Abstract
Chronic intestinal and hepatic colonization with the microaerophilic murine pathogen Helicobacter hepaticus can lead to a range of inflammatory diseases of the lower digestive tract. Colonization is associated with an active cellular immune response and production of oxygen radicals. During colonization, H. hepaticus needs to cope with and respond to oxidative stress, and here we report on the role of the H. hepaticus PerR-regulator (HH0942) in the expression of the peroxidase-encoding katA (HH0043) and ahpC (HH1564) genes. Transcription of katA and ahpC was induced by hydrogen peroxide, and by iron restriction of growth media. This iron- and hydrogen peroxide-responsive regulation of katA and ahpC was mediated at the transcriptional level, from promoters directly upstream of the genes. Inactivation of the perR gene resulted in constitutive, iron-independent high-level expression of the katA and ahpC transcripts and corresponding proteins. Finally, inactivation of the katA gene resulted in increased sensitivity of H. hepaticus to hydrogen peroxide and reduced aerotolerance. In H. hepaticus, iron metabolism and oxidative stress defense are intimately connected via the PerR regulatory protein. This regulatory pattern resembles that observed in the enteric pathogen Campylobacter jejuni, but contrasts with the pattern observed in the closely related human gastric pathogen Helicobacter pylori.
Keywords: catalase, gene regulation, H. hepaticus, iron, oxidative stress defense, peroxide
Introduction
Members of the genus Helicobacter are microaerophilic bacteria that colonize the different organs of the mammalian digestive tract, and owing to their immunostimulative properties they are associated with the development of immunoproliferative diseases [1]. Helicobacter hepaticus is a murine pathogen, which colonizes the intestines, but can also be found in the liver and bile ducts [2]. Infection with H. hepaticus is associated with typhlocolitis, hepatitis and hepatocellular carcinoma [3, 4], and may also induce the formation of cholesterol gallstones [5, 6]. H. hepaticus infection is widespread in mouse colonies used for experimental infections [7], and the inflammatory reactions generated in response to intestinal, biliary, or hepatic colonization may well influence the outcome of murine infection experiments with other enteric pathogens.
Colonization of host tissues by bacterial pathogens often results in an active cellular immune response associated with the production of reactive oxygen species (ROS) by inflammatory cells [8]. ROS can damage lipids, proteins and DNA by oxidation, and thus bacteria will attempt to remove ROS before they cause significant damage, and have evolved protective mechanisms aimed at defusing the antimicrobial activity of ROS [9]. In addition to external sources, ROS are also produced by cellular metabolism, and this is often intimately coupled to cellular iron metabolism [9, 10]. In the presence of oxygen, iron can generate ROS like superoxide anions (O2–), peroxides (RO2), and hydroxyl radicals (OH•) through the Haber–Weiss and Fenton reactions (Fe2+ + O2 → Fe3+ + O2– and Fe2+ + H2O2→ Fe3+ + OH– + OH•). To combat and prevent oxidative stress, bacteria have evolved well-regulated systems that can sense and respond to changes in intracellular or environmental concentrations of iron and ROS, and both stimuli are important signals for pathogenic bacteria to trigger expression of virulence determinants [8].
Peroxide stress is a subset of oxidative stress, and resistance to peroxides is mediated by peroxidases like catalase [9]. Analysis of the H. hepaticus genome sequence [11, 12] has indicated that the components of the H. hepaticus peroxide stress resistance system resemble that of the related pathogen Campylobacter jejuni, and contain several peroxidases including a single catalase [13] and an alkyl hydroperoxide reductase [14, 15]. In addition to this, H. hepaticus also expresses an NADPH quinone reductase (MdaB) that is identified as an oxidative stress-combating enzyme [16].
Expression of oxidative stress defense proteins is usually coupled with the presence of the stressor, and different forms of oxidative stress-responsive regulatory mechanisms have been described [17]. The members of the genus Helicobacter lack an ortholog of the OxyR regulator [11, 17], but some species including H. hepaticus (but not Helicobacter pylori) contain a gene which encodes a putative PerR peroxide stress regulatory protein [18, 19]. The PerR regulatory protein belongs to the ferric uptake regulator (Fur) class of metal-responsive repressor proteins [20], which require a metal-cofactor for their activity. PerR is thought to use either manganese or iron to detect oxidative stress via metal-catalyzed ligand oxidation [21], and changes to the cofactor affect its DNA-binding ability [22].
In this study, we have investigated the function and regulation of the peroxide stress defense proteins KatA and AhpC of H. hepaticus, and demonstrate that catalase is required for survival of peroxide stress and for aerotolerance. The expression of catalase and AhpC is repressed by iron, but induced by peroxide stress, and this regulation is mediated by PerR at the transcriptional level.
