Abstract
Purpose
Melanoma is the most commonly fatal form of skin cancer, with nearly 50,000 annual deaths worldwide. We sought to assess long-term trends in the incidence and mortality of melanoma in a state with complete and consistent registration.
Methods
We used data from the Connecticut Tumor Registry, the original National Cancer Institute SEER site, to determine trends in invasive melanoma (1950-2007), in situ melanoma (1973-2007), tumor thickness (1993-2007), mortality (1950-2007), and mortality to incidence (1950-2007) among the 19,973 and 3,635 Connecticut residents diagnosed with invasive melanoma (1950-2007) and who died as a result of melanoma (1950-2007), respectively. Main outcome measures included trends in incidence and mortality by age, sex, and birth cohort.
Results
In the initial period (1950-1954), a diagnosis of invasive melanoma was rare, with 1.9 patient cases per 100,000 for men and 2.6 patient cases per 100,000 for women. Between 1950 and 2007, overall incidence rates rose more than 17-fold in men (1.9 to 33.5 per 100,000) and more than nine-fold in women (2.6 to 25.3 per 100,000). During these six decades, mortality rates more than tripled in men (1.6 to 4.9 per 100,000) and doubled in women (1.3 to 2.6 per 100,000). Mortality rates were generally stable or decreasing in men and women through age 54 years.
Conclusion
Unremitting increases in incidence and mortality of melanoma call for a nationally coordinated effort to encourage and promote innovative prevention and early-detection efforts.
INTRODUCTION
Melanoma has become an increasingly common cancer throughout much of the developed world.1–5 Prior analyses of Connecticut Tumor Registry (CTR) data for the period of 1950 to 1989 predicted overall increases in melanoma incidence throughout the 1990s. Earlier cohort analyses for the state of Connecticut from the period of 1950 to 1984 indicated a decline in mortality rates among women born in the 1930s and men born since the 1950s. Recent analysis of the US SEER registry (1992-2006) found increasing mortality rates among those age ≥ 65 years, with decreasing mortality rates for younger individuals.5
Assessing long-term trends in the incidence and mortality of melanoma in settings with complete and consistent registration is vital to evaluate the possible effects of prevention or early-detection efforts to plan for future health service requirements, such as workforce demands, and build cancer-control strategies to mitigate the toll of this once-rare cancer.
METHODS
The state of Connecticut has the longest-operating population-based cancer registry in the United States—the CTR—with cancer registrations dating back to 1935, nearly 40 years before the establishment of the US National Cancer Institute SEER program in 1973.6,7 The CTR provides sex-, age-, and time period–specific counts of malignant melanoma incidence, along with breakdowns of rates by anatomic subsite, histologic subtype, and tumor thickness (ie, Breslow's depth). It also provides Connecticut population counts supplemented by data obtained from the US Census.8 According to the 2010 census, Connecticut has a population of 3,574,097, which is an approximately 75% increase over the 1950 population of 2,007,280. Coding of melanoma can be found in the SEER coding and staging manuals.9
This analysis of time trends in melanoma rates included men and women age 20 to 84 years. We restricted the analysis to whites because they comprised 97% of all patient cases. Rates and counts were grouped into the following time periods for the analysis: 1950 to 1954, 1960 to 1964, and 1973 to 1977 (to coincide with start of SEER program in 1973), 1983 to 1987, 1993 to 1997, and 2003 to 2007. The last year of complete data on recorded patient cases and deaths was 2007 at the time of analysis. We used the total state population to calculate rates based on census data and present crude population rates stratified by sex and age.
We classified Breslow thickness categories as ≤ 1.00, 1.01 to 2.00, 2.01 to 4.00, > 4.00 mm, and unknown. Thickness distributions were compared by diagnosis period (1993-1997 v 2003-2007) and sex.
To analyze time trends and evaluate group differences for invasive melanoma incidence, in situ melanoma incidence, and mortality, we performed log-linear modeling using R and generalized linear models.10 We did not model anatomic site or thickness outcome because of missing data from earlier periods. We assumed that the observed number of patient cases followed a Poisson distribution. Data were fit to models containing terms for age, sex, and birth cohort (decade of birth). The models also contained population values that were used as an offset (to force coefficient to be 1). Model-checking methods included Akaike information criterion and likelihood ratio tests for nested models.11 The best-fitting models for each of the three outcomes included sex, age, birth cohort, and interaction between age and birth cohort. In each of the final Poisson regression models, the estimated main effects for age and birth cohort were statistically different from zero using t tests; the significance of the interaction term was tested using changes in the Akaike information. Sex was an important first-, second-, and third-order term; to simplify the interpretation of the regression models, we ran separate age, birth cohort, and age × birth cohort models with the male and female data. Predicted values from the age and birth cohort models were used to create fitted curves across age for birth cohort and time period (time period = cohort + age), showing trends in rates.
