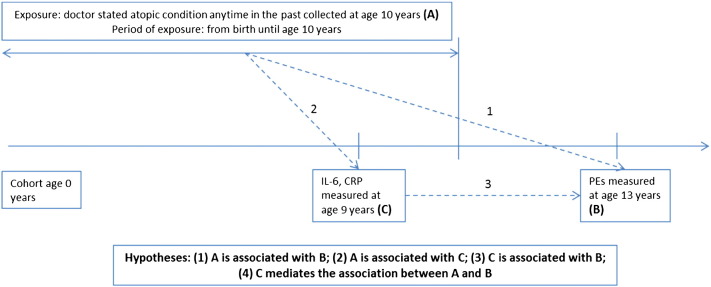
Timing of data collection and hypotheses for the study of atopic disorders, inflammatory markers and risk of psychotic experiences (PEs) in ALSPAC.
2. Method
2.1. Sample
The ALSPAC birth cohort is based on all pregnant women residing in the county of Avon, a geographically defined region in the southwest of England, with expected dates of delivery between April 1991 and December 1992 (www.alspac.bris.ac.uk). The initial ALSPAC cohort consisted of 14,062 live births and 13,988 infants still alive at 12 months (Fraser et al., 2013, Boyd et al., 2013). Avon included both urban and rural areas, and the population was broadly representative of all children in the UK. The parents completed regular postal questionnaires about all aspects of their child's health and development since birth. Since the age of 7 years the children attended an annual assessment clinic during which they participated in a range of face-to-face interviews and physical tests. The current study is based on 6784 individuals who completed the Psychosis-like Symptoms interview (PLIKSi) at age 13 years.
Ethical approval for the study was obtained from ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
2.2. Assessment of psychotic experiences (PEs)
Psychotic experiences were assessed by the semi-structured Psychosis-like Symptoms interview (PLIKSi) at a mean age of 12.9 years (SD = 0.23). The interview instrument comprised 12 ‘core’ questions derived from the Diagnostic Interview Schedule for Children–IV (DISC–IV) (Shaffer et al., 2000), and the Schedules for Clinical Assessment in Neuropsychiatry version 2.0 (SCAN 2.0) (WHO, 1994). It included twelve key symptoms covering the three main domains of positive psychotic symptoms: hallucinations (visual and auditory); delusions (delusions of being spied on, persecution, thoughts being read, reference, control, grandiose ability, and other unspecified delusions); and bizarre symptoms (thought broadcasting, insertion and withdrawal). Interviewees were asked about the presence of these symptoms and frequency of symptoms. We used the observer based rating for the presence or absence of any psychotic experiences in the past six months as the main outcome (i.e. a binary dependent variable). We also examined twelve specific PEs individually as outcomes. The group with PEs was compared with the rest of the cohort. We chose the binary definition of outcome rather than a dimensional approach based on symptom frequency in order to maximize power in multivariable regression analysis.
The interviews were carried out by 13 psychology graduates who were trained by two experienced clinicians and SCAN trainers (a child psychiatrist and a general adult psychiatrist) (Horwood et al., 2008). The interviewers were required to reach 95% agreement whilst scoring two ‘gold standard’ interview videotapes that were prepared by the trainers. Monthly booster training sessions and workshops were arranged to discuss scoring of complex cases and consolidate training. All interviews were audiotaped for each interviewer until they reached eight interviews containing several items rated ‘positive’ (i.e. PEs present). These tapes were independently rated by a second interviewer. The average inter-rater reliability for PLIKS interviews was good (kappa = 0.7) (Horwood et al., 2008). Parents were not interviewed.
2.3. Assessment of atopic disorders
When ALSPAC participants were 10 years old their parents completed a postal questionnaire on various aspects of health and development of the child. This included questions on atopic conditions. Parents were asked whether anytime in the past a doctor had stated that the child suffered from asthma or eczema (original question: “Has a doctor ever actually said that your study child has asthma or eczema?”). Based on response to this question a single categorical exposure (or independent) variable was created, which included four groups: no asthma or eczema (reference group); only asthma; only eczema; both asthma and eczema.
2.4. Assays for inflammatory markers
IL-6, a proinflammatory cytokine, and CRP, an acute phase protein, were measured in blood samples collected at age 9 years. Non-fasting blood samples were taken using standard procedures, with samples immediately spun and frozen at − 80 °C. The measurements were assayed in 2008 after a median of 7.5 years in storage with no previous freeze–thaw cycles during this period (n = 5076). IL-6 was measured by enzyme-linked immunosorbent assay (ELISA) (R&D systems, Abingdon, UK). CRP was measured by automated particle-enhanced immunoturbidimetric assay (Roche UK, Welwyn Garden City, UK). All assay coefficients of variation were < 5% (Falaschetti et al., 2010).
Acute infection is likely to cause a transient increase in serum IL-6 and CRP levels. Since we were interested in inflammatory markers in individuals with atopic disorders in healthy state, those who reported to have an infection at the time of blood collection or in the preceding week were excluded (n = 482). The information regarding infection was gathered in the clinic during blood collection using a questionnaire.
