Summary
Background
The balance of risk and benefit from early neurosurgical intervention for conscious patients with superficial lobar intracerebral haemorrhage of 10–100 mL and no intraventricular haemorrhage admitted within 48 h of ictus is unclear. We therefore tested the hypothesis that early surgery compared with initial conservative treatment could improve outcome in these patients.
Methods
In this international, parallel-group trial undertaken in 78 centres in 27 countries, we compared early surgical haematoma evacuation within 12 h of randomisation plus medical treatment with initial medical treatment alone (later evacuation was allowed if judged necessary). An automatic telephone and internet-based randomisation service was used to assign patients to surgery and initial conservative treatment in a 1:1 ratio. The trial was not masked. The primary outcome was a prognosis-based dichotomised (favourable or unfavourable) outcome of the 8 point Extended Glasgow Outcome Scale (GOSE) obtained by questionnaires posted to patients at 6 months. Analysis was by intention to treat. This trial is registered, number ISRCTN22153967.
Findings
307 of 601 patients were randomly assigned to early surgery and 294 to initial conservative treatment; 298 and 291 were followed up at 6 months, respectively; and 297 and 286 were included in the analysis, respectively. 174 (59%) of 297 patients in the early surgery group had an unfavourable outcome versus 178 (62%) of 286 patients in the initial conservative treatment group (absolute difference 3·7% [95% CI −4·3 to 11·6], odds ratio 0·86 [0·62 to 1·20]; p=0·367).
Interpretation
The STICH II results confirm that early surgery does not increase the rate of death or disability at 6 months and might have a small but clinically relevant survival advantage for patients with spontaneous superficial intracerebral haemorrhage without intraventricular haemorrhage.
Funding
UK Medical Research Council.
Introduction
Spontaneous supratentorial intracerebral haemorrhage is a heterogeneous disorder with clinical manifestations that range from none to rapid death. It affects 4 million patients worldwide each year and median case fatality at 1 month is 40%.1 Many survivors remain severely disabled and therefore are an enormous burden on stroke services with only a quarter having a good outcome.2
Surgery has the potential to reduce the volume of intracerebral haemorrhage and there is clinical and experimental evidence that mass removal might reduce nervous tissue damage, possibly by relieving local ischaemia3, 4, 5, 6 or removal of noxious chemicals.7, 8, 9 Nevertheless, responses to surgery do not seem to be homogeneous, with trial data, expert opinion, and mechanistic reasoning all indicating that early surgery benefits only some clots. For example, large, surgically accessible clots exerting a mass effect might benefit from early surgery; whereas inaccessible clots, with surgical approach paths that cross eloquent speech and motor regions probably do not. Therefore, most neurosurgeons would remove a large frontopolar intracerebral haemorrhage with recent deterioration in consciousness and would not remove a small intracerebral haemorrhage in the internal capsule or basal ganglia. Also some clots are too small or the patient is too well to consider intervention. The hypothesis in the present STICH II study was based on the results of a subgroup analysis from the first STICH trial that accorded with these ideas.10
Several prospective randomised controlled trials11, 12, 13, 14, 15, 16, 17, 18, 19 were undertaken during the previous century, culminating in the first large trial of early surgery for spontaneous supratentorial intracerebral haemorrhage,20 the results of which were neutral. This outcome seemed to occur because some groups of patients did worse with surgery (those with deep-seated bleeds or with intraventricular haemorrhage and hydrocephalus) and some better (patients with superficial lobar haematomas without intraventricular haemorrhage).10 The same effect was noted in a meta-analysis of other studies: a benefit with surgery that was not significant.21
These findings led to the STICH II trial, designed to find out whether early surgery would improve outcomes compared with initial conservative treatment in patients with superficial lobar supratentorial intracerebral haemorrhage without intraventricular haemorrhage. The hypothesis was that early surgery could improve outcome in conscious patients in whom there is a superficial intracerebral haemorrhage of 10–100 mL and no evidence of intraventricular haemorrhage.
Methods
Trial design and participants
STICH II was an international, multicentre, prospective, randomised, parallel group, pragmatic trial as described in the protocol.21 129 neurosurgical units in 39 countries completed all regulatory requirements and registered for participation in this trial.
For the UK, ethics approval was obtained from the Scotland Multicentre Research Ethics Committee and the Newcastle and North Tyneside Local Research Ethics Committee. Elsewhere, appropriate local ethical approval was sought from each participating centre and the trial was undertaken according to the UK Medical Research Council's good clinical practice guidelines and local ethical and research and development procedures.
Patients were eligible if they had a spontaneous lobar intracerebral haemorrhage on CT scan (≤1 cm from the cortical surface of the brain) with a volume of between 10 mL and 100 mL, were within 48 h of ictus, had a best motor score on the Glasgow Coma Score (GCS) of 5 or 6, and had a best eye score of 2 or more (ie, were conscious at randomisation). They were ineligible if the haemorrhage was due to an aneurysm or angiographically proven arteriovenous malformation; was secondary to tumour or trauma; involved the basal ganglia, thalamic, cerebellar, or brainstem regions; or if there was any intraventricular blood. Patients were also ineligible if they had any severe pre-existing physical or mental disabilities or comorbidities that could interfere with the assessment of the outcome.
Interventions
Patients were randomly allocated to either early surgery or initial conservative treatment. In the early surgery group, surgeons were expected to endeavour to undertake evacuation of the haematomas within 12 h. In the initial conservative treatment group, delayed evacuation was permitted if judged clinically appropriate. All patients were given the best medical treatment as defined according to their hospital settings.
Information about the preintracerebral haemorrhage status and early progress of all patients (including GCS and focal signs for the first 5 days) was gathered by the local investigator at 2 weeks, discharge, or death, whichever was earliest. Surviving patients had an additional CT scan at 5 days (give or take 2 days) to assess changes in the haematoma size with and without surgery. Patients' general practitioners (UK) or their local investigators (outside the UK) were then contacted at 4 months after randomisation to confirm that the patients were alive, their place of residence, and to request completion of a major events form.
Randomisation and masking
The Centre for Healthcare Randomised Trials, Aberdeen, UK, provided an automatic telephone and internet-based randomisation service to assign patients in a 1:1 ratio. Patients were stratified by country group and planned operation (craniotomy or other) and within these strata they were allocated according to a minimisation algorithm based on age (<60 years, 60–69 years, or ≥70 years) and neurological deficit in the worst affected arm or leg (normal, weak, or paralysed) with a random component such that there was a 20% chance of the allocation being reversed.
Patients, relatives, and site investigators were aware of which treatment the patient had been allocated to; however, at the coordinating centre only the data manager was aware of the allocation. Throughout the study, data broken down by treatment assignment were never provided to investigators or to the study team. Outcome was assessed with questionnaires that were completed by the patients or their relatives. If it was necessary to administer these questionnaires to the patients then the interviewer was masked to treatment allocation. Outcome assignment, data cleaning, and CT assessment were all completed before the treatment assignment was unmasked.
Outcomes
The primary outcome was a prognosis-based favourable or unfavourable outcome dichotomised from the Extended Glasgow Outcome Scale (GOSE) at 6 months after randomisation. GOSE was computed from the answers to 14 questions in a postal questionnaire completed by patients or their relatives.22 The postal questionnaires were translated into the appropriate languages and mailed to patients or their relatives or carers at 5 months after randomisation and, if needed, followed with a reminder at 6 months. In the centres where there were problems with the postal systems, there were likely to be literacy or language problems, or when there had been no response to a reminder, questionnaires could be completed by a social worker or research nurse, who did not know the treatment allocation, in interview with the patient or relative.
