Abstract
In the experimental models of intestinal inflammation and humans with inflammatory bowel diseases (IBD), increased levels of the matrix metalloproteinases (MMPs), MMP-2 and -9 (also referred to as gelatinase A and B, respectively), in inflamed tissue sites can be detected. In the presented study, we investigated potential beneficial effects exerted by doxycycline nonselectively blocking MMPs and the selective gelatinase inhibitor RO28-2653 in acute DSS colitis. Treatment with either compound for 8 days ameliorated clinical colitis pathology with a superior outcome in RO28-2653-treated animals. As compared to placebo controls, histopathological changes in the colon were less distinct following MMP blockage and IL-6 secretion in ex vivo biopsies was downregulated, paralleled by a diminished influx of pro-inflammatory immune cells and lack of overgrowth of the colonic lumen by potentially pro-inflammatory Escherichia coli of the commensal colon flora.
We conclude that selective gelatinase inhibition not only exerts beneficial effects by disrupting the vicious cycle of positive feedback between immune cell stimulation and MMP induction but also prevents overgrowth of the colonic lumen by pro-inflammatory E. coli despite a lack of direct anti-bacterial properties, thus unaffecting the commensal gut microbiota. These findings put RO28-2653 into a center stage for development of intervention strategies in human IBD.
Keywords: acute DSS colitis, doxycycline, E. coli, gelatinases, gut microbiota, matrix metalloproteinases, RO28-2653, synthetic blockage
Introduction
Matrix metalloproteinases (MMPs) belong to a large family of zinc- and calcium-dependent endopeptidases that degrade extracellular matrix under tight control of endogenous tissue inhibitors of matrix metalloproteinases (TIMPs) [1–3]. With respect to their substrate specificity, MMPs are categorized into collagenases (MMP-1, -8, -13, -18), gelatinases (MMP-2, -9), stromelysins (MMP-3, -7, -10, -11), elastase (MMP-12), and membrane-type matrix metalloproteinases (MT-MMP-1 through -5) [4]. Under physiological conditions, MMPs are involved in embryonic development and differentiation, proliferation, and regeneration of tissues [1, 3]. An imbalance of activators (e.g. pro-inflammatory molecules such as IL-1, IL-6, and TNF-α) and inhibitors (e.g. anti-inflammatory cytokines IL-4, IL-10, TGF-β besides TIMPs) of MMP expression and function results in tissue destruction [5, 6]. In experimental models of Th1-type inflammation (e.g. rheumatoid arthritis, atherosclerosis, and colitis) [7–10], as well as in humans with inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis [11–16], increased levels of gelatinases A (MMP-2) and B (MMP-9) in inflamed tissue sites associated with increased mRNA levels of IL-1, IL-6, and TNF-α could be detected. Most interestingly, MMPs are known to shed biologically active IL-1, IL-6, and TNF-α molecules from the surfaces of macrophages, which, in turn, induce MMP expression from immune, epithelial, and parenchymal cells [17, 18]. Thus, from a therapeutic point of view, it would be highly appreciable to cut the vicious pro-inflammatory cycles exerted by this positive MMP-mediated feedback loop pharmacologically. A potential synthetic compound should therefore effectively inhibit or downregulate expression and function of MMPs, immune cells (e.g. macrophages), and pro-inflammatory cytokines without compromising potential health-promoting MMP properties (e.g. tissue regeneration). Anti-inflammatory properties of synthetic zinc-chelating substances, such as hydroxamate and thiol, that nonselectively (universally) block MMPs [19] have been demonstrated in various experimental models of IBD [18, 20–22]. The application of these compounds in clinical settings, however, has been hampered because of lacking effectiveness and/or exerting critical side-effects such as serious musculoskeletal symptoms and arthralgia as a consequence of collagenase blockage [23–27].
We have recently shown that nonselective (doxycycline) and selective (RO28-2653) gelatinase inhibitors ameliorated hyper-acute small-intestinal immunopathology (Th1-type-driven pan-ileitis) in Toxoplasma gondii-infected mice when given either prophylactically or therapeutically [28]. It is of note that RO28-2653 did not show significant side effects in rat and monkey toxological studies [29]. Furthermore, we demonstrated that acute ileitis [28, 30–32] and DSS-colitis [33, 34] were aggravated by overgrowing Escherichia coli from the luminal commensal gut microbiota resembling key feature of acute episodes of IBD [30, 32]. We were therefore interested in potential beneficial effects of the MMP-blocking compounds doxycycline and RO28-2653 in acute DSS colitis. In the study presented here, we demonstrate that in mice treated with either doxycycline or RO28-2653 (1) clinical colitis pathology was ameliorated with an even better outcome following RO28-2653 treatment, (2) histopathological changes in the colon were less severe, which was paralleled by less secretion of the pro-inflammatory cytokine IL-6, (3) immune cell influx into mucosa and submucosa was reduced, and, finally, (4) E. coli loads in the colon did not differ from those seen in naïve, healthy mice. We therefore propose the selective gelatinase blocker RO28-2653 as a promising future option for prophylaxis and treatment of IBD.