IL-6, IL-1β and tumour necrosis factor α (TNF-α) are important cytokines secreted by dendritic cells and macrophages following infection (Janeway et al., 2001). These have a wide spectrum of biological effects that help to coordinate the body's response to infection including induction of acute phase proteins, raising body temperature (Janeway et al., 2001). However, in other circumstances IL-6 also shows anti-inflammatory activities (Scheller et al., 2011), which are consistent with the pleiotropic nature of most cytokines (Janeway et al., 2001). In ALSPAC, apart from IL-6 and CRP no other inflammatory markers were assayed at age 9 years.
2.5. Assessment of covariates
Age at the time of assessment of PEs (in weeks) and gender were associated with atopic disorder (Table 1); these were included as potential confounders. We also included paternal social class and ethnicity, common correlates of psychotic outcomes as potential confounders. As per the UK Office of National Statistics (ONS) classification system paternal social class was recorded in six categories (I, II, III non-manual, III manual, IV, and V), in descending order, with professionals and higher managerial workers representing social class I (Anon, 1980). In addition, ALSPAC recorded a separate category for fathers working for the armed forces (0.3% of the entire cohort). However, this category is likely to represent different ONS social groups, and we did not have sufficient information to reassign these individuals to the original ONS categories. Therefore, the armed forces category was excluded from all analysis.
Table 1.
Baseline characteristics of individuals with and without atopic disorders.
| Groups/characteristics | Asthma | Eczema | Both asthma and eczema | No atopy | p valuea |
|---|---|---|---|---|---|
| n (%) | 1125 (14.4%) | 994 (12.7%) | 574 (7.3%) | 5121 (65.5%) | |
| Age at the time of assessment of PEs, mean (SD) in years | 12.87 (0.24) | 12.86 (0.20) | 12.84 (0.19) | 12.87 (0.21) | 0.02 |
| Male (%) | 58.8 | 44.2 | 54.2 | 49.5 | < 0.001 |
| British White (%) | 98.3 | 97.7 | 98.1 | 98.4 | 0.75 |
| Social class (%) | 0.14 | ||||
| I | 11.9 | 14.7 | 11.2 | 13.3 | |
| II | 33.3 | 35.4 | 39.4 | 36.5 | |
| III non-manual | 11.5 | 12.4 | 12.2 | 12.2 | |
| III manual | 30.7 | 28.0 | 28.0 | 27.8 | |
| IV | 10.4 | 7.2 | 7.7 | 7.7 | |
| V | 2.2 | 2.3 | 1.5 | 2.5 |
Chi-square test, except mean age, which is independent sample T-test.
2.6. Statistical analysis
2.6.1. Atopic disorders and PEs
We used atopic disorders as the exposure/independent variable (categorical) and PEs as the outcome/dependent variable (binary). Multinomial logistic regression was used to calculate odds ratios for developing PEs in the following groups: asthma only; eczema only; both asthma and eczema. Those with no asthma or eczema were used as the reference group. Regression models were adjusted for age at the time of assessment of PEs, gender, social class and ethnicity. We also calculated the odds ratios for twelve specific PEs individually using the same procedure.
2.6.2. Atopic disorders and inflammatory markers
The relationship between atopy and inflammatory markers was examined using linear regression. IL-6 and CRP values were not normally distributed. Normality of distributions was achieved after natural logarithmic transformation. Log transformed values of IL-6 and CRP were used as the dependent variables. Regression models were adjusted for age at the time of blood test, gender, body mass index (BMI), social class and ethnicity, as these were associated with IL-6 (all p < 0.05).
2.6.3. Inflammatory markers and PEs
Median and interquartile range of the observed values of IL-6 and CRP in those with PEs and the rest of the cohort have been presented. Using log transformed values of IL-6 and CRP (standardized) as the independent variable, we carried out binary logistic regression to calculate the odds ratios for PEs. Thus, the odds ratios represent the increase in the risk of PEs at age 13 years for each standard deviation increase in serum levels of inflammatory markers at age 9 years.
2.6.4. Mediation analysis
Any mediating effects of IL-6 or CRP on the association between atopy and PEs were examined using the following procedure (Judd and Kenny, 1981, Baron and Kenny, 1986). First, separate regression models assessed the associations between: (1) exposure (atopy) and outcome (PEs); (2) exposure and mediator (IL-6); (3) mediator and outcome controlling for exposure. If all of these associations were statistically significant, we proceeded to mediation analysis, whereby exposure–outcome, exposure–mediator, mediator–outcome, all three regression lines were fitted simultaneously in a single model using MPlus. It was expected that in the final step, the exposure–outcome relationship would be attenuated (partial mediation), or eliminated (complete mediation). In case of partial mediation, the extent to which the exposure–outcome estimate was attenuated after inclusion of the mediator was also calculated. This procedure was repeated using CRP as the potential mediator.