Materials and methods
Bacterial strains, plasmids and growth conditions
H. hepaticus strain ATCC 51449 [2] and its isogenic perR and katA mutants (this study) were routinely cultured at 37 °C in microaerobic conditions (5% O2, 7.5% CO2, 7.5% H2, and 80% N2) on Dent agar (Oxoid) [23]. Liquid growth was performed in Brucella broth (Difco) supplemented with 0.2% (w/v) β-cyclodextrins (Fluka) (BBC) [24]. For growth under iron-restricted conditions, 20 µM of the iron chelator desferoxamine mesylate (desferal, Sigma) was added to the Brucella medium prior to addition of the β-cyclodextrins. Iron-replete media were obtained by the addition of 100 µM of FeCl3 (Sigma) to the desferal-treated BBC medium [24]. Bacteria were inoculated with a starting OD600 of 0.05. For primer extension analysis, cells were grown overnight (16–24 h) in BBC and were then incubated for 30 min in the presence of 100-µM 2,2’-dipyridyl (Sigma) or 100-µM 2,2’-dipyridyl and 100-µM FeCl3 [24]. Escherichia coli strains DH5a and ER1793 were grown aerobically in Luria Bertani medium [25] at 37 °C. Where appropriate, media were supplemented with ampicillin (final concentration 100 µg ml–1) or chloramphenicol (final concentration 20 µg ml–1).
Peroxide sensitivity and aerotolerance experiments
H. hepaticus strains were grown overnight in BBC. In the case of peroxide shock experiments, bacteria were centrifuged for 10 min at 4000×g, and the pellet was resuspended in PBS (Phosphate buffered saline) to a final OD600 of 0.5, and incubated with 100-µM H2O2 for 15 min. Subsequently, either RNA was isolated for downstream experiments or 5 µl of 10-fold dilutions was spotted on Dent agar for viability counts. Plates were incubated for 2 days at 37 °C under microaerobic conditions [15]. For the aerotolerance assay, bacteria were incubated under either microaerobic or aerobic conditions for 2, 4, 6, 8, 10 or 12 h at 37 °C. At each timepoint, samples were taken and 10-fold dilutions were spotted on Dent agar for viability counts, and incubated for 2 days at 37 °C under microaerobic conditions [15, 26].
RNA analysis
RNA was isolated from H. hepaticus with Trizol reagent (Invitrogen), according to the manufacturer’s instructions. Gel electrophoresis of RNA, transfer to positively charged nylon membranes (Roche), crosslinking, hybridization to digoxygenin (DIG)-labeled specific RNA probes, and detection of bound probe were performed as described previously [23]. Probes specific for H. hepaticus perR and katA were synthesized by in vitro transcription using T7 RNA polymerase (Roche) and PCR products obtained with primers HhahpC-F2+HhahpC-R1-T7 and HhkatA-F2+ HhkatA-R1-T7 for the ahpC and katA genes, respectively. Primer extension analyses were performed using the reverse primers HhahpC-DIG and HhkatA-DIG, respectively, and AMV reverse transcriptase (Promega) as described previously [24]. All primers used are listed in Table 1.
Table 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5’→3’)* |
|---|---|
| Primers used for insertional inactivation | |
| HhperR-mutF1 | TTGGAGAATCTTTAGAG |
| HhperR-mutR1 | GGAGCATTTCACATATTCTG |
| HhperR-outF1 | ATCTTGGAGCGCATTTATGA |
| HhperR-outR1 | CTCACGCGAGATGATTGTAG |
| HhkatA-F1 | AGGAGTTTCTCCAAGTGTAT |
| HhkatA-R1 | TGCGTCTATCGCCAAACTGA |
| Primers used for qRT-PCR | |
| HhahpC-FQ1 | GGAGGGATTGGCGCAGTAAC |
| HhahpC-RQ1 | ATGACTGCGTGGCGAACTAC |
| HhkatA-FQ | GGTTTTGCACTCAAGCTTTA |
| HhkatA-RQ | AAATCCCACATTGCCGTAGG |
| Hh16SFQ | GCGACCTGCTGGAACATTAC |
| Hh16SRQ | CCCAGGCGGGATGCTTAATG |
| Primers used for primer extension analysis | |
| HhkatA-DIG‡ | GCCTGCTGTAATTGAGTTTT |
| HhahpC-DIG‡ | CGCCTCCGCTGTAAAATCTG |
| Primers used for Northern hybridization | |
| HhkatA-F2 | GTGAGCGAGGAGCAGCAGAT |
| HhkatA-R1-T7† | ctaatacgactcactatagggagaGGCGATAACTGCCTCTGA |
| HhahpC-F2 | CCCAATGCTCGCAGGCATAG |
| HhahpC-R1-T7† | ctaatacgactcactatagggagaCGAGCATTGGGCTTTTCCAATA |
| *Primer sequences derived from the
H. hepaticus ATCC 51449 genome sequence
[12]. †Lowercase letters indicate the T7 promoter used for in vitro transcription. ‡Primer labeled with a 5’ digoxygenin (DIG) group for detection purposes. | |
Quantitative reverse transcriptase-PCR analyses
All reverse transcription reactions were performed using 100 ng of RNA, and gene-specific primers (DQ and RQ primers, Table 1), essentially as described previously [27, 28]. cDNA was used directly for quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). qRT-PCR reactions contained 5 µl of cDNA template, 25-pM gene-specific primers (Table 1), 10-nM dNTP, 1 unit Taq polymerase (Promega), and dH2O to a final volume of 25 µl. SYBR green was added in a 10,000-fold dilution as instructed by the supplier (Bio-Rad IQ5 manual). Transcript levels were normalized to the levels of 16S rRNA in each sample and gene expression was calculated using the 2(ΔΔC(T)) analysis method [29]. qRT-PCR assays were performed using RNA isolated from three independent growth experiments.