The prognosis-based outcome was designed as a differential type of outcome with two different levels of success.23 The prognostic score was based on GCS, age, and haemorrhage volume at randomisation, and the algorithm had been developed from patients with a broad range of different intracerebral haemorrhages. It was calculated as:
The predefined cutoff of 27·67224 for supratentorial intracerebral haemorrhage was used to divide the patients into a poor prognosis group and a good prognosis group. The outcome was judged favourable if GOSE was good recovery or moderate disability; additionally, in the poor prognosis group, upper severe disability. This disability represents patients who are completely self-caring within their homes but who are unable to shop or use public transport without assistance.
Secondary outcomes were mortality, time to death, prognosis-based dichotomised Rankin (appendix p 4), and GOSE, and Rankin and EuroQoL; all measured at 6 months.
We also report the crossover and major event rates in each treatment group.
Statistical analysis
Based on findings from previous work that a prognosis-based favourable outcome would be noted in 37% of the conservative treatment group, a sample size of 566 (283 in each group) was needed to show a 12% benefit from surgery (two-sided p<0·05) with 80% power. We therefore proposed a sample size of 600 patients to allow for withdrawals and crossovers.
The independent data monitoring committee reviewed data from the study after 50, 100, 200, and 400 patients had been recruited. These interim reviews were confidential, with only the data manager and the data monitoring committee having access to them. The committee did not plan any formal interim analyses but worked on the principle that a difference of at least 3 SEs in an analysis of a major outcome event (eg, death from all causes or independent survival at 6 months) would be needed to justify halting or modifying the study before the planned recruitment was completed.
The detailed analysis plan has been reported previously.24 Analysis was undertaken on an intention-to-treat basis by treatment allocation. Outcomes were reported as odds ratios (OR) with 95% CI. Absolute differences with 95% CI were also reported.
Primary outcome analysis was a simple categorical frequency comparison by use of the χ2 test for prognosis-based favourable and unfavourable outcome on GOSE. Logistic regression was undertaken to adjust for covariates, age, GCS, volume of haematoma, and neurological deficit.
Secondary analysis consisted of a Kaplan-Meier survival curve with log-rank test, χ2 test for mortality at 6 months, and 6 month prognosis-based Rankin. Additionally, GOSE, Rankin, and EuroQoL were reported by treatment allocation.
We also did a sensitivity analysis based on a proportional odds model.
Prespecified subgroup analyses were also undertaken for age (<65 years, ≥65 years), volume of haematoma (≤35 mL, >35 mL), GCS (8–12, 13–15), time from ictus to randomisation (<21 h, ≥21 h), and severity of neurological deficit in worse limb (normal, weak, paralysed). An additional analysis of the two prognosis groups was undertaken.
This trial is registered, number ISRCTN22153967.
Role of the funding source
Neither the sponsor nor the funder of the study had any role in study design, data gathering, analysis, and interpretation, or writing of the report. The corresponding author and ENR had full access to all the data in the study and all members of the writing committee had responsibility for the decision to submit for publication.
Results
601 patients from 78 centres in 27 countries were randomly assigned between Jan 11, 2007, and Aug 15, 2012: 307 to early surgery and 294 to initial conservative treatment; recruitment by centre is shown in the appendix p 2. Four patients were excluded because they were recruited by two centres that randomly assigned patients after evacuating the haematoma: a serious protocol violation (figure 1). All other patients were included in the analysis irrespective of the decision of the central CT reading committee about their eligibility (the CT committee's findings will be reported later in a separate paper). This analysis, therefore, includes 305 patients assigned to early surgery and 292 to initial conservative treatment (figure 1). Table 1 shows details of the patients' age, sex, previous medical history, and neurological status. The two groups were well matched at baseline. 57% were men and the median age of the patients was 65 years (range 17–94; table 1). Patients were randomly assigned within 48 h of ictus and a quarter (76 [25%] of 305 in the early surgery group and 73 [25%] of 292 in the initial conservative treatment group) were assigned within 12 h (median 21·6 h [IQR 12·0–31·5] and 21·0 [12·0–32·0], respectively). 50% of the patients in the early surgery group and 49% in the initial conservative treatment group had a GCS of 14 or 15 at randomisation (table 1). The planned method of evacuation in 98% of all cases was craniotomy. Table 2 shows the haematoma characteristics reported by site investigators at randomisation. The median volume of the haematoma (with the Broderick method25) was 36 mL (23·0–55·5) and the median depth from the cortex surface was 1 mm (0–2).
Figure 1.
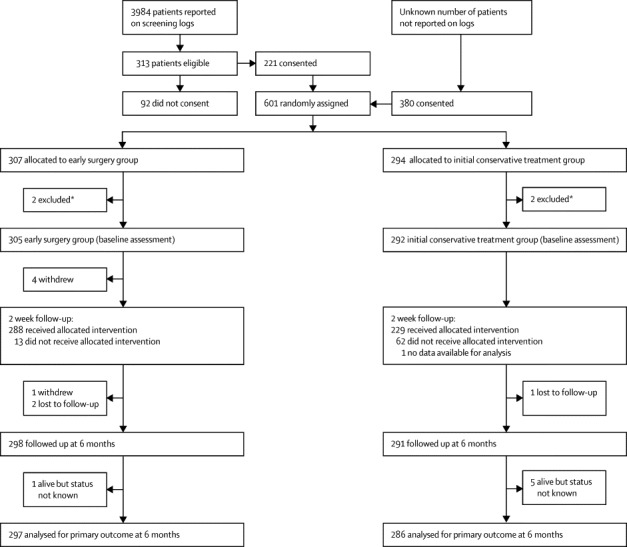
Trial profile
*One site recruited one patient but had undertaken surgery before randomisation (the patient was allocated to initial conservative treatment); another site recruited three patients, and two of these had surgery before randomisation (one allocated to early surgery and one to initial conservative treatment); because of the severe breach of protocol all four patients were excluded.
Tabl91e 1.