3. Results
3.1. Baseline characteristics
Data on atopic disorders stated by a doctor to be present in the child any time since birth until age 10 years were available for 7814 children. On whole, atopic disorders were more common in boys than girls. Baseline characteristics of individuals with and without atopic disorders have been presented in Table 1.
3.2. Atopic disorders and the risk of psychotic experiences at age 13 years
Fig. 2 presents total percentage of individuals with psychotic experiences (PEs) at age 13 years in different groups of atopic disorder at age 10 years.
Fig. 2.
Psychotic experiences among individuals with atopic disorders.
Compared with those with no atopic disorder there was significant increase in risk of psychotic experiences at age 13 years among individuals with a history of atopic disorder (Table 2). Adjustment for age, gender, social class and ethnicity had minimal influence on the odds ratios. With regards to specific PEs, asthma and eczema were associated with the risk of auditory hallucinations. The frequency of individual PEs and their association with atopic disorders have been presented in Table 3.
Table 2.
Atopic disorders and risk of psychotic experiences at age 13 years in ALSPAC cohort.
| Exposure/independent variable (atopy)a | Adjustment for confounding | n | Odds ratio (95% CI) for the outcome/dependent variable (PEs) |
|---|---|---|---|
| Eczema | Unadjusted | 5727 | 1.26 (1.00–1.58) |
| Adjustedb | 4923 | 1.33 (1.04–1.69) | |
| Asthma | Unadjusted | 5727 | 1.35 (1.09–1.86) |
| Adjusted | 4923 | 1.37 (1.08–1.74) | |
| Both asthma and eczema | Unadjusted | 5727 | 1.45 (1.10–1.90) |
| Adjusted | 4923 | 1.44 (1.07–1.95) |
The group with no atopic disorders (i.e. no asthma or eczema) was used as reference (odds ratio = 1).
Adjusted for age at the time of assessment of PEs, gender, social class and ethnicity.
Table 3.
Associations between atopic disorders and specific psychotic experiences in ALSPAC cohort.
| Psychotic experiences (PEs) | Sample (% of PEs) | Odds ratio (95% CI) for PEs in groups of atopic disorder |
||
|---|---|---|---|---|
| Asthma | Eczema | Both | ||
| Auditory hallucinations | 5722 (6.8) | 1.60 (1.21–1.99) | 1.49 (1.11–1.99) | 1.35 (0.93–1.97) |
| Visual hallucinations | 5728 (2.4) | 1.20 (0.74–1.96) | 1.39 (0.87–2.22) | 1.16 (0.61–2.20) |
| Delusions of being spied on | 5723 (2.7) | 1.05 (0.65–1.68) | 1.00 (0.61–1.63) | 1.33 (0.76–2.31) |
| Delusions of persecution | 5723 (0.2) | 0.46 (0.06–3.61) | 0.48 (0.06–3.97) | 0.85 (0.11–6.65) |
| Delusions of thought being read | 5729 (0.6) | 1.01 (0.38–2.66) | 0.84 (0.29–2.44) | 1.11 (0.33–3.72) |
| Delusions of reference | 5729 (0.7) | 0.85 (0.33–2.23) | 0.72 (0.25–2.05) | 0.94 (0.28–3.13) |
| Delusions of control | 5728 (0.6) | 1.01 (0.38–2.65) | 0.84 (0.30–2.44) | 1.86 (0.70–4.92) |
| Delusions of grandiose ability | 5729 (1.1) | 0.51 (0.20–1.30) | 0.75 (0.34–1.67) | 1.33 (0.60–2.97) |
| Other delusions | 5727 (0.4) | 0.38 (0.05–2.96) | 1.21 (0.34–4.31) | 3.57 (1.25–10.20) |
| Thought broadcasting | 5725 (1.3) | 1.51 (0.80–2.84) | 1.84 (1.01–3.34) | 1.50 (0.66–3.36) |
| Thought insertion | 5729 (1.3) | 1.21 (0.64–2.30) | 0.95 (0.46–1.94) | 0.92 (0.36–2.34) |
| Thought withdrawal | 5727 (0.3) | 0.61 (0.14–2.70) | 0.64 (0.18–2.83) | 0.56 (0.07–4.31) |
3.3. Association between atopy and inflammatory markers
Data on both atopy and inflammatory markers were available for about 3850 individuals after excluding those with an infection at the time of blood collection. Atopy was associated with increased serum IL-6 (β = 0.051; SE = 0.014; p < 0.0001). The association was attenuated after adjusting for age, BMI, gender, social class and ethnicity but still remained statistically significant (β = 0.036; SE = 0.015; p = 0.01). Similar findings were observed for serum CRP (unadjusted β = 0.068; SE = 0.019; p < 0.001; adjusted β = 0.039; SE = 0.019; p = 0.03).
3.4. Association between inflammatory markers and psychotic experiences
Data on inflammatory markers at age 9 years (after excluding those with infection) and psychotic experiences at age 13 years were available for 3747 individuals. Out of these, 498 had PEs (13.29%). Medians (interquartile range) of IL-6 for those with PEs and the rest of the cohort were 0.78 (0.47–1.36), and 0.75 (0.47–1.33) pg/ml. Medians (interquartile range) of CRP for those with PEs and the rest of the cohort were 0.22 (0.11–0.45), and 0.20 (0.11–0.47) mg/l.