Construction of H. hepaticus mutants
The perR and katA genes of H. hepaticus strain ATCC 51449 were amplified using primers HhperR-mutF1+HhperR-mutR1 and HhkatAF1+HhkatAR1, respectively, and cloned in pGEM-Teasy (Promega). The genes were subsequently interrupted by insertion of the chloramphenicol (CmR) from pAV35 [30] resistance gene resulting in plasmids pCB31 (perR::CmR) and pCB41(katA::CmR). Plasmids were introduced into E. coli ER1793 and subsequently used for natural transformation of H. hepaticus ATCC 51449 [24]. Two chloramphenicol-resistant colonies, each derived from an independent transformation experiment, were subsequently selected and gave identical results in all experiments. Correct allelic replacement of the perR gene with the interrupted version was confirmed by PCR using the primers HhperR-outF1 and HhperR-outR1. The katA mutant was confirmed by absence of the KatA protein on an SDS-PAGE (Sodium dodecyl sulfate polycrylamide gel electrophoresis) gel and absence of catalase activity in a peroxide assay (data not shown).
Protein analysis
H. hepaticus wild-type strain and its, isogenic mutant cells were grown for 16 h in BBC supplemented with 20-µM desferal or 20-µM desferal and 100-µM FeCl3, centrifuged at 4000×g for 10 min at room temperature and the pellet was resuspended in PBS (pH 7.4) to a final OD600 of 10. H. hepaticus cells were subsequently lysed by sonication with a MSE Soniprep 150 (MSE, Crawley, UK) for 15 s on ice at amplitude 6. Proteins were separated by SDS-PAGE on a 10% (w/v) polyacrylamide gel and stained with Coomassie Brilliant Blue. Proteins were isolated from the SDS-PAGE and identified by MALDI-TOF (Matrix-assisted laser desorption/ionization-time of flight) analysis as described before [24].
Results
The H. hepaticus ahpC and katA promoters are iron repressed
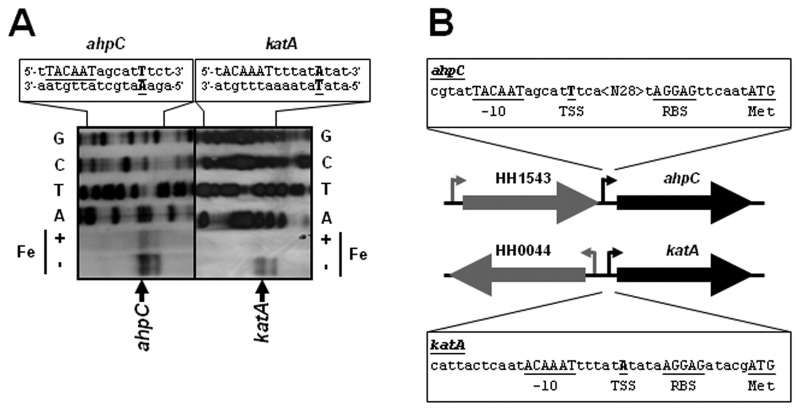
Analysis of the H. hepaticus ATCC 51449 genome sequence [12] allowed the identification of several peroxidases, which include the catalase KatA (HH0043) and the alkyl hydroperoxide reductase AhpC (HH1564, also known as thiol-specific antioxidant TsaA). Expression of these peroxidases is iron repressed in the phylogenetically related enteric pathogen C. jejuni, but it is iron independent in H. pylori [18, 31]. To identify the promoter region controlling transcription of the ahpC and katA genes, we performed primer extension analysis on RNA isolated from H. hepaticus ATCC 51449 grown in iron-restricted and iron-replete conditions (Fig. 1A). For both genes, primer extension products were detected, with the 5’ end directly upstream of the ahpC and katA genes (Fig. 1A). The level of ahpC and katA primer extension product was visibly lower when RNA isolated from iron-replete conditions was used, indicative of iron-repressed transcription (Fig. 1A). For both genes, three primer extension products were obtained, all very close to each other. The products with the highest intensity were located 44 nt and 16 nt upstream of the ahpC and katA ATG start codons, respectively (Fig. 1B), and are preceded by a –10 sequence resembling the TanaAT box of the H. pylori σ70 vegetative sigma factor for RNA polymerase [32].
Fig. 1.