RESULTS
Invasive Melanoma
From 1950 to 2007, 19,973 malignant melanomas (excluding in situ tumors) were diagnosed in white Connecticut residents between the ages of 20 and 84 years. Over this entire period, 54.2% were diagnosed in men. However, profound temporal shifts by sex were evident; women comprised 58% of patient cases in 1950, whereas men comprised 56% by 2007. A shift in median age at diagnosis was also observed; the median age at diagnosis in 1950 was 53 years in men and 52 years in women, and in 2007, it was 65 years in men and 58 years in women.
In the initial period (1950-1954), a diagnosis of invasive melanoma was rare, with 1.9 patient cases per 100,000 for men and 2.6 per 100,000 for women (Table 1; Fig 1). Between 1950 and 2007, the overall incidence rates rose more than 17-fold in men (1.9 to 33.5 per 100,000; Table 1) and more than nine-fold in women (2.6 to 25.3 per 100,000). The average annual increase in melanoma incidence over this period was 0.6 per 100,000 in men and 0.4 per 100,000 in women. The observed rate of increase rose steadily over the years until the last decade, when the increase accelerated to 0.8 per 100,000 per year in men and 0.6 per 100,000 per year in women.
Table 1.
Invasive Melanoma, In Situ Incidence, and Melanoma Mortality in Connecticut Tumor Registry, 1950 to 2007
| Incidence | Rates per 100,000 by Age (years) |
Counts by Age (years) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20-24 | 25-29 | 30-34 | 35-39 | 40-44 | 45-49 | 50-54 | 55-59 | 60-64 | 65-69 | 70-74 | 75-79 | 80-84 | Overall | < 50 | ≥ 50 | |
| Invasive melanoma | ||||||||||||||||
| Men | ||||||||||||||||
| 1950-1954 | 1.7 | 1.3 | 2.8 | 3.4 | 2.4 | 2.1 | 3.0 | 2.2 | 3.9 | 2.2 | 4.9 | 6.9 | 9.6 | 1.9 | 53 | 43 |
| 1960-1964 | 1.9 | 3.1 | 3.7 | 5.4 | 7.6 | 7.7 | 5.7 | 7.8 | 6.3 | 4.7 | 11.3 | 9.4 | 16.8 | 3.8 | 128 | 106 |
| 1973-1977 | 2.9 | 3.1 | 6.6 | 8.0 | 13.7 | 15.9 | 21.0 | 16.5 | 18.0 | 25.8 | 22.8 | 31.0 | 24.3 | 8.6 | 221 | 363 |
| 1983-1987 | 3.3 | 3.1 | 6.6 | 12.5 | 18.2 | 19.0 | 26.9 | 31.4 | 42.2 | 43.4 | 49.3 | 48.7 | 76.2 | 15.1 | 316 | 725 |
| 1993-1997 | 2.3 | 3.1 | 7.4 | 12.2 | 21.2 | 26.3 | 38.0 | 50.4 | 68.6 | 86.8 | 98.0 | 106.7 | 102.9 | 25.5 | 418 | 1311 |
| 2003-2007 | 4.2 | 3.1 | 9.4 | 12.7 | 20.6 | 31.3 | 35.8 | 50.8 | 81.6 | 100.2 | 123.7 | 174.2 | 176.4 | 33.5 | 473 | 1810 |
| Women | ||||||||||||||||
| 1950-1954 | 1.1 | 2.8 | 3.4 | 1.4 | 4.4 | 3.3 | 1.9 | 4.6 | 3.7 | 7.5 | 9.7 | 8.4 | 5.8 | 2.6 | 65 | 68 |
| 1960-1964 | 2.8 | 2.7 | 3.8 | 6.6 | 6.2 | 5.0 | 6.3 | 4.6 | 5.2 | 7.4 | 7.5 | 10.6 | 5.3 | 3.6 | 123 | 106 |
| 1973-1977 | 3.4 | 5.4 | 7.3 | 9.4 | 12.4 | 13.2 | 14.2 | 11.2 | 9.7 | 14.3 | 12.2 | 15.7 | 17.9 | 7.3 | 232 | 276 |
| 1983-1987 | 2.7 | 8.9 | 11.9 | 12.5 | 15.1 | 21.2 | 24.5 | 23.3 | 24.6 | 19.2 | 21.1 | 19.5 | 26.4 | 12.3 | 372 | 504 |
| 1993-1997 | 3.6 | 9.9 | 13.6 | 20.9 | 25.4 | 23.4 | 24.0 | 35.1 | 37.4 | 35.7 | 39.8 | 37.6 | 48.1 | 19.4 | 561 | 794 |