Logistic regression showed that serum IL-6 and CRP levels were not associated with the risk of psychotic experiences. For each standard deviation increase in IL-6 the odds ratio for PEs was 0.98 (95% CI 0.90–1.08); p = 0.75. A similar result was observed for CRP; OR 1.03 (95% CI 0.93–1.14); p = 0.53.
3.5. Effect of inflammatory markers on the association between atopy and PEs
We examined whether the association between atopy (the group with both asthma and eczema) and PEs was mediated by serum IL-6. Step by step analysis showed that: (1) exposure (atopy) was associated with the outcome (PEs) (β = 0.368, SE = 0.14; p = 0.009); (2) exposure (atopy) was associated with the mediator (IL-6) (β = 0.156, SE = 0.05; p = 0.003); but (3) the mediator (IL-6) was not associated with the outcome (PEs) after controlling for the exposure (atopy) (β = 0.034, SE = 0.06; p = 0.61). Therefore, mediation analysis was not attempted (where the exposure–outcome, exposure–mediator, mediator–outcome all three regression lines would have been fitted simultaneously in a single model). However, in step three which included both IL-6 and atopy as predictors of PEs, an attenuation of the effect estimate between atopy and PEs was observed (β = 0.338, SE = 0.18; p = 0.06), compared with that in step one. This equated to only a 3% reduction of the atopy–PEs effect estimate by IL-6. Together, these results indicated that in our sample IL-6 neither mediated nor greatly confounded the association between atopy and PEs. Similar results were observed for CRP.
4. Discussion
Our findings demonstrate that childhood atopic disorders are associated with the risk of developing psychotic experiences in adolescence. Compared to children with no atopic disorder in the first ten years of life, an increased risk of PEs at age 13 years has been observed in all groups with atopy (asthma, eczema, both). These associations are independent of effects of age at the time of assessment of PEs, gender, social class and ethnicity. To our knowledge, this is the first population-based longitudinal study of childhood atopic disorders and psychotic experiences in adolescence. These results are in line with previous reports of high comorbidity of asthma in adult schizophrenia (Chen et al., 2009, Weber et al., 2009), and higher risk of developing schizophrenia on follow-up among individuals with a history of atopic conditions (asthma, atopic dermatitis, urticaria and allergic rhinitis) (Pedersen et al., 2012).
An important methodological difference between our study and the previous studies of atopic disorders and psychosis needs to be acknowledged. The outcome of our study was psychotic experiences in early adolescence rather than diagnosis of schizophrenia. However, several lines of evidence suggest that early-life psychotic experiences may provide a valid paradigm for studying the development of adult psychotic disorders (Kelleher and Cannon, 2011, Murray and Jones, 2012). First, prospective birth cohort studies suggest higher risk of psychotic disorders in adult life among individuals reporting psychotic symptoms in childhood (Poulton et al., 2000, Zammit et al., 2013). Second, childhood psychotic symptoms are associated with a number of risk factors for schizophrenia, such as prenatal maternal infection and obstetric complications (Zammit et al., 2009), low birth weight (Thomas et al., 2009), and poorer childhood IQ (Horwood et al., 2008). Increased prevalence of antibodies to Epstein Barr Virus has been reported in adolescents reporting psychotic experiences (Wang et al., 2011) and in adult schizophrenia (Delisi et al., 1986). A recent study from the ALSPAC birth cohort has reported increased risk of psychotic experiences in adolescence for autistic traits in early childhood (Bevan Jones et al., 2012). Together, these findings indicate that childhood psychotic experiences may have a developmental component to their origin. Third, population-based studies suggest psychotic symptoms in the general population and that observed in psychotic disorders may exist in a continuum (van Os et al., 2009). Finally, neurophysiological studies suggest common underlying mechanisms for psychotic symptoms seen in healthy people and in schizophrenia (Howes et al., 2013).
One explanation for the association between atopy and PEs could be the effect of medication. Individuals with asthma or treatment resistant eczema are sometimes prescribed oral steroids, which are also associated with the risk of psychosis. We did not have the data on steroid prescriptions. Thus the possibility of steroid medication as a mediator of the association between atopy and PEs cannot be ruled out.
Limitations of this study include the use of parent reported data rather than confirmatory tests for asthma or eczema. However, the questionnaire asked whether these conditions had been diagnosed by a doctor. Using a similar question (Have you ever had asthma?) the prevalence of asthma has been reported to be about 10% in 6–7 year old children in the Western Europe including UK (Lai et al., 2009), and about 20% in 12–14 year old children in the UK (Kaur et al., 1998). These are comparable to the prevalence of parent-reported asthma in our sample (14.4% at age 10 years). Both atopy and PEs were assessed prospectively; thus, eliminating any chance of recall bias.