The H. hepaticus ahpC (HH1544) and katA (HH0043) genes have an iron-responsive promoter directly upstream of the coding sequence. (A) Primer extension analysis for the identification of the ahpC and katA transcription start sites. RNA from iron-restricted (–Fe) and iron-replete (+Fe) cultures of wild-type H. hepaticus ATCC 51449 was used for primer extension using a DIG-labeled primer (Table 1). Please note that the primer extension product displays iron-responsive repression of transcription. A standard dideoxy sequencing reaction was performed with the same DIG-labeled primer, and is shown above the primer extension reactions, with the respective dideoxynucleotide indicated next to the lanes, and the sequence of both strands given at the top of the figure. For both genes, the primer extension experiment resulted in three products, of which the product with the strongest band intensity is indicated by an arrow. For sequence annotation, see below. (B) Schematic representation of the genomic locations of the H. hepaticus ahpC and katA genes, with the transcription start site and associated promoter sequences annotated. The underlined sequence annotated as -10 is the sequence most closely resembling the σ70 –10 sequence (5’-TanaAT) identified in H. pylori [32]. The underlined residues represent annotated features in the sequence: TSS represents the transcription start site of the band highlighted in panel A, RBS is the proposed Shine-Dalgarno sequence, Met is the translational start of the gene, <N28> is a stretch of 28 nucleotides, and –10 is the proposed –10 box for the σ70 sigma factor
The PerR regulator controls iron- and H2O2-responsive expression of ahpC and katA
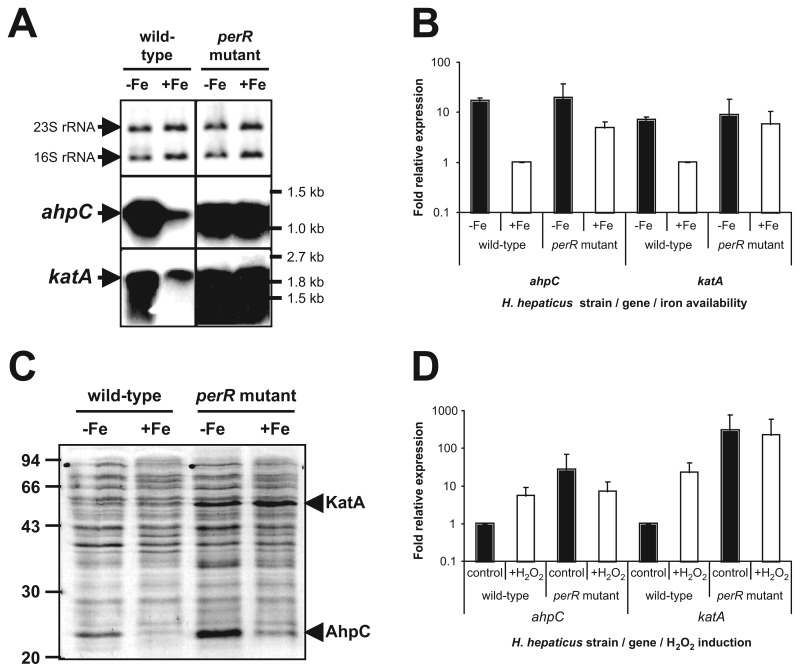
The iron-repressed transcription of the ahpC and katA genes suggested the involvement of a metal-dependent transcriptional regulator. To investigate the role of the proposed PerR regulator in iron-responsive regulation, we inactivated the H. hepaticus perR gene by insertion of a chloramphenic antibiotic resistance cassette and assessed transcription of ahpC and katA in the perR mutant by Northern hybridization (Fig. 2A) and qRT-PCR (Fig. 2B). In the wild-type strain, the ahpC and katA genes were transcribed on mRNAs of ~1.2 kb and 2.0 kb, respectively, which suggests a monocistronic transcript for ahpC and co-transcription of katA and the downstream HH0042 (kapA [31]) gene. Transcription of both ahpC and katA was iron repressed in the wild-type strain but was very high and iron independent in the perR mutant (Fig. 2A). This transcriptional pattern was confirmed using qRT-PCR (Fig. 2B), with transcription of both ahpC and katA being induced more than fivefold by iron restriction in the wild-type strain, while transcription was not significantly affected by iron’s status in the perR mutant (Fig. 2B). Finally, comparison of the whole-cell protein profiles of the H. hepaticus wild-type strain and perR mutant by SDS-PAGE revealed that 25 and 55 kDa proteins showed both iron- and PerR-dependent repression of expression (Fig. 2C). Subsequent identification of these proteins by MALDI-TOF analysis confirmed the identity of the 25-kDa protein as AhpC, and the 55-kDa protein as KatA.
Fig. 2.