| 2003-2007 | 7.1 | 15.0 | 15.3 | 18.1 | 25.5 | 33.6 | 32.0 | 39.8 | 41.3 | 49.8 | 64.0 | 64.0 | 60.4 | 25.3 | 631 | 1158 |
| In situ melanoma | ||||||||||||||||
| Men | ||||||||||||||||
| 1973-1977 | 0.0 | 1.3 | 0.0 | 0.5 | 0.3 | 0.7 | 0.4 | 1.3 | 0.9 | 0.8 | 1.9 | 0.0 | 1.5 | 0.4 | 8 | 16 |
| 1983-1987 | 0.0 | 2.0 | 0.3 | 0.3 | 1.9 | 2.3 | 2.0 | 3.7 | 6.1 | 8.0 | 5.1 | 9.3 | 10.5 | 1.7 | 22 | 99 |
| 1993-1997 | 0.3 | 5.4 | 1.6 | 2.0 | 6.6 | 7.9 | 14.1 | 21.4 | 30.4 | 42.1 | 52.7 | 55.5 | 52.4 | 11.0 | 109 | 622 |
| 2003-2007 | 1.1 | 2.8 | 2.3 | 6.8 | 8.6 | 16.6 | 24.0 | 33.6 | 51.8 | 69.5 | 103.9 | 131.5 | 149.6 | 22.8 | 209 | 1316 |
| Women | ||||||||||||||||
| 1973-1977 | 0.4 | 0.2 | 0.2 | 0.7 | 0.7 | 0.7 | 0.6 | 1.0 | 0.3 | 0.3 | 1.3 | 1.1 | 0.7 | 0.4 | 13 | 15 |
| 1983-1987 | 0.5 | 0.6 | 1.0 | 1.4 | 1.9 | 1.6 | 3.2 | 1.2 | 3.8 | 5.7 | 2.6 | 2.6 | 7.1 | 1.6 | 36 | 78 |
| 1993-1997 | 0.7 | 8.2 | 4.2 | 6.1 | 10.3 | 9.8 | 13.9 | 17.1 | 17.7 | 16.7 | 21.5 | 25.7 | 14.3 | 8.4 | 198 | 403 |
| 2003-2007 | 3.0 | 4.7 | 7.6 | 13.7 | 18.8 | 22.0 | 26.3 | 31.0 | 40.2 | 53.9 | 50.0 | 54.7 | 52.9 | 19.5 | 400 | 1005 |
| Melanoma mortality | ||||||||||||||||
| Men | ||||||||||||||||
| 1950-1954 | 0.3 | 0.5 | 1.2 | 1.9 | 0.8 | 2.1 | 0.7 | 1.5 | 3.9 | 2.2 | 4.1 | 6.9 | 1.6 | 26 | 30 | |
| 1960-1964 | 0.3 | 0.8 | 0.7 | 2.8 | 1.7 | 2.1 | 4.3 | 4.9 | 3.9 | 1.9 | 6.9 | 9.4 | 2.6 | 37 | 66 | |
| 1973-1977 | 0.0 | 1.5 | 1.5 | 1.8 | 2.9 | 2.9 | 5.1 | 6.9 | 6.8 | 7.6 | 9.3 | 9.4 | 10.0 | 2.5 | 46 | 126 |
| 1983-1987 | 0.0 | 1.0 | 1.4 | 1.8 | 2.9 | 2.9 | 7.4 | 9.3 | 11.2 | 11.1 | 15.8 | 9.3 | 19.7 | 3.5 | 58 | 193 |
| 1993-1997 | 0.0 | 0.0 | 1.0 | 1.6 | 1.4 | 2.7 | 4.2 | 8.7 | 9.0 | 12.7 | 13.6 | 24.8 | 22.6 | 3.8 | 44 | 215 |
| 2003-2007 | 0.0 | 0.0 | 0.0 | 0.0 | 1.8 | 2.2 | 3.3 | 7.6 | 9.4 | 14.5 | 25.1 | 32.2 | 35.2 | 4.9 | 32 | 276 |
| Women | ||||||||||||||||
| 1950-1954 | 0.3 | 0.9 | 0.7 | 1.2 | 0.8 | 1.2 | 1.0 | 2.5 | 2.5 | 4.5 | 2.8 | 1.1 | 1.3 | 20 | 30 | |
| 1960-1964 | 0.0 | 1.0 | 1.1 | 1.9 | 1.2 | 0.9 | 1.6 | 1.2 | 3.8 | 3.1 | 5.0 | 3.8 | 1.7 | 28 | 44 | |
| 1973-1977 | 0.0 | 0.0 | 1.7 | 2.5 | 2.0 | 3.4 | 2.8 | 2.8 | 3.6 | 5.6 | 4.6 | 4.9 | 5.2 | 1.8 | 41 | 82 |
| 1983-1987 | 0.0 | 0.0 | 1.0 | 1.8 | 1.6 | 4.1 | 2.2 | 5.4 | 4.2 | 6.8 | 6.7 | 4.8 | 6.4 | 2.4 | 43 | 115 |
| 1993-1997 | 0.0 | 0.0 | 0.0 | 1.2 | 2.0 | 1.7 | 2.5 | 5.3 | 4.7 | 3.9 | 6.5 | 10.4 | 14.2 | 2.5 | 35 | 138 |
| 2003-2007 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.8 | 2.0 | 3.8 | 4.4 | 4.8 | 9.3 | 10.1 | 8.3 | 2.6 | 28 | 137 |
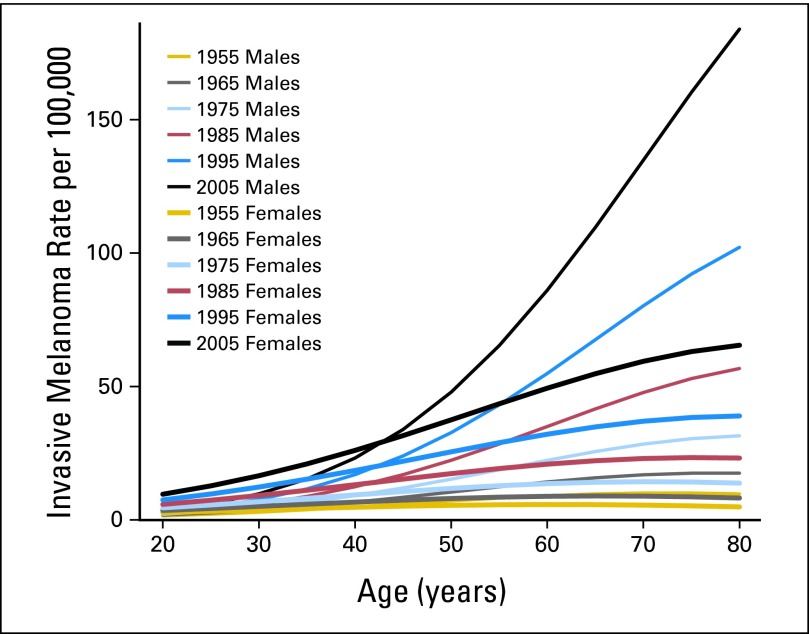
Fig 1.
Invasive melanoma rates in Connecticut Tumor Registry from 1950 to 2007 by sex, age, and time period (estimates from Poisson regression model). Time periods centered on mid-decade year; note that first two time periods actually straddle second year of decade.
