Abstract
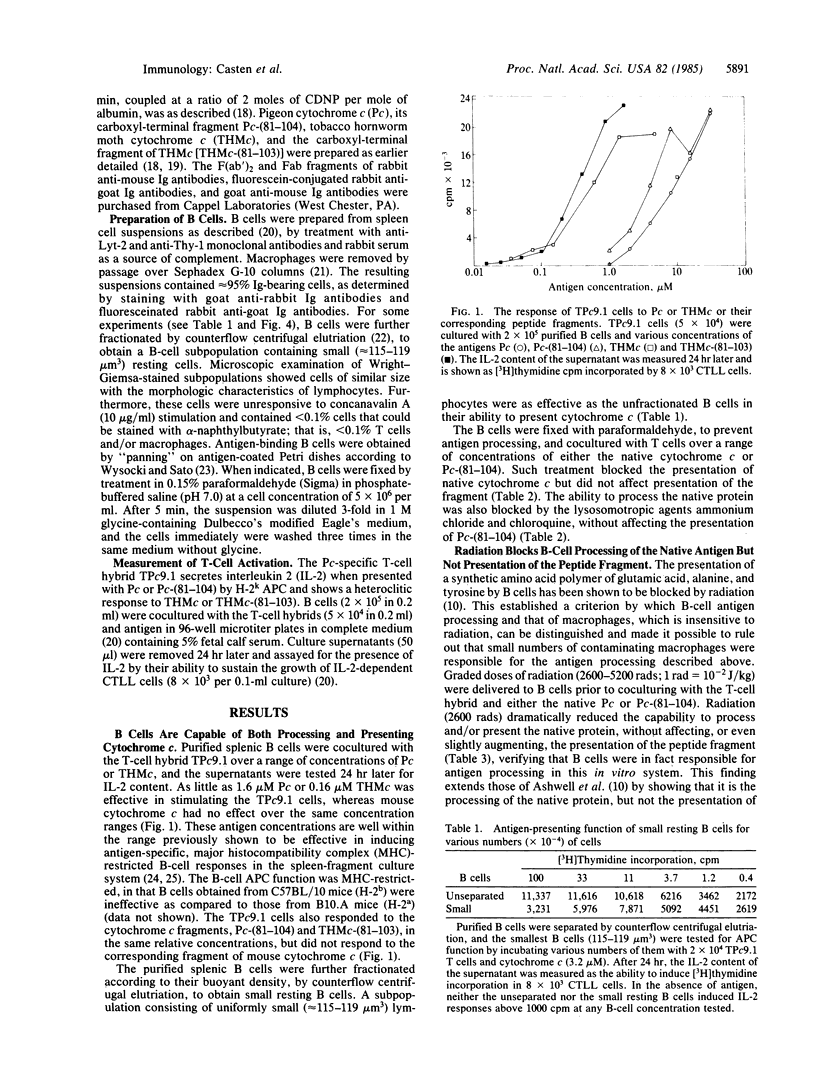
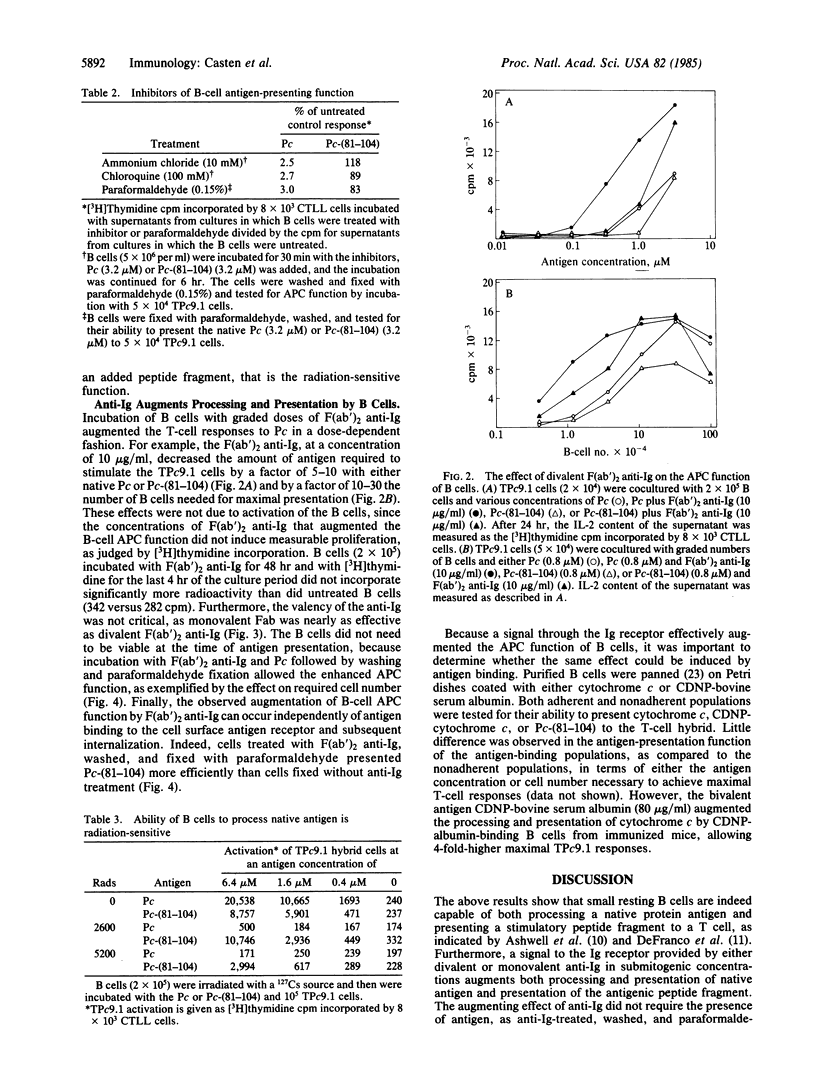
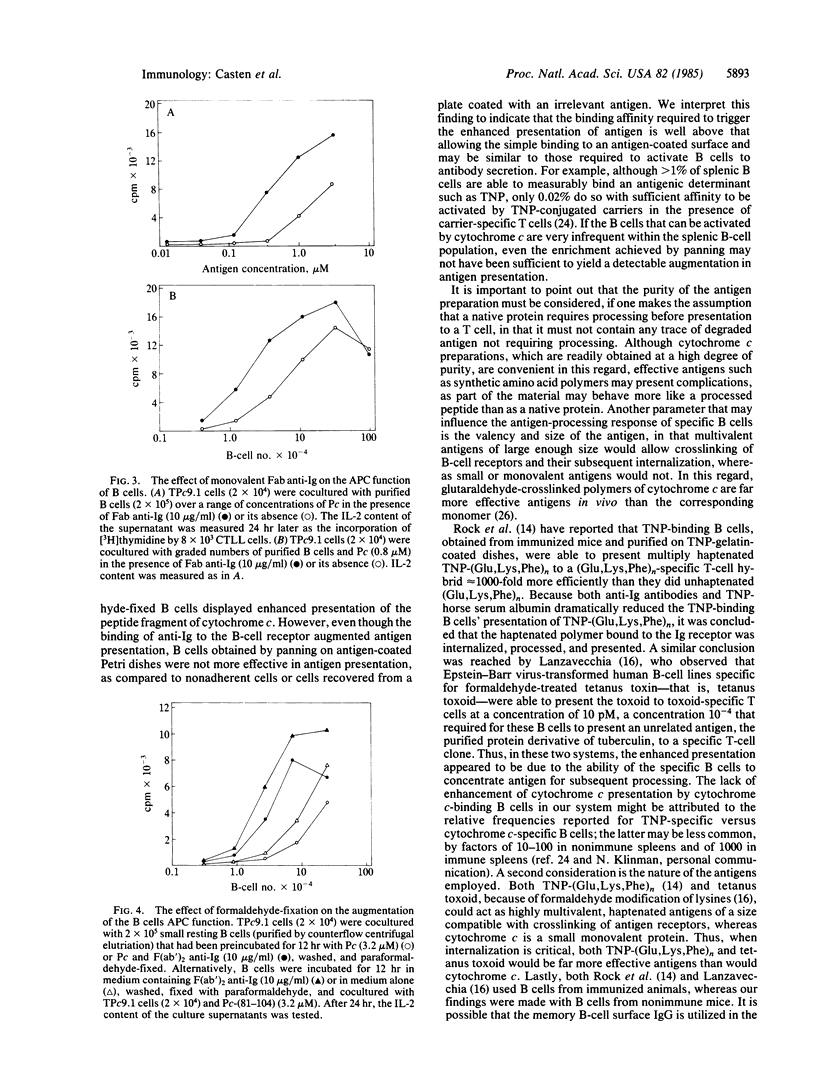
All mouse splenic B cells, including small resting B cells, process and present the native globular protein antigens, pigeon and tobacco hornworm moth cytochromes c, to a cytochrome c-specific T-cell hybrid in a major histocompatibility complex-restricted fashion, in the micromolar to nanomolar antigen-concentration range. As is the case for macrophages, treatment with paraformaldehyde or the lysosomotropic agents chloroquine and ammonium chloride blocked processing of the native pigeon protein but did not affect the presentation of a carboxyl-terminal peptide fragment of pigeon cytochrome c (residues 81-104) which contained the T-cell antigenic determinant. However, in contrast to macrophages, whose antigen-processing and -presentation functions are insensitive to radiation, radiation blocked the processing of the native protein but not the presentation of the peptide fragment. The processing and presentation function of the B cells was augmented by F(ab')2 of rabbit anti-mouse Ig antibodies, in that 1/10th to 1/30th as many cells and 1/10th as much antigen were required to maximally activate the T-cell hybrid. This augmentation did not appear to be due to either crosslinking of the Ig receptors or to B-cell activation, as monovalent Fab fragments were nearly as effective as the bivalent reagent, and the concentrations of F(ab')2 anti-Ig used did not induce measurable proliferative responses. Furthermore, enhancement can occur in the absence of cytochrome c binding and internalization, since B cells that were fixed with paraformaldehyde after treatment with F(ab')2 anti-Ig were more effective in presenting the carboxyl-terminal peptide than were untreated fixed cells. The same phenomenon followed the binding of an irrelevant antigen (carboxydinitrophenylated bovine serum albumin) by antigen-binding B cells, resulting in enhanced processing and/or presentation of native pigeon cytochrome c. Thus, nonspecific enhancement of antigen processing and presentation can