Baseline characteristics of patients
| Early surgery group (n=305) | Initial conservative treatment group (n=292) | |
|---|---|---|
| Age (years) | ||
| Median (IQR; range) | 65 (55 to 74; 17 to 90) | 65 (56 to 74; 23 to 94) |
| Mean (SD) | 63·9 (13·0) | 63·9 (13·7) |
| <60 | 105 (34%) | 106 (36%) |
| 60–69 | 89 (29%) | 70 (24%) |
| ≥70 | 111 (36%) | 116 (40%) |
| Sex | ||
| Male | 174 (57%) | 166 (57%) |
| Female | 131 (43%) | 126 (43%) |
| Preintracerebral haemorrhage Rankin* | ||
| 0 | 240 (80%) | 236 (81%) |
| 1 | 41 (14%) | 37 (13%) |
| 2 | 17 (6%) | 11 (4%) |
| 3 | 2 (<1%) | 5 (2%) |
| 4 | 1 (<1%) | 2 (<1%) |
| Preintracerebral haemorrhage mobility*† | ||
| Able to walk 200 m | 283 (94%) | 275 (95%) |
| Able to walk indoors | 17 (6%) | 13 (4%) |
| Unable to walk | 1 (<1%) | 2 (<1%) |
| Glasgow Coma Score, eye | ||
| 2 | 26 (9%) | 27 (9%) |
| 3 | 69 (23%) | 65 (22%) |
| 4 | 210 (69%) | 200 (68%) |
| Glasgow Coma Score, verbal | ||
| 1 | 40 (13%) | 44 (15%) |
| 2 | 36 (12%) | 25 (9%) |
| 3 | 37 (12%) | 35 (12%) |
| 4 | 93 (30%) | 96 (33%) |
| 5 | 99 (32%) | 92 (32%) |
| Glasgow Coma Score, motor | ||
| 5 | 83 (27%) | 71 (24%) |
| 6 | 222 (73%) | 221 (76%) |
| Glasgow Coma Score, total | ||
| 8 | 12 (4%) | 4 (1%) |
| 9 | 9 (3%) | 15 (5%) |
| 10 | 23 (8%) | 21 (7%) |
| 11 | 32 (10%) | 32 (11%) |
| 12 | 32 (10%) | 34 (12%) |
| 13 | 46 (15%) | 43 (15%) |
| 14 | 70 (23%) | 68 (23%) |
| 15 | 81 (27%) | 75 (26%) |
| Localising arm | ||
| Normal | 84 (28%) | 82 (28%) |
| Weak | 129 (42%) | 116 (40%) |
| Paralysed | 92 (30%) | 94 (32%) |
| Localising leg | ||
| Normal | 94 (31%) | 96 (33%) |
| Weak | 141 (46%) | 121 (41%) |
| Paralysed | 70 (23%) | 75 (26%) |
| Neurological deficit | ||
| Normal | 80 (26%) | 81 (28%) |
| Weak | 129 (42%) | 112 (38%) |
| Paralysed | 96 (31%) | 99 (34%) |
| Medical history | ||
| Documented hypertension (>140 mm Hg/90 mm Hg)* | 204 (68%) | 196 (67%) |
| On antihypertensive medication* | 144 (48%) | 150 (52%) |
| Previous myocardial infarction* | 18 (6%) | 14 (5%) |
| Previous stroke* | 30 (10%) | 33 (11%) |
| Medication before intracerebral haemorrhage* | ||
| Anticoagulant drugs | 22 (7%) | 20 (7%) |
| Antiplatelet drugs | 42 (14%) | 31 (11%) |
| Thrombolytic drugs | 4 (1%) | 2 (<1%) |
| Prognostic score | ||
| Median (IQR; range) | 42·5 (20 to 62; −57 to 120) | 41·2 (16 to 63; −53 to 121) |
| Mean (SD) | 38·6 (30·7) | 39·0 (31·7) |
| Prognostic score category | ||
| Poor | 104 (34%) | 105 (36%) |
| Good | 201 (66%) | 187 (64%) |
For continuous variables, data are median (IQR; range) and mean (SD); for categorical variables, data are number (%).
Data were missing for four patients in the early surgery group who withdrew after randomisation and for one patient in the initial conservative treatment group for whom no 2 week data were obtained.
One patient in the initial conservative treatment group did not provide this information.
Table 2.
Characteristics of haematomas
| Early surgery group (n=305) | Initial conservative treatment group (n=292) | |
|---|---|---|
| Volume (mL) | ||
| Median (IQR; range) | 38 (24–54; 10–100) | 36 (22–58; 10–100) |
| Mean (SD) | 41·4 (21·2) | 41·0 (22·9) |
| Depth (mm) | ||
| Median (IQR; range) | 1 (0–2; 0–10) | 1 (0–2; 0–10) |
| Mean (SD) | 1·6 (2·4) | 1·6 (2·5) |
| Side of haemorrhage* | ||
| Left | 158 (52%) | 149 (51%) |
| Right | 147 (48%) | 142 (49%) |
For continuous variables, data are median (IQR; range) and mean (SD); for categorical variables, data are number (%).
Data were missing for one patient in the initial conservative group for whom no forms were returned.
Four patients withdrew from the study after allocation to early surgery group because they or their relatives refused surgery and further involvement, and all data for one patient in the initial conservative treatment group were lost by the centre (figure 1). Thus, process data were available for 301 patients in the early surgery group and 291 in the initial conservative treatment group. Another patient withdrew (self-discharged) in the early surgery group between 2 weeks and the 6 month follow-up because the patient did not want to have surgery (figure 1). Nine patients were lost to follow-up (although six were known to be alive at 6 months and could be included in survival analyses; figure 1). Thus, 583 patients had complete follow-up at 6 months for the primary outcome analysis.
Of the 301 assessable patients in the early surgery group, 288 (96%) had surgery and 281 (93%) had surgery within 12 h (table 3). 13 (4%) patients did not have surgery because their families refused (n=6), or they had a rebleed or intraventricular haemorrhage (3), cardiac problem, respiratory problem, or fever (3), and logistical problems (1). Of 291 assessable patients in the initial conservative treatment group, 62 (21%) had surgery (table 3). Reasons for these patients requiring operation were deterioration in GCS (n=36), oedema (1), rebleed (3), deterioration and oedema (7), deterioration and rebleed (5), deterioration, oedema, and rebleed (4), rise in intracranial pressure (2), surgeon error (1), family request (2), and an underlying cause (1). Craniotomy was the most commonly used surgical technique for evacuation in 343 (98%) of 350 of cases.
Table 3.
Surgery details
| Early surgery group (n=288) | Initial conservative treatment group (n=62) | ||
|---|---|---|---|
| Surgery | |||
| Craniotomy | 284 (99%) | 59 (95%) | |
| Craniectomy | 1 (<1%) | 3 (5%) | |
| Minimally invasive* | 3 (1%) | .. | |
| Any other procedure | 15 (5%) | 6 (10%) | |
| Paralysed and sedated | 20 (7%) | 7 (11%) | |
| Preoperative Glasgow Coma Score, eye† | |||
| 1 | 7 (3%) | 20 (36%) | |
| 2 | 23 (9%) | 20 (36%) | |
| 3 | 62 (23%) | 11 (20%) | |
| 4 | 176 (66%) | 4 (7%) | |
| Preoperative Glasgow Coma Score, verbal† | |||
| 1 | 35 (13%) | 24 (44%) | |
| 2 | 30 (11%) | 11 (20%) | |
| 3 | 33 (12%) | 12 (22%) | |
| 4 | 83 (31%) | 6 (11%) | |
| 5 | 87 (33%) | 2 (4%) | |
| Preoperative Glasgow Coma Score, motor† | |||
| 1 | .. | .. | |
| 2 | .. | 1 (2%) | |
| 3 | 1 (<1%) | 11 (20%) | |
| 4 | 5 (2%) | 14 (25%) | |
| 5 | 69 (26%) | 24 (44%) | |
| 6 | 193 (72%) | 5 (9%) | |
| Preoperative arm† | |||
| Normal | 75 (28%) | 2 (4%) | |
| Weak | 116 (43%) | 10 (19%) | |
| Paralysed | 76 (28%) | 40 (77%) | |
| Preoperative leg† | |||
| Normal | 82 (31%) | 2 (4%) | |
| Weak | 129 (48%) | 12 (24%) | |
| Paralysed | 56 (21%) | 38 (73%) | |
| Preoperative speech† | |||
| Normal | 121 (48%) | 5 (15%) | |
| Dysarthric | 60 (24%) | 14 (41%) | |
| Aphasic | 70 (28%) | 15 (44%) | |
| Time randomisation to surgery (h) | |||
| Median (IQR; range) | 3·3 (1·9–5·7; 0·2–34·6) | 22·8 (5·6–68·8; 0·6–249·6) | |
| Mean (SD) | 4·5 (4·3) | 45·5 (56·0) | |
| Surgery within 12 h of randomisation | 281 (98%) | 23 (37%) | |
| Time from ictus to surgery (h) | |||
| Median (IQR; range) | 26 (15·3–35·3; 2·5–69·0) | 46 (21·7–81·9; 5·0–266·5) | |
| Mean (SD) | 26·7 (13·6) | 64·2 (58·9) | |
| Surgery within 12 h of ictus | 48 (17%) | 9 (15%) | |
For continuous variables, data are median (IQR; range) and mean (SD); for categorical variables, data are number (%).
Minimally invasive procedures were burrhole with endoscopic evacuation in two patients and keyhole evacuation in one patient.
These questions were not answered for patients who were paralysed and sedated at that time and data were not recorded for some other patients.