Degradation of inflammatory markers over time is possible. However, serum samples were frozen at − 80 °C, which is recommended as the optimum temperature for storage of these samples for cytokine assays at a later stage (Holland et al., 2003, de Jager et al., 2009). There was no evidence of prior freeze–thaw cycles. Cytokine levels in blood samples collected at a non-fasting state could be affected by diurnal effect (de Jager et al., 2009). However, the use of non-fasting samples would increase measurement error likely to be random in relation to outcome. We acknowledge that inflammatory markers were measured at age 9 years, and data on atopy were collected slightly later at age 10 years (Fig. 1). However, this is justified as the questionnaire asked about the diagnosis of atopy anytime in the past since birth, and atopy is considered as a long-term attribute in many individuals (Boulay and Boulet, 2003). The approach is also consistent with our hypothesis and the direction of association, i.e. atopy leads to increased IL-6/CRP.
To our knowledge, this is the first longitudinal study of childhood inflammatory markers and subsequent risk of psychotic outcomes. Meta-analyses have reported increased levels of inflammatory markers including IL-6 and CRP in schizophrenia (Miller et al., 2011, Miller et al., 2013). However, due to their cross sectional design these studies cannot establish whether increased inflammatory markers is a cause or consequence of the illness. We found no association between IL-6/CRP at age 9 years and risk of PEs at age 13 years. This could be due to a number of reasons. Measurement error as a result of non-fasting serum samples would contribute to the null finding. A previous meta-analysis suggests that IL-6 may be a state marker of acute psychosis (Miller et al., 2011). Since IL-6 is the primary inducer of CRP, the latter might also be a state marker. Thus, measurement of IL-6 and CRP several years before the assessment of PEs might explain why we did not observe an association between inflammatory markers and PEs. The same meta-analysis reported that other cytokines, including sIL-2R, IL-12, and TNF-α, appeared to be trait markers for schizophrenia (Miller et al., 2011). However, these cytokines were not included when the serum samples were analysed a few years ago in ALSPAC. In the future, studies should include a more comprehensive panel of inflammatory markers including cytokines and white blood cells measured over a period of time.
Effect of inflammatory cytokines on the developing brain has been proposed as a possible mechanism underlying the association between schizophrenia and early life infection. Birth cohort studies suggest that inflammatory cytokines (TNF-α) or chemokines (IL-8) may mediate the risk of schizophrenia in adult offspring associated with prenatal maternal infection (Buka et al., 2001b, Brown et al., 2004b). A possible role of inflammation in the pathogenesis of psychosis is also supported by animal studies. Simulated viral or bacterial infection or direct injection with the inflammatory cytokine IL-6 in pregnant mice has been reported to produce intermediate phenotypes related to schizophrenia in the adult offspring (Meyer and Feldon, 2010). Some of these phenotypes, such as deficits in sensory gating and abnormal latent inhibition are reversible by the antipsychotic clozapine (Smith et al., 2007). However, in our sample there was no indication that inflammatory markers explained the association between atopy and PEs. This could be related to the outcome studied. Adolescent participants with PEs in our sample are almost certainly a heterogeneous group. In many cases these symptoms are likely to be part of normal development, whilst in some they may be more pathological (De Loore et al., 2011, Rubio et al., 2012). Thus, any underlying biological effect (such as mediation by inflammatory markers) may have become diluted. Nevertheless, we found that atopic disorders were associated with higher levels of inflammatory markers and increased risk of PEs. Follow-up of these individuals will be useful to explore further the effect of atopy and inflammation on different developmental trajectories of early-life PEs.
Possible explanations for the observed association between atopy and psychotic experiences include an overlap of genetic risk between atopy and psychosis. For example, shared genetic susceptibility for schizophrenia and asthma has been proposed (Harrison and Law, 2006, Moffatt et al., 2007, Akhabir and Sandford, 2011). Recent genome wide association studies have reported significant associations between schizophrenia and several markers spanning the major histocompatibility complex region on chromosome 6p21.3–22.1. This is consistent with an immune component to schizophrenia risk (Shi et al., 2009, Stefansson et al., 2009). Thus, it is possible that atopic conditions, in the presence of pre-existing genetic liability, can lead to a distinct or pathological immune response. In turn, this may lead to CNS alterations making these individuals susceptible to developing psychotic illness in adult life. In the future, prospective epidemiological studies with genetic and immunological data as well as detailed phenotypic characterisation during childhood and adult life are required to test this hypothesis. Animal models will be useful to identify specific mechanisms underlying behavioural and cognitive phenotypes relevant to schizophrenia involving genes and immunity.
Role of funding source
This study was funded through a Clinical Research Training Fellowship awarded to Dr Golam Khandaker from the Wellcome Trust (Clinical PhD Programme, grant number 094790/Z/10/Z). Dr Zammit holds a Clinician Scientist Award from the National Assembly for Wales. Prof Jones is supported by the Wellcome Trust (095844/Z/11/Z & 088869/Z/09/Z), and NIHR (RP-PG-0606-1335). The UK Medical Research Council (Grant ref: 74882), the Wellcome Trust (Grant ref: 092731), and the University of Bristol provide core support for the ALSPAC birth cohort. The funding bodies had no role in the analyses or writing of the manuscript, or the decision to submit this work for publication.