Materials and methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, Germany). Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice, colitis induction, and determination of clinical scores
Female C57BL/6j wild-type mice were bred and maintained under specific pathogen-free (SPF) conditions in the Forschungsinstitut für Experimentelle Medizin (FEM, Charité, Berlin, Germany). For colitis induction, mice 3 months of age were treated with 3.5% (wt/vol) DSS (40 kDa, MP Biomedicals, Illkirch, France) in drinking water ad libitum for 7 days. Prior to necropsy, mice received water without DSS for 24 h. The intake of the DSS solution was controlled and mice were weighed daily. Total clinical scores with a maximum of 12 were generated daily by combined data of weight loss, occurrence of blood in stool (as determined by the Guajak method using Haemoccult, Beckman Coulter/PCD, Krefeld, Germany), and stool consistency, as described in Refs [33, 35].
Treatment with doxycycline or RO28-2653
C57BL/6j wild-type mice were treated perorally by gavage twice daily with either doxycycline (50 mg/kg body weight/day; Sigma, Germany) or RO28-2653 (75 mg/kg body weight/day; Roche, Penzberg, Germany) in 0.3 ml starting at day 0 for 8 days until necropsy. PBS-treated animals (0.3 ml perorally twice daily for the respective period of time) served as negative controls (placebo).
Sampling procedures and histologic scoring
Mice were sacrificed by isofluran treatment (Abbott, Germany) on day 8 after induction of colitis. Colon samples from each mouse were removed under sterile conditions and collected in parallel for histopathological, immunohistochemical, and microbiological analyses, as well as for detection of cytokines (protein level). For immunohistochemical stainings, colon samples were immediately fixed in 5% formalin and embedded in paraffin, and sections (5 µm) were stained with the respective antibodies as described below. Histopathology was investigated in paraffin-embedded HE-stained tissue sections. A published standardized histologic score ranging from 0 to 6 was used for blinded evaluation of the inflammatory processes in the colon [33, 35].
Immunohistochemistry
In situ immunohistochemical analysis of colon paraffin sections was performed as described previously [36, 37]. Primary antibodies against CD3 (#N1580, Dako, Denmark, dilution 1:10), myeloperoxidase-7 (MPO-7, # A0398, Dako, 1:10,000), FOXP-3 (FJK-16s, eBioscience, 1:100), B220 (eBioscience, San Diego, CA, USA, 1:200), and F4/80 (#14–4801, clone BM8, eBioscience, 1:50) were used. For each animal, the average number of positively stained cells within at least six high power fields (HPF, 400× magnification) were determined light-microscopically by three independent investigators (MMH, CL, AAK).
Cytokine detection in colon culture supernatants
Colon biopsies were cut longitudinally, washed with PBS, and strips of 1 cm2 were placed in 24-flat-bottom-well culture plates (Nunc, Wiesbaden, Germany) containing 500-µl serum-free RPMI 1640 medium supplemented with penicillin (100 U/ml) and streptomycin (100 µg/ml; PAA Laboratories). After 18 h at 37 °C, culture supernatants were tested for IL-6 by ELISA (BD Biosciences, Heidelberg, Germany) as described previously [30, 31, 33].
Analysis of the intestinal microflora
Cultural analysis and biochemical identification of luminal E. coli loads from colon samples were performed as previously described [30, 31, 33].
Statistical analysis
Medians, mean values, standard error of the means (SEM), and levels of significance were determined using appropriate tests as indicated (two-tailed Student’s t-test, Mann–Whitney-U test). Two-sided probability (P) values ≤ 0.05 were considered significant. All experiments were repeated at least twice.