Comparison of patients in the initial conservative treatment group who had surgery with those who did not showed that they were more likely to undergo surgery if they had a paralysed limb at randomisation (34 [55%] of 62 vs 65 [28%] of 229; p<0·0001), lower GCS (median 13 [IQR 10–14] vs 14 [12–15]; p<0·0001), larger haematoma (54 mL [35–74] vs 32 mL [20–50]; p<0·0001), or were in the poor prognosis group (34 [55%] vs 71 [31%]; p=0·0005). Patients who crossed over to surgery were more likely than were those allocated to early surgery to have a GCS of at least one point lower just before their operation than at randomisation (47 [82%] of 57 vs 50 [19%] of 269; p<0·0001).
At 2 weeks, the status of patients was classified as dead, still on a neurosurgical ward, transferred to another unit or hospital, or discharged. There were significant differences between the early surgery group and initial conservative group (p=0·02). In the early surgery group, 16 (5%) of 301 patients had died, 85 (28%) were still on a neurosurgical ward, 80 (27%) were transferred, and 120 (40%) were discharged, whereas in the initial conservative treatment group 29 (10%) of 291 patients had died, 102 (35%) were still on a neurosurgical ward, 60 (21%) were transferred, and 100 (34%) were discharged. Few patients in either group (four [1%] of 301 in the early surgery group and one [<1%] of 291 in the initial conservative treatment group) were treated with factor VIIa. Patients in the initial conservative group were more likely to have an angiogram (108 [37%] of 291 vs 87 [29%] of 301; p=0·034). However, clinically significant lesions were only found in ten patients: three with an arteriovenous malformation and three with an aneurysm in the early surgery group, and three patients with an arteriovenous malformation and one with an aneurysm in the initial conservative treatment group. Post-randomisation adverse events reported during the hospital stay before the 2 week point were similar in the two groups: overall 37 (6%) of 592 patients had a further intracerebral haemorrhage increasing the volume by at least 20% (14 in the early surgery group and 23 in the initial conservative treatment), six (1%) had an ischaemic stroke (five and one, respectively), six (1%) a pulmonary embolism (one and five, respectively), 16 (3%) a major cardiac event (nine and seven, respectively), and 71 (12%) pneumonia (31 and 40, respectively).
With the prognosis-based dichotomy of GOSE, 123 (41%) of 297 patients in the early surgery group had a favourable outcome at 6 months compared with 108 (38%) of 286 patients in the initial conservative treatment group (OR 0·86, 95% CI 0·62 to 1·20; p=0·367). Early surgery had an absolute benefit of 3·7% (table 4) and a relative benefit of 9·7% (–11·4 to 30·8). Adjustment for the covariates age, GCS, haemorrhage volume, and neurological deficit made little difference to the prognosis-based outcome (0·85, 0·59 to 1·22; p=0·384).
Table 4.
Prespecified outcomes at 6 months
| Early surgery group | Initial conservative treatment group | p value | Absolute difference (95% CI) | |||
|---|---|---|---|---|---|---|
| Primary outcome | 297 | 286 | ||||
| Prognosis based | 0·367* | 3·7% (−4·3 to 11·6) | ||||
| Unfavourable | 174 (59%) | 178 (62%) | .. | .. | ||
| Favourable | 123 (41%) | 108 (38%) | .. | .. | ||
| Secondary outcomes | 298 | 291 | ||||
| Mortality at 6 months | 0·095* | 5·6% (−1·0 to 12·2) | ||||
| Dead | 54 (18%) | 69 (24%) | .. | .. | ||
| Alive | 244 (82%) | 222 (76%) | .. | .. | ||
| Prognosis-based modified Rankin | 0·456* | 3·1% (−5·0 to 11·2) | ||||
| Unfavourable | 155 (53%) | 158 (56%) | .. | .. | ||
| Favourable | 140 (47%) | 126 (43%) | .. | .. | ||
| GOSE | 0·091*; 0·075† | .. | ||||
| Dead | 54 (18%) | 69 (24%) | .. | .. | ||
| Vegetative | 0 | 0 | .. | .. | ||
| Lower severe disability | 64 (22%) | 66 (23%) | .. | .. | ||
| Upper severe disability | 72 (24%) | 59 (21%) | .. | .. | ||
| Lower moderate disability | 20 (7%) | 15 (5%) | .. | .. | ||
| Upper moderate disability | 32 (11%) | 35 (12%) | .. | .. | ||
| Lower good recovery | 37 (12%) | 26 (9%) | .. | .. | ||
| Upper good recovery | 18 (6%) | 16 (6%) | .. | .. | ||
| Rankin | 0·128*; 0·147† | .. | ||||
| 0 | 20 (7%) | 16 (6%) | .. | .. | ||
| 1 | 54 (18%) | 57 (20%) | .. | .. | ||
| 2 | 58 (20%) | 41 (14%) | .. | |||
| 3 | 35 (12%) | 32 (11%) | .. | .. | ||
| 4 | 40 (14%) | 28 (10%) | .. | .. | ||
| 5 | 34 (12%) | 41 (14%) | .. | .. | ||
| Dead | 54 (18%) | 69 (24%) | .. | .. | ||
| EuroQoL Index | 235 | 210 | 0·751‡ | .. | ||
| Median (IQR; range) | 0·64 (0·20 to 0·85; −0·59 to 1·00) | 0·69 (0·08 to 0·82; −0·59 to 1·00) | .. | .. | ||
Data are number or number (%), unless otherwise indicated. EuroQol utility index was calculated with UK weightings provided by the EuroQol Group Foundation. Absolute differences (95% CIs) are provided for binary outcomes. Rankin was not available for three patients in the early surgery group and for seven in the initial conservative group. GOSE was not available for one patient in the early surgery group and five patients in the initial conservative group. GOSE=Extended Glasgow Outcome Scale.
χ2 test.
Proportional odds model.
Mann-Whitney test.
The mortality rate at 6 months was 18% in the early surgery group and 24% in the initial conservative treatment group (table 4; OR 0·71, 95% CI 0·48 to 1·06; p=0·095); absolute difference in favour of early surgery was 5·6% (table 4) and the relative difference was 7·3% (–1·3 to 16·0). The actual survival advantage during the first 6 months with early surgery was not significant (figure 2). 27 (9%) of 298 patients died at 30 days and 43 (14%) at 90 days in the early surgery group, whereas 43 (15%) of 291 patients died at 30 days and 63 (22%) at 90 days in the initial conservative treatment group.
Figure 2.
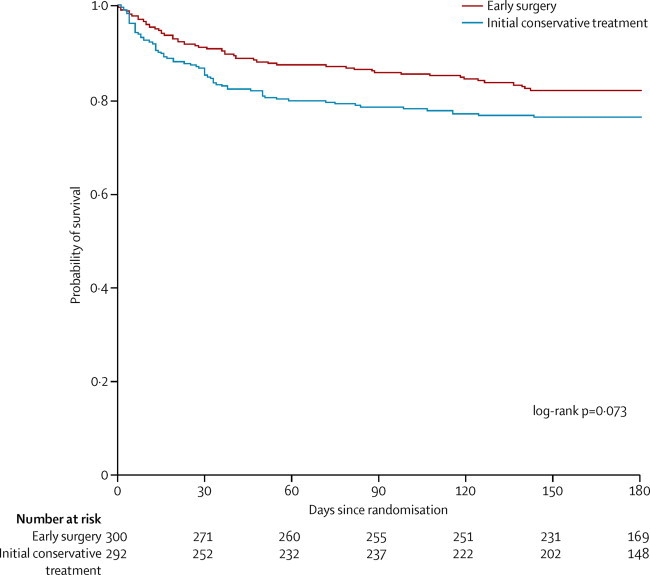
Kaplan–Meier survival curve
Table 4 shows the full extended GOSE, Rankin, and EuroQoL by treatment group. The prognosis-based Rankin showed favourable outcome in 47% of the patients in the early surgery group and in 44% of those in the initial conservative treatment group (p=0·46; table 4); the absolute difference in favour of early surgery was 3·1% (table 4) and the relative difference was 7·0% (95% CI −11·4 to 25·3). The actual distribution of GOSE was more favourable for the early surgery group than for the initial conservative treatment group (figure 3), although the difference was not significant (p=0·091; table 4). The proportional odds model analysis of these data (OR 0·77, 95%CI 0·58 to 1·03; p=0·075) was consistent with the χ2 trend analysis.