Contributors
GMK designed the study, analysed data and wrote the first draft. SZ contributed in data collection, analysis, and revision of the draft. GL and PBJ contributed in design, analysis, revision, and provided overall supervision for the study.
Conflict of interest
Prof Peter Jones is co-inventor on patent PCT/GB2005/003279 (methods for assessing psychotic disorders), and received research support from GlaxoSmithKline 2006–10. He directs the National Institute for Health Research Collaborations for Leadership in Applied Health Research and Care for Cambridgeshire and Peterborough (CLAHRC-CP) of which this work forms part. Dr Zammit has received contributions from pharmaceutical companies as honoraria for talks. None of the authors has any conflicts of interest to declare.
Acknowledgment
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, including interviewers, computer and laboratory technicians, clerical workers, research scientists, statisticians, volunteers, managers, receptionists and nurses.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Akhabir L., Sandford A.J. Genome-wide association studies for discovery of genes involved in asthma. Respirology. 2011;16(3):396–406. doi: 10.1111/j.1440-1843.2011.01939.x. [DOI] [PubMed] [Google Scholar]
- Anon . Office of Population Censuses and Surveys. Her Majesty's Stationery Office; London: 1980. Classification of occupations and coding index; p. x. ( http://www.ons.gov.uk/ons/guide-method/classifications/archived-standard-classifications/soc-and-sec-archive/index.html) [Google Scholar]
- Baron R.M., Kenny D.A. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bevan Jones R., Thapar A., Lewis G., Zammit S. The association between early autistic traits and psychotic experiences in adolescence. Schizophr. Res. 2012;135(1–3):164–169. doi: 10.1016/j.schres.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Boulay M.E., Boulet L.P. The relationships between atopy, rhinitis and asthma: pathophysiological considerations. Curr. Opin. Allergy Clin. Immunol. 2003;3(1):51–55. doi: 10.1097/00130832-200302000-00009. [DOI] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., Lawlor D.A., Fraser A., Henderson J., Molloy L., Ness A., Ring S., Davey Smith G. Cohort profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., Derkits E.J. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., Begg M.D., Gravenstein S., Schaefer C.A., Wyatt R.J., Bresnahan M., Babulas V.P., Susser E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown A.S., Hooton J., Schaefer C.A., Zhang H., Petkova E., Babulas V., Perrin M., Gorman J.M., Susser E.S. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry. 2004;161(5):889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown A.S., Schaefer C.A., Quesenberry C.P., Jr., Liu L., Babulas V.P., Susser E.S. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am. J. Psychiatry. 2005;162(4):767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Buka S.L., Tsuang M.T., Torrey E.F., Klebanoff M.A., Bernstein D., Yolken R.H. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry. 2001;58(11):1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Buka S.L., Tsuang M.T., Torrey E.F., Klebanoff M.A., Wagner R.L., Yolken R.H. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun. 2001;15(4):411–420. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Lee H.C., Lin H.C. Prevalence and risk of atopic disorders among schizophrenia patients: a nationwide population based study. Schizophr. Res. 2009;108(1–3):191–196. doi: 10.1016/j.schres.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Chen S.J., Chao Y.L., Chen C.Y., Chang C.M., Wu E.C., Wu C.S., Yeh H.H., Chen C.H., Tsai H.J. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br. J. Psychiatry J. Ment. Sci. 2012;200(5):374–380. doi: 10.1192/bjp.bp.111.092098. [DOI] [PubMed] [Google Scholar]
- Dalman C., Allebeck P., Gunnell D., Harrison G., Kristensson K., Lewis G., Lofving S., Rasmussen F., Wicks S., Karlsson H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am. J. Psychiatry. 2008;165(1):59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- de Jager W., Bourcier K., Rijkers G.T., Prakken B.J., Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loore E., Gunther N., Drukker M., Feron F., Sabbe B., Deboutte D., van Os J., Myin-Germeys I. Persistence and outcome of auditory hallucinations in adolescence: a longitudinal general population study of 1800 individuals. Schizophr. Res. 2011;127(1–3):252–256. doi: 10.1016/j.schres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Delisi L.E., Smith S.B., Hamovit J.R., Maxwell M.E., Goldin L.R., Dingman C.W., Gershon E.S. Herpes simplex virus, cytomegalovirus and Epstein–Barr virus antibody titres in sera from schizophrenic patients. Psychol. Med. 1986;16(4):757–763. doi: 10.1017/s0033291700011764. [DOI] [PubMed] [Google Scholar]