PerR controls iron-responsive repression and H2O2-responsive induction of ahpC and katA expression in H. hepaticus, as demonstrated by (A) Northern hybridization, (B) qRT-PCR, and (C) protein separation by SDS-PAGE analysis. (A) Northern hybridization of H. hepaticus total RNA isolated from cultures grown in iron-restricted (–Fe) and iron-replete (+Fe) conditions with specific probes for ahpC (middle panel) and katA (lower panel) is shown. The top panel displays a methylene blue staining of transferred rRNA, to show that equal amounts of RNA were loaded. The positions of the 16S and 23S rRNA are indicated on the left and relevant marker sizes on the right. (B) qRT-PCR analysis of ahpC and katA transcript levels after overnight growth of H. hepaticus wild type strain and perR mutant, in either iron-restricted medium (black bars, –Fe) or iron-replete medium (white bars, +Fe). The levels of mRNA were calculated from the Ct-value observed in the qPCR reaction, after normalization to the levels of 16S rRNA [29], and are expressed relative to wild-type transcript levels in iron-replete medium, which was set to 1. Error bars represent standard deviations, from two biological replicates. (C) Total protein of H. hepaticus ATCC 51449 and perR mutant, grown in iron-restricted medium (–Fe) and iron-replete medium (+Fe), was separated by SDS-PAGE and stained using Coomassie Brilliant Blue. Relevant marker sizes are indicated on the left. The protein bands identified as the H. hepaticus KatA and AhpC proteins by MALDI-TOF analysis are indicated on the right. (D) qRT-PCR analysis of ahpC and katA transcript levels after a 15-min incubation of H. hepaticus wild type strain and perR mutant without H2O2 (control) or with 100-µM H2O2 (+H2O2). The levels of mRNA observed in the qRT-PCR were normalized to the levels of 16S rRNA [29], and expressed as relative to wild-type transcript levels without exposure to H2O2, which were set to 1. Error bars represent standard deviations, from two biological replicates
To investigate whether expression of the peroxidases AhpC and KatA is responsive to peroxide stress, the effect of short (15 min) incubation with 100-µM H2O2 on transcription of ahpC and katA was determined by qRT-PCR in the wild-type strain and perR mutant (Fig. 2D). In the wild-type strain, transcription of ahpC and katA was induced sevenfold and 22-fold, respectively, by exposure to H2O2, while transcript levels were already much higher in the perR mutant and did not change significantly upon exposure to H2O2(Fig. 2D).
Catalase contributes to hydrogen peroxide resistance and aerotolerance
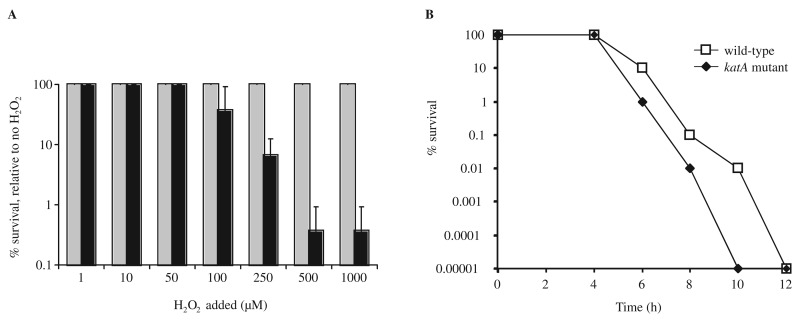
The role of KatA in peroxide stress resistance and aerotolerance of H. hepaticus was investigated using an isogenic H. hepaticus katA mutant. H. hepaticus wild-type ATCC 51449 and the katA mutant were exposed to a range of H2O2 concentrations (1–1000 µM) for 30 min, after which the viability of the cells was analyzed (Fig. 3A). The wild-type strain did not show a decrease in viability with any of the used H2O2 concentrations, but the katA mutant strain was much more sensitive to hydrogen peroxide, with up to a 3-log decrease in viability at H2O2 concentrations exceeding 100 µM (Fig. 3A).
Fig. 3.
The H. hepaticus catalase is required for resistance to hydrogen peroxide and contributes to aerotolerance. (A) H. hepaticus peroxide sensitivity was measured by incubating strain ATCC 51449 (gray bars) and its isogenic katA mutant (black bars) in PBS supplemented with H2O2 concentrations ranging from 0 to 1000 mM. Survival of cells was monitored by spotting 10-fold dilutions on Dent plates, and visual assessment of growth after 2 days. Survival is expressed as percentage of the cfu count of the wild-type strain or katA mutant in PBS without H2O2 supplementation. The wild-type strain did not show any killing by H2O2 at these concentrations, whereas the katA mutant showed decreased survival at H2O2 concentrations exceeding 100 µM. Data shown are the average of three independent experiments, and error bars denote standard deviation. (B) Inactivation of katA reduces aerotolerance of H. hepaticus. Aerotolerance of H. hepaticus wild-type strain (open squares) and its isogenic katA mutant (black diamonds) was determined by exposing half of an overnight culture to atmospheric oxygen conditions [15, 26]. Survival of cells was monitored by plating 10-fold dilutions on Dent plates, and is expressed as percentage of the other half of the culture incubated microaerobically. Data shown are from a single experiment, representative for the three experiments performed
To examine the role of KatA in aerotolerance of H. hepaticus, wild-type and katA mutant cells were exposed to atmospheric oxygen levels, and viability was compared with cells incubated in microaerophilic conditions. The katA mutant displayed a reduced tolerance to atmospheric oxygen conditions when compared to the wild-type strain (Fig. 3B), with cfu counts decreasing more rapidly and going below detection levels ~2 h earlier than the wild-type strain.
Discussion
Obligate microaerobic organisms like H. hepaticus are continuously faced with a conundrum: while they require oxygen for metabolism, they cannot tolerate atmospheric levels of oxygen, and hence require systems to defend themselves from the deleterious reaction products of oxidative metabolism. Furthermore, pathogenic microorganisms often induce an active host immune response to their presence, which includes production of ROS by immune cells, and successful pathogens need to be able to survive exposure to these ROS. Peroxidases constitute a part of the standard set of oxidative stress defenses of virtually all cells, with catalase likely to be the best studied peroxidase. Catalase mediates the breakdown of hydrogen peroxide to water and oxygen. This protects the cells against the deleterious features of peroxides, which can lead to production of hydroxyl radicals or other ROS, which in turn can lead to DNA strand breaks, lipid peroxidation and oxidative damage of proteins [9].