Figure 3.
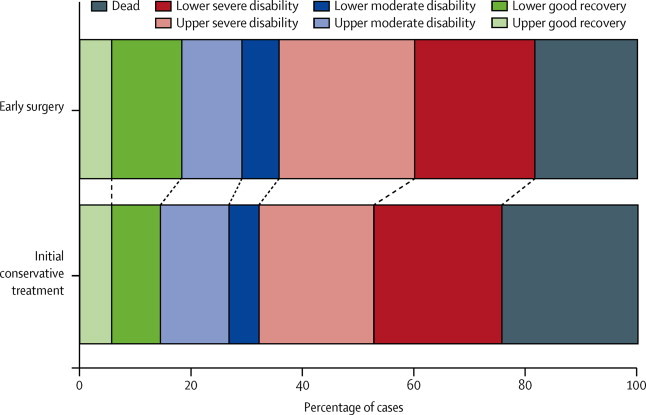
Extended Glasgow Outcome Scale at 6 months
Proportional odds model p=0·075.
Figure 4 shows prespecified subgroup analyses and analyses for the poor and good prognosis groups. No prespecified subgroups showed heterogeneity of treatment response.
Figure 4.
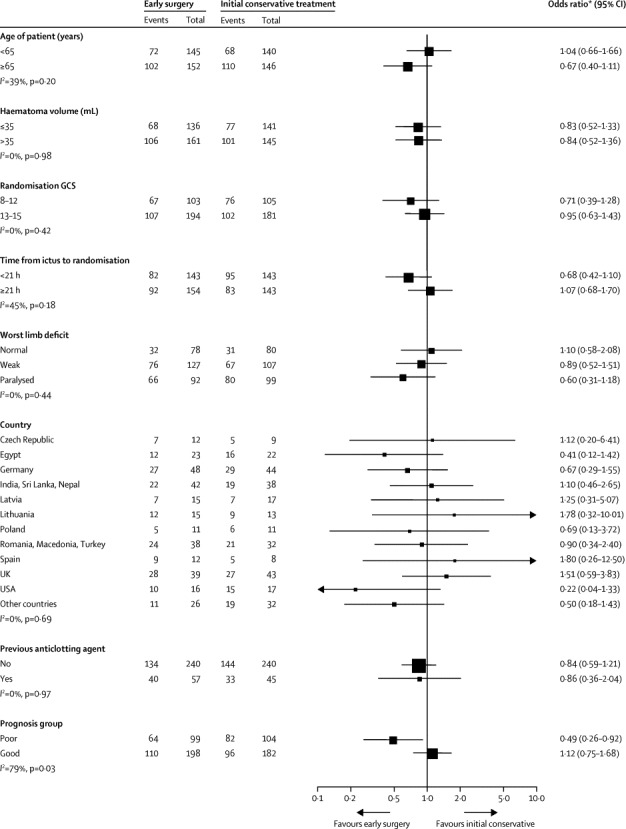
Subgroup analysis Forest plot
GCS=Glasgow Coma Score.
At 6 months, 79 (39%) of 203 patients in the poor prognosis group died and 67 (33%) had lower severe disability, whereas 44 (12%) of 380 patients died in the good prognosis group and 63 (17%) had lower severe disability. Patients in the good prognosis group were much more likely to have a good recovery (85 [22%] of 380) or moderate disability (89 [23%]) than were those in the poor prognosis group (12 [6%] of 203 and 13 [6%], respectively). Subgroup analysis of the prognosis-based prediction group showed significant heterogeneity (I2=79%, p=0·03). Patients in the poor prognosis group were more likely to have a favourable outcome with early surgery than with initial conservative treatment (OR 0·49, 95% CI 0·26–0·92; p=0·02; figure 4). By contrast, there was no advantage for surgery in the good prognosis group (1·12, 0·75–1·68; p=0·57).
There were differences in the causes of death between the two groups. Patients in the early surgery group were more likely to die from cardiac events (14 [26%] of 54 vs five [7%] of 69) and less likely to die from intracerebral haemorrhage or rebleed (eight [15%] vs 20 [29%]), chest infection (13 [24%] vs 20 [29%]), or a pulmonary embolism (two [4%] vs nine [13%]) than were those in the initial conservative treatment group. There were an additional 139 serious adverse events reported in 107 patients (appendix p 1), with no differences between the treatment groups. The most common adverse events were respiratory infection in eight patients in the early surgery group and 12 in the initial conservative group, intracerebral haemorrhage or rebleed in nine and 12, respectively, neurological deterioration in nine and 17, respectively, seizures in ten and five, respectively, and cardiac events in four and eight, respectively.
Discussion
In STICH II, using the prognosis based outcome, we did not find significant evidence to support our hypothesis that early surgery compared with initial conservative treatment (with delayed surgery if the patient deteriorates) improves outcome in conscious patients in whom there is a superficial intracerebral haemorrhage of 10–100 mL and no evidence of intraventricular haemorrhage.
The prespecified recruitment target was attained in the STICH II trial and a follow-up rate of 98% was achieved at 6 months. Historically, recruitment and power are difficult and ubiquitous issues in surgical trials. The proportional odds model is sensitive to differences across the outcome scale rather than just to those in the primary dichotomy (favourable or unfavourable). That this analysis gives a lower p value than the primary outcome suggests insufficient power might have been an issue; however, this trial was powered to detect a 12% difference in outcome and took 67 months to reach the target sample size. If the 4% difference was regarded as clinically significant it would require many more patients.
Interpretation of many surgical trials is also complicated by crossovers from conservative treatment to surgery as commonly seen in spinal, cardiac, and neurosurgical trials.20, 26, 27 In STICH II, 62 (21%) of 291 patients assigned to initial conservative treatment went on to have delayed surgery. At the time of the delayed surgery, the patients were in deeper coma with worse neurological deficits than were those in the early surgery group (table 3). The crossover to surgery from initial conservative treatment might therefore have rescued these patients from what otherwise might have been a fatal outcome, but because of the intention-to-treat analysis they remained in the initial conservative treatment group.
The absence of a significant difference between the two groups in this trial might be because of the case mix of patients: more than half were fully conscious or just confused; such patients perhaps could be safely observed and delayed surgery undertaken only if their state deteriorates. There is evidence that the lack of effect could be a consequence of surgery being beneficial for some and not for other patients with an overall average showing little difference. In secondary analyses, a survival advantage (with no vegetative survivors) was noted for surgery throughout the 6 month follow-up (figure 2), but it was not significant. Mortality rate remained lower with early surgery than with initial conservative treatment throughout. This pattern contrasts with the Kaplan-Meier plot from the first STICH trial in which a more diverse group of patients with intracerebral haemorrhage was examined and there was no difference.20 Because we had dichotomised patients' prognoses as part of the primary outcome in the STICH II trial, we looked at the post hoc effects of surgery in the two prognostic groups. Patients in the STICH II trial with a poor prognosis did better with early surgery, whereas those with a good prognosis did not (figure 4). The interaction test was significant (p=0·03), but this result needs to be interpreted cautiously because the analysis was not prespecified. However, the result accords with some surgeons' preference to initially observe patients with a good prognosis thus avoiding surgery unless a patient deteriorates later.