- Eaton W.W., Byrne M., Ewald H., Mors O., Chen C.Y., Agerbo E., Mortensen P.B. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am. J. Psychiatry. 2006;163(3):521–528. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- Falaschetti E., Hingorani A.D., Jones A., Charakida M., Finer N., Whincup P., Lawlor D.A., Davey Smith G., Sattar N., Deanfield J.E. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur. Heart J. 2010;31(24):3063–3072. doi: 10.1093/eurheartj/ehq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Davey Smith G., Henderson J., Macleod J., Molloy L., Ness A., Ring S., Nelson S.M., Lawlor D.A. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P.J., Law A.J. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol. Psychiatry. 2006;60(2):132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Holland N.T., Smith M.T., Eskenazi B., Bastaki M. Biological sample collection and processing for molecular epidemiological studies. Mutat. Res. 2003;543(3):217–234. doi: 10.1016/s1383-5742(02)00090-x. [DOI] [PubMed] [Google Scholar]
- Horwood J., Salvi G., Thomas K., Duffy L., Gunnell D., Hollis C., Lewis G., Menezes P., Thompson A., Wolke D., Zammit S., Harrison G. IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. Br. J. Psychiatry J. Ment. Sci. 2008;193(3):185–191. doi: 10.1192/bjp.bp.108.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Shotbolt P., Bloomfield M., Daalman K., Demjaha A., Diederen K.M., Ibrahim K., Kim E., McGuire P., Kahn R.S., Sommer I.E. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr. Bull. 2013;39(4):807–814. doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Travers P., Walport M., Shlomchik M.J. 5th ed. Garland Science; New York: 2001. Immunobiology: The Immune System in Health and Disease. [Google Scholar]
- Judd C.M., Kenny D.A. Process analysis: estimating mediation in treatment evaluations. Eval. Rev. 1981;5(5):602–619. [Google Scholar]
- Kaur B., Anderson H.R., Austin J., Burr M., Harkins L.S., Strachan D.P., Warner J.O. Prevalence of asthma symptoms, diagnosis, and treatment in 12–14 year old children across Great Britain (international study of asthma and allergies in childhood, ISAAC UK) BMJ. 1998;316(7125):118–124. doi: 10.1136/bmj.316.7125.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher I., Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol. Med. 2011;41(1):1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- Khandaker G.M., Zimbron J., Dalman C., Lewis G., Jones P.B. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr. Res. 2012;139(1–3):161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Zimbron J., Lewis G., Jones P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol. Med. 2012:1–19. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.K., Beasley R., Crane J., Foliaki S., Shah J., Weiland S., International Study of, A., Allergies in Childhood Phase Three Study, G Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64(6):476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- Meyer U., Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 2010;90(3):285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.J., Culpepper N., Rapaport M.H. C-reactive protein levels in schizophrenia. Clin. Schizophr. Relat. Psychoses. 2013:1–22. doi: 10.3371/CSRP.MICU.020813. [DOI] [PubMed] [Google Scholar]
- Moffatt M.F., Kabesch M., Liang L., Dixon A.L., Strachan D., Heath S., Depner M., von Berg A., Bufe A., Rietschel E., Heinzmann A., Simma B., Frischer T., Willis-Owen S.A., Wong K.C., Illig T., Vogelberg C., Weiland S.K., von Mutius E., Abecasis G.R., Farrall M., Gut I.G., Lathrop G.M., Cookson W.O. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- Mortensen P.B., Norgaard-Pedersen B., Waltoft B.L., Sorensen T.L., Hougaard D., Torrey E.F., Yolken R.H. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol. Psychiatry. 2007;61(5):688–693. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Mortensen P.B., Pedersen C.B., Hougaard D.M., Norgaard-Petersen B., Mors O., Borglum A.D., Yolken R.H. A Danish National Birth Cohort study of maternal HSV-2 antibodies as a risk factor for schizophrenia in their offspring. Schizophr. Res. 2010;122(1–3):257–263. doi: 10.1016/j.schres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Murray G.K., Jones P.B. Psychotic symptoms in young people without psychotic illness: mechanisms and meaning. Br. J. Psychiatry J. Ment. Sci. 2012;201(1):4–6. doi: 10.1192/bjp.bp.111.107789. [DOI] [PubMed] [Google Scholar]
- Pedersen M.S., Benros M.E., Agerbo E., Borglum A.D., Mortensen P.B. Schizophrenia in patients with atopic disorders with particular emphasis on asthma: a Danish population-based study. Schizophr. Res. 2012;138(1):58–62. doi: 10.1016/j.schres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Poulton R., Caspi A., Moffitt T.E., Cannon M., Murray R., Harrington H. Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch. Gen. Psychiatry. 2000;57(11):1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Rantakallio P., Jones P., Moring J., Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int. J. Epidemiol. 1997;26(4):837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- Rothermundt M., Arolt V., Bayer T.A. Review of immunological and immunopathological findings in schizophrenia. Brain Behav. Immun. 2001;15(4):319–339. doi: 10.1006/brbi.2001.0648. [DOI] [PubMed] [Google Scholar]