H. hepaticus produces high levels of catalase, of almost 1% of total protein content [13]. The high energy cost to the organism suggests that catalase is an important enzyme for H. hepaticus, and this is confirmed by the aerotolerance experiments shown in Fig. 3B. Absence of catalase shortens the survival time of H. hepaticus in aerobic conditions, possibly because of their inability to breakdown peroxides formed during oxidative reactions induced by the high oxygen levels. The results obtained are in agreement with other studies on oxidative stress resistance in H. hepaticus [13, 14] and C. jejuni [15, 33]. Disruption of the katA gene also results in sensitivity to hydrogen peroxide (Fig. 3A), and confirms the results of earlier studies [9, 13]. Mice infected with H. hepaticus demonstrated immune responses to murine and H. hepaticus catalase, suggesting that apart from oxidative stress defense mediated by H. hepaticus catalase, it may also contribute to autoimmune responses [34].
In this study, we have demonstrated that transcription of the H. hepaticus katA and ahpC genes is increased in response to iron restriction and to the presence of hydrogen peroxide and that this regulation is mediated via the PerR regulatory protein. Insertional inactivation of the perR gene results in high level, iron- and peroxide-independent transcription of ahpC and katA (Fig. 2a, b, c), which is reflected in the protein levels of AhpC and KatA (Fig. 2C). This is similar to the role of PerR in other organisms like C. jejuni, Streptococcus pyogenes, Staphylococcus aureus and Bacillus subtilis [18, 33, 35–37].
PerR is a member of the Fur family of metalloregulatory proteins, which normally bind to promoters when complexed with a regulatory metal cofactor but are unable to bind to target promoters in the absence of this metal. The best known example of this family is the Fur, which controls transcription of iron-acquisition, iron-storage, and iron-utilization genes in many bacteria [20]. PerR was originally described in B. subtilis and was subsequently found in both Gram-positive and Gram-negative organisms, and can be seen as a functional replacement of the OxyR regulatory system present in many bacteria [17]. The mechanism by which PerR senses peroxide stress is not yet completely clear, but both oxidation of histidine amino acids in the PerR protein and the oxygen-labile iron metal and more stable manganese metal ions are thought to play important roles in allowing PerR to dissociate from its metal cofactor and, as a result, lose the ability to repress its target promoters upon peroxide stress [20–22, 38, 39]. However, all these mechanisms have been characterized using the B. subtilis PerR protein, and it is not clear yet whether PerR proteins from other bacteria are using some or all of these mechanisms or may have evolved to use alternative mechanisms. There are differences in iron and manganese homeostasis mechanisms between the different PerR-positive bacterial species, and the amino acid sequences of the PerR proteins may allow for functional differentiation between PerR from Gram-positive bacteria like B. subtilis and Gram-negative bacteria like H. hepaticus. As we have been unable to recombinantly express H. hepaticus PerR, we were not able to study the protein itself (data not shown).
The regulatory pattern described for the H. hepaticus PerR regulon resembles that seen in the closely related enteric pathogen C. jejuni, but is not a general mechanism of Helicobacter species, as the genome of the human gastric pathogen H. pylori does not encode a recognizable PerR ortholog [11]. It is possible that the Fur contributes to oxidative stress responses, and a role for Fur has been reported for the H. pylori catalase and superoxide dismutase [31, 40]. In C. jejuni, both Fur and PerR control the ahpC and katA genes, although inactivation of PerR has the most dramatic effect on AhpC and KatA expression [18]. Inactivation of the fur gene in H. hepaticus did not significantly affect ahpC or katA transcription (data not shown), suggesting either functional diversification of the Fur and PerR systems in H. hepaticus or, as in C. jejuni [18], a more subtle effect of Fur on regulation of oxidative stress defense.
In conclusion, we have demonstrated the involvement of the KatA catalase in H. hepaticus oxidative stress resistance, and its regulation by iron and peroxide via the PerR regulatory protein. Iron restriction and increased peroxide levels may be linked, since peroxides can oxidize iron, thus preventing the metal cofactor from associating with the PerR protein. Alternatively, iron restriction can be a signal for colonization of host tissues and a signal indicating the necessity to protect the cell against peroxides that will be produced by the immune cells. Therefore, iron-dependent regulation of peroxide stress defense may be an advantage in host colonization.
Acknowledgments
We thank Jeroen Stoof, Raymond Pot and Mark Verbeek for technical support.
Contributor Information
C. Belzer, 1Department of Gastroenterology and Hepatology, Erasmus MC-University Medical Center, Rotterdam, The Netherlands; 4Laboratory of Microbiology, Wageningen University, Wageningen, The Netherlands.
B. A. M. van Schendel, 1Department of Gastroenterology and Hepatology, Erasmus MC-University Medical Center, Rotterdam, The Netherlands.