This trend was particularly pronounced in middle-age and older people. After age 50 years, rates for men in all age groups increased more than 20-fold, highlighted by 45-fold increases for men age 65 to 69 years. From the 1950s to 1970s, incidence rates were relatively consistent for younger versus older individuals. By the mid 1980s, a shift toward onset at middle and older age began, particularly for men. By 2003 to 2007, diagnoses among men age > 50 years were nearly 4× greater than those among younger men (Table 1; Fig 1).
The overall acceleration in rate increases over the most recent 10 years is explained largely by increases at the oldest and youngest ages. Incidence rates for many younger-age subgroups (age 30-59 years) have stabilized, whereas elderly men and women (age 75-79 years) experienced rate increases of 64% and 72%, respectively. Compared with young men (age 20-29 years), incidence rates rose sharply for young women (age 20-29 years), nearly doubling between 1993 to 1997 and 2003 to 2007. In 1950, rates in men exceeded those in women at approximately age > 34 years, whereas in 2007, rates in men first exceed rates in women at age 50-54 years.
In modeling incidence, best results were obtained from models containing age and sex interactions. Time trends were significant in both sexes and in all age groups except the youngest (age < 35 years). Slopes for time trends increased with age consistently, except for a somewhat steeper slope for men age 65 to 70 years.
Birth cohort analysis (Fig 2) clearly showed the trends in melanoma incidence rates in the CTR data. The expected pattern of increasing incidence with age was observed for all patients across all birth cohorts, but there were also progressive increases at all ages with increasing decade of birth. The increases were largest at older ages and in the more recent birth cohorts. There was no apparent decrease or even plateauing in the rates for the most recent birth cohorts. Although there were only modest increases or even slight decreases in a few age groups (Table 1), invasive melanoma rates among those age < 40 years continued to increase in the most recent time periods, particularly among women.
Fig 2.
Invasive melanoma rates in Connecticut Tumor Registry from 1890 to 1970 by sex, age, and birth cohort (estimates from Poisson regression model). Time periods centered on mid-decade year, with birth cohorts centered on decade.
In Situ Melanoma
Ascertainment of in situ melanoma first became available in 1973. The rate of increase in incidence of in situ melanoma increased over time, with the greatest increase in the most recent decade. For those age < 50 years, the ratio of in situ melanoma to invasive melanoma rose from 0.36 to 0.66 in men and from 0.54 to 1.05 in women (Table 1).
Anatomic Site
From the mid 1970s to the most recent period, the proportion of melanomas occurring on the head and neck and upper limbs and shoulders increased slightly, whereas the proportion of melanomas on the trunk remained essentially the same (Table 2).
Table 2.