Two-thirds of patients in this trial had documented hypertension before the intracerebral haemorrhage. Almost a quarter of these hypertensive patients were not on antihypertensive medication at the time of their ictus.
In STICH II, almost all the patients undergoing surgery had craniotomy. Other operative procedures include decompressive craniectomy and minimal access procedures. Use of decompressive craniectomies have been reported for patients with intracerebral haemorrhage28 but no prospective randomised controlled trials have yet been undertaken to compare their effect with conservative treatment. Other minimally invasive techniques are being tested in ongoing trials, in particular stereotactic delivery of tissue plasminogen activator to clots to dissolve them29 and tissue plasminogen activator assisted clearance of ventricular haemorrhage.30 Almost half of all intracerebral haemorrhages are associated with intraventricular haemorrhage and preliminary results from CLEAR IVH,31 the precursor to CLEAR III, have shown a benefit for treatment with intraventricular tissue plasminogen activator. In the STICH and STICH II trials, craniotomy was assessed whereas in the CLEAR III and MISTIE trials minimal access techniques were assessed. Figure 5 shows the updated meta-analyses for the trials of surgery for intracerebral haemorrhage and for the subgroup of patients with lobar intracerebral haemorrhage and no intraventricular haemorrhage; the results are discussed in the panel. However, minimal access techniques might be more beneficial for deeper clots and intraventricular haemorrhage. Further research is warranted to assess the benefits of these other surgeries.
Figure 5.
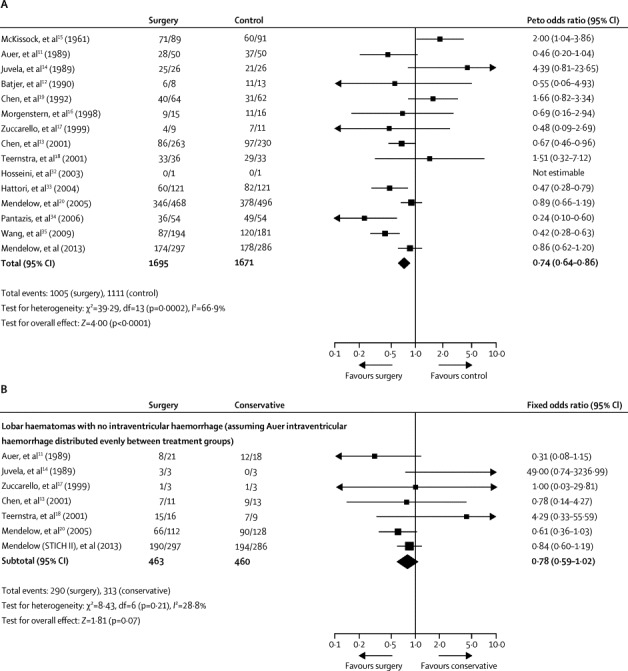
Updated meta-analysis of 15 trials of surgery in patients with intracerebral haemorrhage (A) and individual patient data in cases of lobar haematomas without intraventricular haemorrhage (B)
Data are n/N, unless otherwise indicated. Reported outcomes were unfavourable outcome in (A) and death or disability in (B). df=degrees of freedom.
Panel. Research in context.
Systematic review
Previously reported meta-analyses36, 37 were updated with the addition of data from STICH II after confirmation that there had been no other reports of trials of surgery versus initial conservative treatment. STICH II is the 15th pragmatic randomised controlled trial of surgery for intracerebral haemorrhage compared with conservative treatment to be reported. STICH II (n=597) is of moderate size compared with occlusive stroke trials but in intracerebral haemorrhage it is second only in size to STICH (n=1033). Incorporation of the results from STICH II (583 patients) with the previous meta-analysis of 14 trials of surgery37 gives a total sample size of 3366. The result shows a significant advantage for surgery with an odds ratio of 0·74 (95% CI 0·64–0·86; p<0·0001), although there is significant heterogeneity (p=0·0002) because the studies included different patient groups and different types of surgery. Addition of the STICH II data to a previous individual patient data meta-analysis subgroup of patients with a lobar intracerebral haemorrhage and no intraventricular haemorrhage38 did not show evidence of heterogeneity (p=0·21) but there was still not a significant benefit from surgery (n=923; 0·78, 0·59–1·02; p=0·07).
Interpretation
The results of the meta-analyses suggest that there is a role for surgery in patients with intracerebral haemorrhage, but that there is still some uncertainty about which patients benefit most. The STICH studies differ from the other trials in that early surgery was compared with the option of delayed surgery for patients who later deteriorate. The survival advantage for conscious patients with lobar haematomas seems to be greatest when the prognosis is poorer (Glasgow Coma Score 9–12) and when randomly assigned within 21 h. This slight advantage is lost in patients with a better prognosis perhaps because there is time to observe them initially and only operate in those that later deteriorate.
The results of STICH II confirm that early surgery does not increase the rate of death or disability at 6 months and might have a clinically relevant survival advantage for patients with spontaneous superficial intracerebral haemorrhage without intraventricular haemorrhage.
This online publication has been corrected. The corrected version first appeared at thelancet.com on Aug 2, 2013
Acknowledgments
Acknowledgments
STICH II was funded by the UK Medical Research Council (MRC; grant number G0501444), now managed by the National Institutes of Health Research (NIHR; grant number 09-800-18). This report is independent research funded by the MRC and managed by the NIHR on behalf of the MRC and NIHR partnership. The views expressed in this report are those of the authors and not necessarily those of the MRC, National Health Service, NIHR, or the Department of Health.
Contributors
ADM, BAG, PMM, GDM, and AG conceived the idea for and designed the study. All listed investigators contributed to enrolment of patients and interpretation of the data. BAG, ENR, and GDM analysed the study. ADM, ENR, and BAG drafted the report, but all listed contributors edited and revised the report.
STICH II Investigators
Writing Committee: A D Mendelow, B A Gregson, E N Rowan, G D Murray, A Gholkar, P Mitchell. Steering Committee: P Sandercock, G Ford, D Barer, A Strong, P M Mitchell, A R Gholkar, G D Murray, A D Mendelow, B A Gregson. Data Monitoring Committee: D Hanley, D T Hope, A Skene, H M Fernandes. Radiology Committee: S Metcalfe, A Iqbal, A Gholkar, K S M Prasad. Management Committee: A D Mendelow (principal investigator), B A Gregson (principal investigator and trial director), E N Rowan (data manager 2008–09, trial manager 2009–13), G M Kenyon (trial administrator 2007–13), L Chilton (data manager 2006–08), Z Liao (data manager 2009–10), A Andras (data manager 2010–11), R Francis (data manager 2012), L Bailey (trial administrator 2006–07). National Coordinators: Armenia Ruben Fanarjyan; Australia Andrew Kaye; Egypt Abd-Elhafiz Shehab-Eldien; England, UK Andrew King; Germany Hansdetlef Wassmann; Greece George Stranjalis; India Bhawani S Sharma; Israel Leon Levi; Latvia Egils Valeinis; Russia Alex Krivoshapkin; Scotland, UK Sam Eljamel; Spain Alfonso Vazquez-Barquero; Turkey Orhan Barlas; USA Christopher Loftus. Physician Champions: Armenia Ararat Minasyan; Australia Stephen Davis; Egypt Nabil Kitchener; England, UK Philippa Tyrrell; Germany Thorsten Steiner; Greece Kostas Vemmos; India Kameshwar Prasad; Israel Sagi Har Nof; Latvia Andrejs Millers; Russia Tatyana Makhovskaya; Scotland, UK Rustam Al-Shahi Salman; Spain Ruben Martin Laez; Turkey Sara Bahar; USA Benjamin Eidelman; Wales, UK Anne Freeman.