T. Hoogenboezem, 3Department of Pediatrics, Erasmus MC-University Medical Center, Rotterdam, The Netherlands.
J. G. Kusters, 5Department of Medical Microbiology, University Medical Center Utrecht, Utrecht, The Netherlands.
P. W. M. Hermans, 6Laboratory of Pediatric Infectious Diseases, Radboud University Nijmegen Medical Centre, The Netherlands.
A. H. M. van Vliet, 7Institute of Food Research, Norwich, United Kingdom.
E. J. Kuipers, 1Department of Gastroenterology and Hepatology, Erasmus MC-University Medical Center, Rotterdam, The Netherlands; 2Department of Internal Medicine, Erasmus MC-University Medical Center, Rotterdam, The Netherlands.
References
- 1.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006 Jul;19(3):449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ, Jr., Gorelick PL, Ward JM. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994 May;32(5):1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward JM, Anver MR, Haines DC, Melhorn JM, Gorelick P, Yan L, Fox JG. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996 Feb;46(1):15–20. [PubMed] [Google Scholar]
- 4.García A, Zeng Y, Muthupalani S, Ge Z, Potter A, Mobley MW, Boussahmain C, Feng Y, Wishnok JS, Fox JG. Helicobacter hepaticus-induced liver tumor promotion is associated with increased serum bile acid and a persistent microbial-induced immune response. Cancer Res. 2011 Apr 1;71(7):2529–2540. doi: 10.1158/0008-5472.CAN-10-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer KJ, Ihrig MM, Rogers AB, Ng V, Bouchard G, Leonard MR, Carey MC, Fox JG. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology. 2005 Apr;128(4):1023–1033. doi: 10.1053/j.gastro.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Belzer C, Kusters JG, Kuipers EJ, van Vliet AH. Urease induced calcium precipitation by Helicobacter species may initiate gallstone formation. Gut. 2006 Nov;55(11):1678–1679. doi: 10.1136/gut.2006.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor NS, Xu S, Nambiar P, Dewhirst FE, Fox JG. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J Clin Microbiol. 2007 Jul;45(7):2166–2172. doi: 10.1128/JCM.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron - a metal at the host-pathogen interface. Cell Microbiol. 2010 Dec;12(12):1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006 Aug;61(4):847–860. doi: 10.1111/j.1365-2958.2006.05302.x. [DOI] [PubMed] [Google Scholar]
- 10.Helmann JD, Wu MF, Gaballa A, Kobel PA, Morshedi MM, Fawcett P, Paddon C. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J Bacteriol. 2003 Jan;185(1):243–253. doi: 10.1128/JB.185.1.243-253.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belzer C, Stoof J, van Vliet AH. Metal-responsive gene regulation and metal transport in Helicobacter species. Biometals. 2007 Jun;20(3-4):417–429. doi: 10.1007/s10534-006-9028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suerbaum S, Josenhans C, Sterzenbach T, Drescher B, Brandt P, Bell M, Droge M, Fartmann B, Fischer HP, Ge Z, Horster A, Holland R, Klein K, Konig J, Macko L, Mendz GL, Nyakatura G, Schauer DB, Shen Z, Weber J, Frosch M, Fox JG. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7901–7906. doi: 10.1073/pnas.1332093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong Y, Wang G, Maier RJ. A Helicobacter hepaticus catalase mutant is hypersensitive to oxidative stress and suffers increased DNA damage. J Med Microbiol. 2007 Apr;56(Pt 4):557–562. doi: 10.1099/jmm.0.46891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta NS, Benoit SL, Mysore J, Maier RJ. In vitro and in vivo characterization of alkyl hydroperoxide reductase mutant strains of Helicobacter hepaticus. Biochim Biophys Acta. 2007 Feb;1770(2):257–265. doi: 10.1016/j.bbagen.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Baillon ML, van Vliet AH, Ketley JM, Constantinidou C, Penn CW. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J Bacteriol. 1999 Aug;181(16):4798–4804. doi: 10.1128/jb.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Y, Wang G, Maier RJ. The NADPH quinone reductase MdaB confers oxidative stress resistance to Helicobacter hepaticus. Microb Pathog. 2008 Feb;44(2):169–174. doi: 10.1016/j.micpath.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mongkolsuk S, Helmann JD. Regulation of inducible peroxide stress responses. Mol Microbiol. 2002 Jul;45(1):9–15. doi: 10.1046/j.1365-2958.2002.03015.x. [DOI] [PubMed] [Google Scholar]