Distribution of Melanoma Characteristics by Diagnosis Period*
| Characteristic | 1950-1954 |
1960-1964 |
1973-1977 |
1983-1987 |
1993-1997 |
2003-2007 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Behavior | ||||||||||||
| In situ | — | — | 52 | 4.5 | 235 | 10.9 | 1,332 | 30.2 | 2,930 | 30.9 | ||
| Invasive | 229 | 100.0 | 463 | 100.0 | 1,092 | 95.5 | 1,917 | 89.1 | 3,084 | 69.8 | 4,072 | 69.1 |
| Sex | ||||||||||||
| Female | 133 | 58.1 | 229 | 49.5 | 508 | 46.5 | 876 | 45.7 | 1,355 | 43.9 | 1,789 | 44.5 |
| Male | 96 | 41.9 | 234 | 50.5 | 584 | 53.5 | 1,041 | 54.3 | 1,729 | 56.1 | 2,283 | 55.5 |
| Age at diagnosis, years | ||||||||||||
| < 50 | 118 | 51.5 | 251 | 54.2 | 453 | 41.5 | 688 | 35.9 | 979 | 31.7 | 1,104 | 27.1 |
| ≥ 50 | 111 | 48.5 | 212 | 45.8 | 639 | 58.5 | 1,229 | 64.1 | 2,105 | 68.3 | 2,968 | 72.9 |
| Anatomic site | ||||||||||||
| C44.0-C44.4, head and neck | — | — | 169 | 15.5 | 317 | 16.5 | 523 | 17.0 | 738 | 18.1 | ||
| C44.5, skin of trunk | — | — | 378 | 34.6 | 688 | 35.9 | 1,149 | 37.3 | 1,388 | 34.1 | ||
| C44.6, skin of upper limb and shoulder | — | — | 237 | 21.7 | 403 | 21.0 | 707 | 22.9 | 993 | 24.4 | ||
| C44.7, skin of lower limb and hip | — | — | 232 | 21.2 | 408 | 21.3 | 578 | 18.7 | 818 | 20.1 | ||
| C44.8-C44.9, overlapping and skin, NOS | — | — | 76 | 7.0 | 101 | 5.3 | 127 | 4.1 | 135 | 3.3 | ||
| Breslow thickness, mm | ||||||||||||
| ≤ 1 | — | — | — | — | 1,870 | 60.6 | 2,722 | 66.8 | ||||
| 1.01-2 | — | — | — | — | 377 | 12.2 | 493 | 12.1 | ||||
| 2.01-4 | — | — | — | — | 223 | 7.2 | 258 | 6.3 | ||||
| > 4 | — | — | — | — | 108 | 3.5 | 153 | 3.8 | ||||
| No mass/tumor found | — | — | — | — | 3 | 0.1 | 36 | 0.9 | ||||
| Unknown/not stated/microinvasion | — | — | — | — | 503 | 16.3 | 410 | 10.1 | ||||
Abbreviation: NOS, not otherwise specified.
For melanomas diagnosed in white Connecticut residents age 20 to 84 years. All characteristics except behavior refer to invasive melanoma only.
Tumor Thickness
The proportion of melanomas diagnosed at ≥ 2.01 mm differed between 2003 to 2007 (11.3%) and 1993 to 1997 (12.8%) when excluding melanomas with unknown thickness and tumors with no mass or tumor found. Thinner melanomas (< 1 mm) were more common in the most recent period when including (67.6% v 61%) and when excluding melanomas with unknown thickness (75.1% v 72.7%; Table 2).
Mortality
During the study period, 3,635 white Connecticut residents died as a result of melanoma. Melanoma deaths were more common in men (58%) than women (42%) and increased more steeply over time in men. Although women had steady increases of approximately 0.01 to 0.02 per 100,000 per year, melanoma mortality in men increased by 0.05 to 0.06 per 100,000 per year until 1993, when it accelerated to 0.11 per 100,000 per year. Overall, from the period 1950 to 1954 to the period of 2003 to 2007, mortality rates doubled in women (1.3 to 2.6 per 100,000) and tripled in men (1.6 to 4.9 per 100,000). The median age of melanoma death changed from 55 years in men and 58 years in women in 1950 to 1954 to 72 years in men and 70.5 years in women in 2003 to 2007.
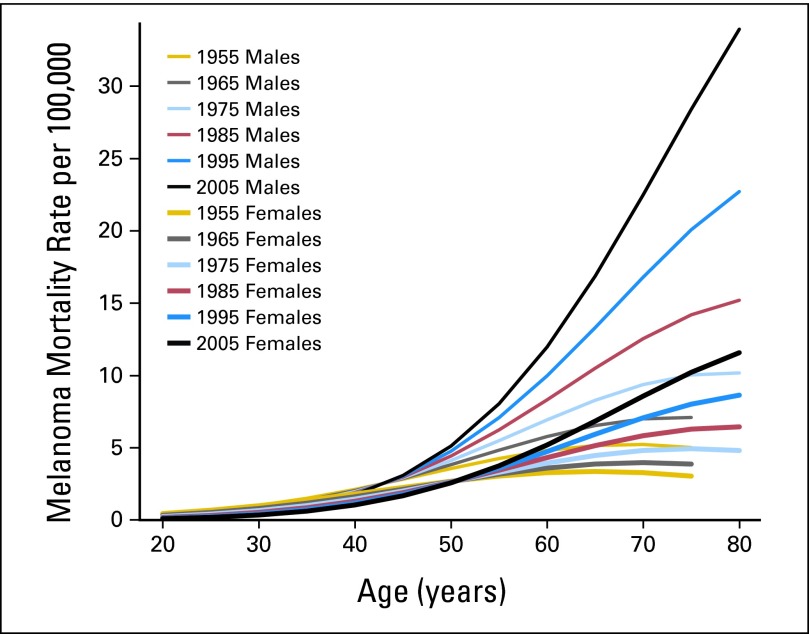
These mortality trends across > 50 years were not uniform across age groups, with mortality rates generally stable or dropping in men and women below age 55 years (Fig 3). Conversely, above these ages, mortality rates increased over time, particularly among men age > 65 years, who had a six-fold increase in melanoma mortality.