be obtained by either antigen or anti-Ig binding to the B-cell antigen receptor, both treatments presumably delivering the same signal without requiring internalization of the specifically bound antigen for subsequent processing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., DeFranco A. L., Paul W. E., Schwartz R. H. Antigen presentation by resting B cells. Radiosensitivity of the antigen-presentation function and two distinct pathways of T cell activation. J Exp Med. 1984 Mar 1;159(3):881–905. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–164. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- DeFranco A. L., Ashwell J. D., Schwartz R. H., Paul W. E. Polyclonal stimulation of resting B lymphocytes by antigen-specific T lymphocytes. J Exp Med. 1984 Mar 1;159(3):861–880. doi: 10.1084/jem.159.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M., Cowing C. Presentation of antigen by B cells: functional dependence on radiation dose, interleukins, cellular activation, and differential glycosylation. J Immunol. 1985 Apr;134(4):2269–2275. [PubMed] [Google Scholar]
- Glimcher L. H., Kim K. J., Green I., Paul W. E. Ia antigen-bearing B cell tumor lines can present protein antigen and alloantigen in a major histocompatibility complex-restricted fashion to antigen-reactive T cells. J Exp Med. 1982 Feb 1;155(2):445–459. doi: 10.1084/jem.155.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. R., Kindle C. S., Abruzzini A. F., Pierce C. W., Cullen S. E. Antigen presentation by the BCL1 murine B cell line: in vitro stimulation by LPS. J Immunol. 1984 Sep;133(3):1202–1208. [PubMed] [Google Scholar]
- Jelachich M. L., Grusby M. J., Clark D., Tasch D., Margoliash E., Pierce S. K. Synergistic effects of antigen and soluble T-cell factors in B-lymphocyte activation. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5537–5541. doi: 10.1073/pnas.81.17.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi T., Chesnut R. W., Grey H. M. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983 Jul;131(1):109–114. [PubMed] [Google Scholar]
- Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Press J. L. The B cell specificity repertoire: its relationship to definable subpopulations. Transplant Rev. 1975;24:41–83. doi: 10.1111/j.1600-065x.1975.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Wortis H. H. Role of antigen-specific B cells in the induction of SRBC-specific T cell proliferation. J Immunol. 1984 May;132(5):2253–2258. [PubMed] [Google Scholar]
- McKean D. J., Infante A. J., Nilson A., Kimoto M., Fathman C. G., Walker E., Warner N. Major histocompatibility complex-restricted antigen presentation to antigen-reactive T cells by B lymphocyte tumor cells. J Exp Med. 1981 Nov 1;154(5):1419–1431. doi: 10.1084/jem.154.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Seghal E., Kung J., Finkelman F. D. Increased expression of I-region-associated antigen (Ia) on B cells after cross-linking of surface immunoglobulin. J Immunol. 1981 Sep;127(3):881–888. [PubMed] [Google Scholar]
- Pierce S. K., Klinman N. R., Maurer P. H., Merryman C. F. Role of the major histocompatibility gene products in regulating the antibody response to dinitrophenylated poly(L-Glu55,L-Ala35,L-Phe9)n. J Exp Med. 1980 Aug 1;152(2):336–349. doi: 10.1084/jem.152.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M., Nisonoff A., Margoliash E. Immunological activity of cytochrome c. 3. Enhancement of antibody detection and immune response initiation by cytochrome c polymers. J Biol Chem. 1970 Mar 10;245(5):947–954. [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984 Oct 1;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Ryan J. J., Sieckmann D. G., Finkelman F. D., Mond J. J., Scher I. A method for size separation of murine spleen cells using counterflow centrifugation. J Immunol Methods. 1983 Oct 28;63(3):299–307. doi: 10.1016/s0022-1759(83)80003-9. [DOI] [PubMed] [Google Scholar]
- Tony H. P., Parker D. C. Major histocompatibility complex-restricted, polyclonal B cell responses resulting from helper T cell recognition of antiimmunoglobulin presented by small B lymphocytes. J Exp Med. 1985 Jan 1;161(1):223–241. doi: 10.1084/jem.161.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