- Rottem M., Shoenfeld Y. Asthma as a paradigm for autoimmune disease. Int. Arch. Allergy Immunol. 2003;132(3):210–214. doi: 10.1159/000074301. [DOI] [PubMed] [Google Scholar]
- Rubio J.M., Sanjuan J., Florez-Salamanca L., Cuesta M.J. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr. Res. 2012;138(2–3):248–254. doi: 10.1016/j.schres.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Lucas C.P., Dulcan M.K., Schwab-Stone M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shi J., Levinson D.F., Duan J., Sanders A.R., Zheng Y., Pe'er I., Dudbridge F., Holmans P.A., Whittemore A.S., Mowry B.J., Olincy A., Amin F., Cloninger C.R., Silverman J.M., Buccola N.G., Byerley W.F., Black D.W., Crowe R.R., Oksenberg J.R., Mirel D.B., Kendler K.S., Freedman R., Gejman P.V. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460(7256):753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. Off. J. Soc. Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H., Ophoff R.A., Steinberg S., Andreassen O.A., Cichon S., Rujescu D., Werge T., Pietilainen O.P., Mors O., Mortensen P.B., Sigurdsson E., Gustafsson O., Nyegaard M., Tuulio-Henriksson A., Ingason A., Hansen T., Suvisaari J., Lonnqvist J., Paunio T., Borglum A.D., Hartmann A., Fink-Jensen A., Nordentoft M., Hougaard D., Norgaard-Pedersen B., Bottcher Y., Olesen J., Breuer R., Moller H.J., Giegling I., Rasmussen H.B., Timm S., Mattheisen M., Bitter I., Rethelyi J.M., Magnusdottir B.B., Sigmundsson T., Olason P., Masson G., Gulcher J.R., Haraldsson M., Fossdal R., Thorgeirsson T.E., Thorsteinsdottir U., Ruggeri M., Tosato S., Franke B., Strengman E., Kiemeney L.A., Melle I., Djurovic S., Abramova L., Kaleda V., Sanjuan J., de Frutos R., Bramon E., Vassos E., Fraser G., Ettinger U., Picchioni M., Walker N., Toulopoulou T., Need A.C., Ge D., Yoon J.L., Shianna K.V., Freimer N.B., Cantor R.M., Murray R., Kong A., Golimbet V., Carracedo A., Arango C., Costas J., Jonsson E.G., Terenius L., Agartz I., Petursson H., Nothen M.M., Rietschel M., Matthews P.M., Muglia P., Peltonen L., St Clair D., Goldstein D.B., Stefansson K., Collier D.A. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K., Harrison G., Zammit S., Lewis G., Horwood J., Heron J., Hollis C., Wolke D., Thompson A., Gunnell D. Association of measures of fetal and childhood growth with non-clinical psychotic symptoms in 12-year-olds: the ALSPAC cohort. Br. J. Psychiatry J. Ment. Sci. 2009;194(6):521–526. doi: 10.1192/bjp.bp.108.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey E.F., Bartko J.J., Yolken R.H. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr. Bull. 2012;38(3):642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J., Linscott R.J., Myin-Germeys I., Delespaul P., Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness–persistence–impairment model of psychotic disorder. Psychol. Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- Wang H., Yolken R.H., Hoekstra P.J., Burger H., Klein H.C. Antibodies to infectious agents and the positive symptom dimension of subclinical psychosis: the TRAILS study. Schizophr. Res. 2011;129(1):47–51. doi: 10.1016/j.schres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Weber N.S., Cowan D.N., Millikan A.M., Niebuhr D.W. Psychiatric and general medical conditions comorbid with schizophrenia in the National Hospital Discharge Survey. Psychiatr. Serv. 2009;60(8):1059–1067. doi: 10.1176/ps.2009.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Weizman R., Bessler H. CRC Press; 1999. Cytokines: Stress and Immunity. [Google Scholar]
- WHO . Psychiatric Publishers International/American Psychiatric Press Inc.; Geneva, Switzerland: 1994. SCAN: Schedules for Clinical Assessment in Neuropsychiatry Version 2.0. [Google Scholar]
- Zammit S., Odd D., Horwood J., Thompson A., Thomas K., Menezes P., Gunnell D., Hollis C., Wolke D., Lewis G., Harrison G. Investigating whether adverse prenatal and perinatal events are associated with non-clinical psychotic symptoms at age 12 years in the ALSPAC birth cohort. Psychol. Med. 2009;39(9):1457–1467. doi: 10.1017/S0033291708005126. [DOI] [PubMed] [Google Scholar]
- Zammit S., Kounali D., Cannon M., David A.S., Gunnell D., Heron J., Jones P.B., Lewis S., Sullivan S., Wolke D., Lewis G. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am. J. Psychiatry. 2013;170(7):742–750. doi: 10.1176/appi.ajp.2013.12060768. [DOI] [PubMed] [Google Scholar]
- Zandi M.S., Irani S.R., Lang B., Waters P., Jones P.B., McKenna P., Coles A.J., Vincent A., Lennox B.R. Disease-relevant autoantibodies in first episode schizophrenia. J. Neurol. 2011;258(4):686–688. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