Fig 3.
Melanoma rates in Connecticut Tumor Registry from 1950 to 2007 by sex, age, and time period (estimates from Poisson regression model). Time periods centered on mid-decade year, with birth cohorts centered on decade.
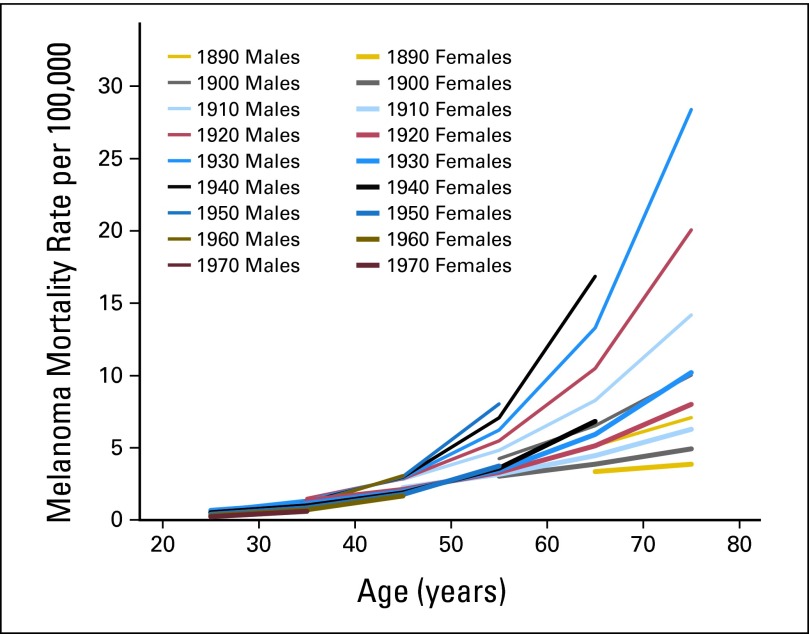
The birth cohort analysis (Fig 4) showed that with each successive cohort from 1910 to 1950, mortality rates rose for men and women age > 50 years, with the largest increases found for men. These increases in risk by birth cohort were particularly steep at older ages. For example, for men age 55 years, there was approximately a two-fold increase between 1900 and 1950, whereas for men age 65 years, there was approximately a three-fold increase (1890 to 1940). Below 50 years of age, mortality rates increased with birth cohort for those born before 1950 but plateaued or decreased slightly for those born in 1950 or later.
Fig 4.
Melanoma rates in Connecticut Tumor Registry from 1890 to 1970 by sex, age, and birth cohort (estimates from Poisson regression model). Time periods centered on mid-decade year, with birth cohorts centered on decade.
From 2003 to 2007, not one death was recorded for women age < 45 or for men age < 40 years. This compares with 40 deaths in women and 36 deaths in men for these age groups in the first two time periods (1950-1954 and 1960-1964 combined).
Mortality-Incidence Ratios
The ratio of melanoma deaths to patient cases decreased steadily over the nearly 60 years. During the period of 1950 to 1954, there was one death for every 1.5 patient cases, decreasing to one death for every 7.8 patient cases during 2003 to 2007. In the most recent period, for men age 70 to 74 years, there was one death per 4.9 patient cases and one death per 5.4 patient cases for men age 75 to 79 years. For men age 45 to 49 years, there was one death per 14.2 patient cases and one death per 12.0 patient cases for women in the same age group (data not shown).
DISCUSSION
With nearly 60 years of data from the state with the longest-running population-based cancer registry, we have described the evolution of a cancer from its relative insignificance to one with major public health importance. Incidence rates in white Connecticut residents have risen nearly 2,000% since 1950; the population of the state has increased by 75%. Mortality rates tripled in men during these six decades and doubled in women.
Three major points emerge. First, there has been a marked shift from a cancer striking younger individuals to one that disproportionately burdens individuals age > 50 years; however, most recent data indicate a resurgence of melanoma in the youngest women.12 Second, the ratio of deaths to patient cases has dropped markedly. Third, examining successive birth cohorts, from 1910 to 1945, mortality rates begin to rise at age 45 years for women and age 50 years for men and increase precipitously by age and time period; moderation of this increase appears only for those born in 1950 or later. Mortality rate increases are particularly steep for men; for example, for men born in 1930, mortality rates exceed 30 deaths per 100,000.