Centre Investigators (recruited at least one patient or completed screening logs; appendix pp 2–3): Australia S Davis, P Hand (Royal Melbourne Hospital, Melbourne, VIC); Austria G Kleinpeter (Rudolfstiftung Wein, Vienna); Canada M Findlay (University of Alberta Hospital, Edmonton, ALB); China Y Zhao (Beijing Tiantan Hospital, Beijing), Y Sin, J Hu (Huashan Hospital, Shanghai); Czech Republic T Krejci, S Poticny (Faculty Hospital of Ostrava, Ostrava), V Benes, O Bradac, M Mohapl (Charles University and Military University Hospital, Prague), M Smrcka, V Juran, K Svoboda (University Hospital, Brno), P Buchvald, V Benes 3rd (Liberec Regional Hospital, Liberec); Egypt O S Abdelaziz, I Zidan (Alexandria University Hospital, Alexandria), A-E Shehab-Eldien, E M Kandil, H M Taher, M F El-Faresy (Mansoura International Specialised Hospital, Mansoura); Germany A Sepehrnia (Clemens Hospital, Münster), J Kiwit, S Schreiber, F Youssef (Helios Klinikum Berlin Buch, Berlin), M Buchfelder, F Swozil (Universitatsklinikum Erlangen, Erlangen); A Kleindienst, M Marin, M Megele (Hospital Klinikum Amberg, Amberg), D Hänggi, K Beseoglus (Medical Faculty, Heinrich-Heine-University, Düsseldorf); K Kiening, B Orakcioglu, P Schiebel (University Hospital Heidelberg, Heidelberg), M Holling, H Wassmann (University Hospital Münster, Münster), K Schwerdtfeger, J Szczygielski (Saarland University Medical Centre, Homburg-Saar), G Nowak, S Spuck (University Schleswig-Holstein, Lübeck), K Sadowy (Neurochirurgische Klinik, Dessau), H W S Schroder, C Müller (Greifswald University, Greifswald), G F Hamann, R Schönmayr (Dr Horst Schmidt Kliniken, Wiesbaden), E Juettler, J Woitzik H Neugebauer (Charite—University Medicine Berlin, Berlin), A Waschke, R Kalff (Universitatsklinikum Jena, Jena), Y Chehade (Asklepios Klinik Altona, Hamburg); Greece P Tsitsopoulos (Ippokration General Hospital, Thessaloniki), G Stranjalis, L Stavrinou (Evangelismos Hospital, Athens); Hungary A Buki, L Szapary Pecs (University Hospital, Pecs), J Dobai (Borsod County and University Teaching Hospital, Borsod); India A Agrawal, A Kakani (Acharya Vinoba Bhave Rural Hospital, Maharashtra), S A V Rao, N K Venkataramana, A L Naik (BGS Global Hospitals, Bangalore), S S Grewal, B Gupta (Christian Medical College and Hospital, Ludhiana), R Bhattacharya (AMRI Hospitals, Dhakuria), T V R Murty (Care Hospitals, Hyderabad), B Indira Devi, G Jagath Lal (National Institute of Mental Health and Neurosciences, Bangalore), P S Chandra, M Tripathi, B S Sharma (All India Institute of Medical Sciences, New Delhi), K Sridhar, R Sengupta (National Neurosciences Centre, Calcutta), S Nair, G Menon (Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum), P V Ramana, P M Jagannath (Care Hospital, Visakhapatnam); Israel L Levi, M Zaaroor (Rambam Hospital, Haifa); Italy R Delfini, A Pichierri (University Hospital Sapienza, Rome); Japan A Morita, T Kimura (Hospital NTT Medical Center Tokyo, Tokyo); Latvia K Auslands (Riga East University Hospital, Clinic Gailezers, Riga), E Valeinis, R Mikijanskis (Pauls Stradins Clinical University Hospital, Riga); Lithuania A Gvazdaitis, K Jacikevicius, D Liutkus (Klaipeda University Hospital, Klaipeda); Macedonia K Lozance, A Chaparoski, I Pangovski (University Neurosurgical Clinic Skopje, Skopje); Malaysia N Rahman (Hospital Sultanah Aminah, Johor Bahru), J M Abdullah, S K Sim (Hospital Universiti Sains Malaysia, Kubang Kerian); Mexico S Romero-Vargas, D Mendez-Rosito (Instituto Nacional de Neurologia y Neurochirugia, Mexico City), J Ruiz-Sandoval (Hospital Civil de Guadalajara, Guadalajara); Nepal Y B Roka (B P Koirala Institute of Health Sciences, Dharan), K Sharma (B & B Hospital, Lalitpur); Pakistan K Mahmood, T Salahuddin (Lahore General Hospital, Lahore), M T Khan, F F Khan (Northwest General Hospital and Research Centre, Peshawar); Poland S Nowak, B Sokol (Karol Marcinkowski University of Medical Sciences, Poznan), P Szydlik, Z Mariak, J Kochanowicz (Medical University Hospital, Bialystok); Romania I S Florian, P A Kiss (Cluj County Emergency Hospital, Cluj-Napoca), H Ples, M A Angelescu, M S Hanas (County Hospital, Timisoara); Russia A Krivoshapkin (Novosibirsk State Medical University, Novosibirsk); Saudi Arabia E Elgamal (King Khalid University Hospital, Riyadh); Singapore D K S Choy, K J Teo (National University Hospital, Singapore); South Africa S Mokgokong (Steve Biko Academic Hospital, Pretoria); Spain R S Sarabia, I A Reganon (Hospital Universitario Rio Hortega, Valladolid), M Galarza (University Hospital Murcia, Murcia), A Vazquez-Barquero, I Pinto Rafael (University Hospital Marques de Valdecilla, Santander), J Garibi, I Pomposo (Cruces University Hospital, Bilbao), R Sarabia (Hospital Clinico Universitario, Valladolid), J Ibanez, E Gonzalez (Son Espases University Hospital, Palma de Mallorca), C Dominguez, L Muñoz (Germans Trias I Pujol Hospital, Badalona-Barcelona); Sri Lanka H S Kularathne, S D Perera, P Kamani (National Hospital of Sri Lanka); Turkey O Barlas, N Y Barlas (Istanbul University Faculty of Medicine, Istanbul); UK J Timothy, R Mathew (Leeds General Infirmary, Leeds), R Strachan, S Metcalfe (James Cook Univesity Hospital, Middlesbrough), A King, H Patel (Salford Royal, Salford), B A Bell, T L Jones (Atkinson Morley Department of Neurosurgery, London), D Bulters, A Belli, S Ross (Southampton University Hospital, Southampton), S Eljamel, F Falcone (Ninewells Hospital and Medical School, Dundee), G Critchley (Hurstwood Park Neurosurgical Centre, Brighton), P Kirkpatrick (Addenbrooke's Hospital, Cambridge), J Crossman, A D Mendelow, P Mitchell, N Ross, P Bhattathiri, S Metcalfe, D Holliman (Royal Victoria Infirmary, Newcastle), P Bhatt, M Kamel (Aberdeen Royal Infirmary, Aberdeen), P Eldridge (Walton Centre, Liverpool), N Gurusinghe, N Kumarasinghe (Lancashire Teaching Hospital NHS Trust, Preston), R A Salman, I Whittle (Western General Hospital, Edinburgh); USA I Kureshi, L Hosig (Hartford Hospital, Hartford), M Weaver, F Sultan, D Laske, P Connolly (Temple University Hospital, Philadephia), G Zipfel (Washington University School of Medicine, St Louis), J German (Albany Medical Centre, Albany), M Schneck, C Loftus (Loyola University Hospital, Chicago), K M Cockroft (Penn State Hershey Medical Centre, Hershey), B H Eidelman, J F Meschia (Mayo Clinic, Jacksonville), S Amin-Hanjani, K Slavin (University of Illinois Hospital and Health Sciences System, Chicago).