- 18.van Vliet AH, Baillon ML, Penn CW, Ketley JM. Campylobacter jejuni contains two fur homologs: characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J Bacteriol. 1999 Oct;181(20):6371–6376. doi: 10.1128/jb.181.20.6371-6376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danielli A, Scarlato V. Regulatory circuits in Helicobacter pylori : network motifs and regulators involved in metal-dependent responses. FEMS Microbiol Rev. 2010 Sep;34(5):738–752. doi: 10.1111/j.1574-6976.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals. 2007 Jun;20(3-4):485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 21.Jacquamet L, Traoré DA, Ferrer JL, Proux O, Testemale D, Hazemann JL, Nazarenko E, El Ghazouani A, Caux-Thang C, Duarte V, Latour JM. Structural characterization of the active form of PerR: insights into the metal-induced activation of PerR and Fur proteins for DNA binding. Mol Microbiol. 2009 Jul;73(1):20–31. doi: 10.1111/j.1365-2958.2009.06753.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006 Mar 16;440(7082):363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 23.Belzer C, Stoof J, Beckwith CS, Kuipers EJ, Kusters JG, van Vliet AH. Differential regulation of urease activity in Helicobacter hepaticus and Helicobacter pylori. Microbiology. 2005 Dec;151(Pt 12):3989–3995. doi: 10.1099/mic.0.28188-0. [DOI] [PubMed] [Google Scholar]
- 24.Belzer C, van Schendel BA, Kuipers EJ, Kusters JG, van Vliet AH. Iron-responsive repression of urease expression in Helicobacter hepaticus is mediated by the transcriptional regulator Fur. Infect Immun. 2007 Feb;75(2):745–752. doi: 10.1128/IAI.01163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, a laboratory manual. New York: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.van Vliet AH, Baillon MA, Penn CW, Ketley JM. The iron-induced ferredoxin FdxA of Campylobacter jejuni is involved in aerotolerance. FEMS Microbiol Lett. 2001 Mar 15;196(2):189–193. doi: 10.1111/j.1574-6968.2001.tb10563.x. [DOI] [PubMed] [Google Scholar]
- 27.Belzer C, Stoof J, Breijer S, Kusters JG, Kuipers EJ, van Vliet AH. The Helicobacter hepaticus hefA gene is involved in resistance to amoxicillin. Helicobacter. 2009 Feb;14(1):72–79. doi: 10.1111/j.1523-5378.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- 28.Stoof J, Breijer S, Pot RG, van der Neut D, Kuipers EJ, Kusters JG, van Vliet AH. Inverse nickel-responsive regulation of two urease enzymes in the gastric pathogen Helicobacter mustelae. Environ Microbiol. 2008 Oct;10(10):2586–2597. doi: 10.1111/j.1462-2920.2008.01681.x. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.van Vliet AH, Wooldridge KG, Ketley JM. Iron-responsive gene regulation in a campylobacter jejuni fur mutant. J Bacteriol. 1998 Oct;180(20):5291–5298. doi: 10.1128/jb.180.20.5291-5298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris AG, Hinds FE, Beckhouse AG, Kolesnikow T, Hazell SL. Resistance to hydrogen peroxide in Helicobacter pylori: role of catalase (KatA) and Fur, and functional analysis of a novel gene product designated 'KatA-associated protein', KapA (HP0874) Microbiology. 2002 Dec;148(Pt 12):3813–3825. doi: 10.1099/00221287-148-12-3813. [DOI] [PubMed] [Google Scholar]
- 32.Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, Chabas S, Reiche K, Hackermüller J, Reinhardt R, Stadler PF, Vogel J. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010 Mar 11;464(7286):250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- 33.Palyada K, Sun YQ, Flint A, Butcher J, Naikare H, Stintzi A. Characterization of the oxidative stress stimulon and PerR regulon of Campylobacter jejuni. BMC Genomics. 2009 Oct 18;10:481. doi: 10.1186/1471-2164-10-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alyamani EJ, Brandt P, Pena JA, Major AM, Fox JG, Suerbaum S, Versalovic J. Helicobacter hepaticus catalase shares surface-predicted epitopes with mammalian catalases. Microbiology. 2007 Apr;153(Pt 4):1006–1016. doi: 10.1099/mic.0.29184-0. [DOI] [PubMed] [Google Scholar]
- 35.King KY, Horenstein JA, Caparon MG. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J Bacteriol. 2000 Oct;182(19):5290–5299. doi: 10.1128/jb.182.19.5290-5299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998 Jul;29(1):189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 37.Horsburgh MJ, Clements MO, Crossley H, Ingham E, Foster SJ. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect Immun. 2001 Jun;69(6):3744–3754. doi: 10.1128/IAI.69.6.3744-3754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traoré DA, El Ghazouani A, Jacquamet L, Borel F, Ferrer JL, Lascoux D, Ravanat JL, Jaquinod M, Blondin G, Caux-Thang C, Duarte V, Latour JM. Structural and functional characterization of 2-oxo-histidine in oxidized PerR protein. Nat Chem Biol. 2009 Jan;5(1):53–59. doi: 10.1038/nchembio.133. [DOI] [PubMed] [Google Scholar]
- 39.Faulkner MJ, Helmann JD. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal. 2011 Jul 1;15(1):175–189. doi: 10.1089/ars.2010.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ernst FD, Homuth G, Stoof J, Mäder U, Waidner B, Kuipers EJ, Kist M, Kusters JG, Bereswill S, van Vliet AH. Iron-responsive regulation of the Helicobacter pylori iron-cofactored superoxide dismutase SodB is mediated by Fur. J Bacteriol. 2005 Jun;187(11):3687–3692. doi: 10.1128/JB.187.11.3687-3692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]