Incidence rates for most age groups continue to increase, including the youngest age groups, and there is little evidence to support a projected drop in melanoma incidence in Connecticut as the youngest birth cohorts age. In fact, increases in incidence were observed for the youngest residents, particularly in women, and these differences by sex may be related to new trends in sun exposure or use of tanning beds.13–16 Increases were also particularly high for elderly residents, who likely used little sun protection in their childhood and early adult years. Of most concern, mortality continues to rise in men starting at age 65 and in women at age 70 years, with little evidence of stabilization.
For the time period covered by both SEER and Connecticut data (1973-2007), strikingly similar patterns emerge: notably sharply rising incidence rates, a shift in the burden of disease from younger to older populations, and an overall mortality rate that has only begun to stabilize.7 Generally, results presented herein are similar to those found in nearly all analyses of melanoma trends in Europe and Australia.1,4 For this analysis, we used CTR data for many reasons. An enhanced historical perspective can be achieved with more than 20 years of additional data from 1950 to 1972; these extra years are invaluable in examining various cohorts as they navigated through historical and cultural events, such as overseas wars exposing fair-skinned populations to intermittent bursts of ultraviolet exposure16 and changes in clothing habits and recreational and workplace exposures that may have influenced both sun protection habits, exposure, and rates of disease. As one of the original SEER registries, Connecticut has had a long-standing commitment to retrieving patient cases from among Connecticut residents diagnosed in nearby states and to ensuring that dermatopathology laboratories report patient cases to the state registry.
Although there has undoubtedly been improved reporting of melanoma of the skin in Connecticut as well as in the SEER program areas beginning in 1973, we are confident that the overall trends in both incidence and mortality are real. Improved reporting in the last few decades cannot explain the consistent overall trends beginning from the earliest time periods for which data are available.
Going forward, these results clearly recommend strengthening and promoting the two central foci of melanoma control: primary prevention and early detection of melanoma.17,18 Reasonably high numbers of cases in young people, particularly young women, speak to the need for comprehensive, multisite community-based sun protection programs in schools, recreational sites, and pediatricians' offices.19,20 Such programs should advocate for adoption of sun protection policies as well as avoidance of and restrictions to tanning bed use for youth. Identification of populations at highest risk for thick, late-stage melanoma is crucial and can also be led and inspired by community-based programs and public health department initiatives that target messages for the prompt and early detection of melanoma.
There is room for cautious optimism about these programs because the overall mortality rate has stabilized for women since the mid 1980s in Connecticut and elsewhere in the United States.6 Moreover, mortality rates for Connecticut men and women between 20 and 50 years of age have been dropping since the 1970s. Conversely, Connecticut data strongly reinforce the fact that middle-age and older men have not shared these encouraging trends and suffer a disproportionate burden of melanoma deaths. The Institute of Medicine has recommended that physicians pay particular attention to the skin of older men,21 and public education efforts have urged middle-age and older men to ask their significant others and physicians to examine hard-to-see areas.22
There is the possibility that some component of the increase in incidence may be attributed to improved registration of melanomas that would not have been recorded earlier. An upswing in the diagnosis of in situ disease may be attributed to both its recognition and classification as a separate entity than to severely dysplastic nevi or severely atypical melanocytic hyperplasia. Differential classification over time is of concern, but it is likely more so for melanoma in situ and is less likely to be affecting the ratio of invasive cases to deaths.23 There is also the possibility that some melanomas diagnosed in Connecticut more recently were subject to the overdiagnosis of melanoma that has been reported elsewhere.24,25 If this is the case, and if it has occurred in the more recent period, the use of a mortality-to-incidence ratio that depends on an accurate historical and current estimate of incidence may be affected. The fact that mortality rates and incidence of melanoma > 2 mm are stable in Connecticut and elsewhere6 provides some evidence against a disease marked primarily by the overdiagnosis or surveillance bias that has been observed elsewhere.24 On the basis of pathology from the United States and other countries and covering the 50-year period from 1930 to 1980, one review concluded that “these findings argue against changes in histological appearance as being responsible for more than a small portion of the continuous increase of some 3% to 8% per annum observed in malignant melanoma incidence.”26p483 Uniform coding of Breslow thickness has only been available since 1988; hence, examination of change in Breslow thickness over time was limited to the two most recent study periods. Finally, there seems to be strong concordance for the reporting of melanoma as a cause of death; validation of cause of death certification was 93%.27
In conclusion, in Connecticut, a state with exemplary recording of invasive melanoma, in situ disease, and deaths, mortality rates for men begin to rise at age 55 years, with the largest increases for men age > 65 years. In light of the recent reduction of 40% in melanoma deaths in a large screening program in Germany,28 large-scale educational and screening campaigns targeting high-risk populations in the United States must be implemented to drive down the incidence and mortality of this preventable cancer.