Conflicts of interest
ADM is a director of Newcastle Neurosurgical Foundation, which is a non-profit organisation for academic research and education, and he is an adviser to Stryker (craniofacial surgery committee). BAG and ENR received salary support from the STICH II grant but not from any commercial organisations. The other authors declare that they have no conflicts of interest. All surgeons in fee-for-service health-care systems receive additional fees for undertaking surgery.
Supplementary Material
References
- 1.van Asch CJJ, Luitse MJA, Rinkel GJE, van der Tweel I, Algra A, Klijn CJM. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 3.Siddique MS, Fernandes HM, Arene NU, Wooldridge TD, Fenwick JD, Mendelow AD. Changes in cerebral blood flow as measured by HMPAO SPECT in patients following spontaneous intracerebral haemorrhage. Acta Neurochir Suppl. 2000;76:517–520. doi: 10.1007/978-3-7091-6346-7_108. [DOI] [PubMed] [Google Scholar]
- 4.Nehls DG, Mendelow DA, Graham DI, Teasdale GM. Experimental intracerebral hemorrhage: early removal of a spontaneous mass lesion improves late outcome. Neurosurgery. 1990;27:674–682. [PubMed] [Google Scholar]
- 5.Mendelow AD, Bullock R, Teasdale GM, Graham DI, McCulloch J. Intracranial haemorrhage induced at arterial pressure in the rat: part 2. Short term changes in local cerebral blood flow measured by autoradiography. Neurol Res. 1984;6:189–193. doi: 10.1080/01616412.1984.11739688. [DOI] [PubMed] [Google Scholar]
- 6.Mendelow AD. Mechanisms of ischaemic brain damage with intracerebral haemorrhage. Stroke. 1993;24(suppl I):I115. I1I7. [PubMed] [Google Scholar]
- 7.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 8.Keep RF, Xi G, Hua Y, Hoff JT. The deleterious or beneficial effects of different agents in intracerebral hemorrhage: think big, think small, or is hematoma size important? Stroke. 2005;36:1594–1596. doi: 10.1161/01.STR.0000170701.41507.e1. [DOI] [PubMed] [Google Scholar]
- 9.Xi G, Wagner KR, Keep RF. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 10.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, STICH Investigators Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 11.Auer LM, Deinsberger W, Niederkorn K. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70:530–535. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 12.Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990;47:1103–1106. doi: 10.1001/archneur.1990.00530100071015. [DOI] [PubMed] [Google Scholar]
- 13.Chen XC, Wu JS, Zhou XP. The randomized multicentric prospective controlled trial in the standardized treatment of hypertensive intracerebral hematomas: the comparison of surgical therapeutic outcomes with conservative therapy. Chin J Clin Neurosci. 2001;9:365–368. [Google Scholar]
- 14.Juvela S, Heiskanen O, Poranen A. The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg. 1989;70:755–758. doi: 10.3171/jns.1989.70.5.0755. [DOI] [PubMed] [Google Scholar]
- 15.McKissock W, Richardson A, Taylor J. Primary Intracerebral haematoma: a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;278:221–226. [Google Scholar]
- 16.Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359–1363. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- 17.Zuccarello M, Brott T, Derex L. Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. 1999;30:1829–1833. doi: 10.1161/01.str.30.9.1833. [DOI] [PubMed] [Google Scholar]
- 18.Teernstra O, Evers S, Lodder J, Leffers P, Franke C, Blaaw G. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA) Stroke. 2003;34:968–974. doi: 10.1161/01.STR.0000063367.52044.40. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Yang H, Cheng Z. A prospective randomised trial of surgical and conservative treatment of hypertensive intracerebral haemorrhage. Acta Acad Shanghai Med. 1992;19:237–240. [Google Scholar]
- 20.Mendelow AD, Gregson BA, Fernandes HM, for the STICH investigators Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 21.Mendelow AD, Gregson BA, Mitchell PM. Surgical Trial in Lobar Intracerebral Haemorrhage (STICH II) Protocol. Trials. 2011;12:124. doi: 10.1186/1745-6215-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JT, Edwards P, Fiddes H, Stewart E, Teasdale GM. Reliability of postal questionnaires for the Glasgow Outcome Scale. J Neurotrauma. 2002;19:999–1005. doi: 10.1089/089771502760341910. [DOI] [PubMed] [Google Scholar]
- 23.Murray GD, Barer D, Choi S. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J Neurotrauma. 2005;22:511–517. doi: 10.1089/neu.2005.22.511. [DOI] [PubMed] [Google Scholar]
- 24.Gregson BA, Murray GD, Mitchell PM, Rowan EN, Gholkar AR, Mendelow AD. Update on the Surgical Trial in Lobar Intracerebral Haemorrhage (STICH II): statistical analysis plan. Trials. 2012;13:222. doi: 10.1186/1745-6215-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broderick JP, Brott TG, Grotta JC. Intracerebral hemorrhage volume measurement. Stroke. 1994;25:1081. [PubMed] [Google Scholar]
- 26.Fairbank J, Frost H, Wilson-MacDonald J, Yu LM, Barker K, Collins R. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ. 2005;330:1233. doi: 10.1136/bmj.38441.620417.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein JN, Tosteson TD, Lurie JD. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296:2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fung C, Murek M, Z'Graggen WJ. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke. 2012;43:3207–3211. doi: 10.1161/STROKEAHA.112.666537. [DOI] [PubMed] [Google Scholar]
- 29.Hanley DF. MISTIE III: Minimally Invasive Surgery plus rTPA for ICH Evacuation Phase III. 2013. http://braininjuryoutcomes.com/mistie-iii-about (accessed May 17, 2013).
- 30.Hanley DF. CLEAR-III Clot lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III. 2013. http://braininjuryoutcomes.com/clear-about (accessed April 12, 2013). [PMC free article] [PubMed]
- 31.Naff N, Williams MA, Keyl PM. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the Intraventricular Hemorrhage Thrombolysis Trial. Stroke. 2011;42:3009–3016. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad K, Mendelow AD, Gregson B. Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev. 2008;4 doi: 10.1002/14651858.CD000200.pub2. CD000200. [DOI] [PubMed] [Google Scholar]
- 37.Mendelow A, Gregson B. Surgery for intracerebral hemorrhage. In: Mohr J, Wolf P, Grotta JC, Moskowitz M, Mayberg M, von Kummer R, editors. Stroke: pathophysiology, diagnosis and management. 5th edn. WB Saunders; Philadelphia: 2011. [Google Scholar]
- 38.Gregson BA, Broderick JP, Auer LM. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012;43:1496–1504. doi: 10.1161/STROKEAHA.111.640284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited References
- 32.Hosseini H, Leguerinel C, Hariz M. Stereotactic aspiration of deep intracerebral hematomas under computed tomographic control: a multicentric prospective randomised trial. Cerebrovas Dis. 2003;16S:57. [Google Scholar]
- 33.Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomised study. J Neurosurg. 2004;101:417–420. doi: 10.3171/jns.2004.101.3.0417. [DOI] [PubMed] [Google Scholar]
- 34.Pantazis G, Tsitsopoulos P, Mihas C. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: a prospective randomized study. Surg Neurol. 2006;66:492–501. doi: 10.1016/j.surneu.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 35.Wang WZ, Jiang B, Liu HM. Minimally invasive craniopuncture therapy vs conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke. 2009;4:11–16. doi: 10.1111/j.1747-4949.2009.00239.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