Footnotes
Processed as a Rapid Communication manuscript
The Connecticut Tumor Registry is supported by Contract No. HHSN261201000024C between the National Cancer Institute and State of Connecticut Department of Public Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Arthur J. Sober, MelaSciences Expert Testimony: Arthur J. Sober, various law firms (C) Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Alan C. Geller, Richard W. Clapp, Lou Gonsalves
Collection and assembly of data: Lou Gonsalves, Lloyd Mueller
Data analysis and interpretation: Alan C. Geller, Richard W. Clapp, Arthur J. Sober, Lou Gonsalves, Cindy L. Christiansen, Waqas Shaikh, Donald R. Miller
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Bulliard JL, Cox B. Cutaneous malignant melanoma in New Zealand: Trends by anatomical site, 1969-1993. Int J Epidemiol. 2000;29:416–423. [PubMed] [Google Scholar]
- 2.Geller AC, Miller DR, Annas GD, et al. Melanoma incidence and mortality among US whites, 1969-1999. JAMA. 2002;288:1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 3.Coory M, Baade P, Aitken J, et al. Trends for in situ and invasive melanoma in Queensland, Australia, 1982-2002. Cancer Causes Control. 2006;17:21–27. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- 4.de Vries E, Bray FI, Coebergh JW, et al. Changing epidemiology of malignant cutaneous melanoma in Europe 1953-1997: Rising trends in incidence and mortality but recent stabilizations in Western Europe and decreases in Scandinavia. Int J Cancer. 2003;107:119–126. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(suppl 1):S17–S25. e1–e3. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Roush GC, McKay L, Holford TR. A reversal in the long-term increase in deaths attributable to malignant melanoma. Cancer. 1992;69:1714–1720. doi: 10.1002/1097-0142(19920401)69:7<1714::aid-cncr2820690712>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Heston JF, Kelly JAB, Meigs JW, et al. Bethesda, MD: National Cancer Institute; 1986. Forty-Five Years of Cancer Incidence in Connecticut 1935-79: Monograph No. 70. [PubMed] [Google Scholar]
- 8.US Department of Commerce. US Census Bureau: Data access tools. http://www.census.gov/main/www/access.html.
- 9.National Cancer Institute. Surveillance, Epidemiology, and End Results: SEER coding and staging manuals. http://www.seer.cancer.gov/tools/codingmanuals/
- 10.McCullagh P, Nelder J. Generalized Linear Models. ed 2. Boca Raton, FL: Chapman and Hall; 1989. [Google Scholar]
- 11.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 12.Purdue MP, Freeman LE, Anderson WF, et al. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Héry C, Tryggvadóttir L, Sigurdsson T, et al. A melanoma epidemic in Iceland: Possible influence of sunbed use. Am J Epidemiol. 2010;172:762–767. doi: 10.1093/aje/kwq238. [DOI] [PubMed] [Google Scholar]
- 14.Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425–2435. doi: 10.1002/ijc.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: A case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;196:1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 17.Emmons KM, Colditz GA. Preventing excess sun exposure: It is time for a national policy. J Natl Cancer Inst. 1999;91:1269–1270. doi: 10.1093/jnci/91.15.1269. [DOI] [PubMed] [Google Scholar]
- 18.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich AJ, Olson AL, Sox CH, et al. Persistent increase in children's sun protection in a randomized controlled community trial. Prev Med. 2000;31:569–574. doi: 10.1006/pmed.2000.0746. [DOI] [PubMed] [Google Scholar]
- 20.Miller DR, Geller AC, Wood MC, et al. The Falmouth Safe Skin Project: Evaluation of a community program to promote sun protection in youth. Health Educ Behav. 1999;26:369–384. doi: 10.1177/109019819902600307. [DOI] [PubMed] [Google Scholar]
- 21.Field MJ, Lawrence RL, Zwanziger L, editors. Washington, DC: National Academies Press; 2000. Extending Medicare Coverage for Preventive and Other Services. [PubMed] [Google Scholar]
- 22.Geller AC, Miller DR, Swetter SM, et al. A call for the development and implementation of a targeted national melanoma screening program. Arch Dermatol. 2006;142:504–507. doi: 10.1001/archderm.142.4.504. [DOI] [PubMed] [Google Scholar]
- 23.Frangos JE, Duncan LM, Piris A, et al. Increased diagnosis of thin superficial spreading melanomas: A 20-year study. J Am Acad Dermatol. 2012;67:387–394. doi: 10.1016/j.jaad.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: Population based ecological study. BMJ. 2005;331:481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swerlick RA, Chen S. The melanoma epidemic: More apparent than real? Mayo Clin Proc. 1997;72:559–564. doi: 10.4065/72.6.559. [DOI] [PubMed] [Google Scholar]
- 26.van der Esch EP, Muir CS, Nectoux J, et al. Temporal change in diagnostic criteria as a cause of the increase of malignant melanoma over time is unlikely. Int J Cancer. 1991;47:483–489. doi: 10.1002/ijc.2910470402. [DOI] [PubMed] [Google Scholar]
- 27.Weinstock MA, Reynes JF. Validation of cause-of-death certification for outpatient cancers: The contrasting cases of melanoma and mycoides fungoides. Am J Epidemiol. 1998;148:1184–1186. doi: 10.1093/oxfordjournals.aje.a009607. [DOI] [PubMed] [Google Scholar]
- 28.Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? An observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395–5402. doi: 10.1002/cncr.27566. [DOI] [PubMed] [Google Scholar]