Results
Less intestinal immunopathology following synthetic MMP blockage in mice with acute DSS colitis
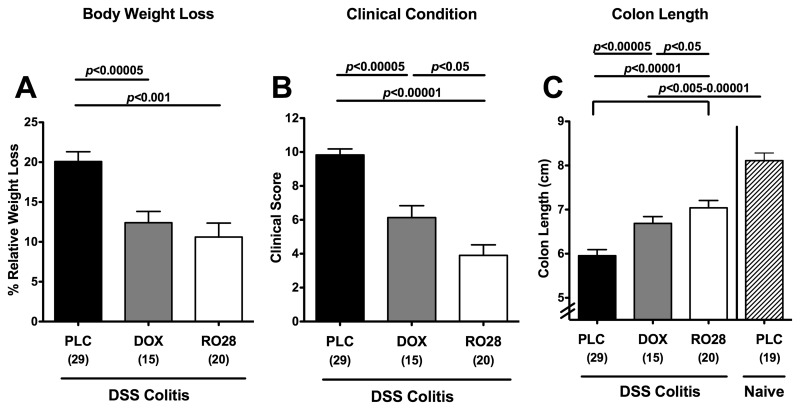
Given that pharmacologic MMP blockage was shown effective in preventing as well as treating acute small intestinal inflammation [28], we were interested in knowing whether the used compounds could ameliorate acute inflammation in the large intestine as well. Thus, mice were treated either with the nonselective MMP-inhibitor doxycycline or with RO28-2653, a compound selectively blocking gelatinases for 8 days starting upon induction of acute DSS colitis (day 0). Mice treated with either doxycycline or RO28-2653 displayed significantly less body weight loss during colitis development (12.3 ± 1.3% and 10.6 ± 1.8%, respectively) as compared to placebo (19.7 ± 1.2%) (Fig. 1A). In addition, applying a “cumulative” clinical colitis score including body weight loss, occurrence of blood in stool, and consistency of stool revealed that mice benefitted from either treatment as compared to placebo controls suffering from severe colitis at day 8 p.i. In addition, mice treated with the selective gelatinase blocker exhibited an even better clinical outcome as indicated by significantly lower clinical scores as compared to mice following nonselective MMP blockage with doxycycline (Fig. 1B).
Fig. 1.
Better clinical outcome of acute DSS colitis after treatment with doxycycline or RO28-2653. (A) Relative body weight loss, (B) clinical condition (as indicated by a clinical colitis score, see the section titled Materials and methods), and (C) absolute colon length at day 8 were recorded after oral treatment with placebo (PLC; black bars), doxycycline (DOX; gray bars), or RO28-2653 (RO28; white bars) from day 0 until day 8 in mice treated with DSS for 7 days and compared to placebo-treated healthy mice (naïve; hatched bars) when applicable (C). Numbers of analyzed animals are given in parentheses. Mean values, standard errors of the mean (SEM), and significance levels as indicated were determined by the Student’s t-test. Data are pooled from at least four independent experiments
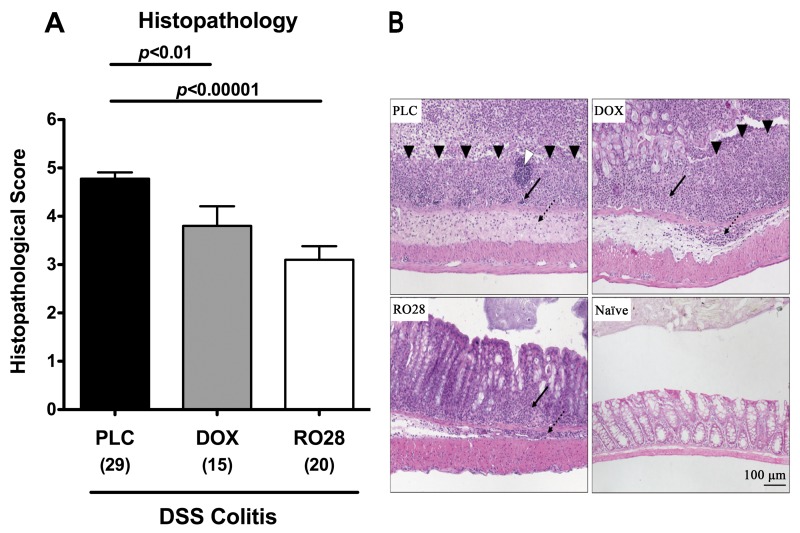
Given that colonic inflammation is accompanied by a significant shortening of the lower intestinal tract, we determined the lengths of the large intestines in treated and control animals. Induction of acute DSS colitis resulted in less significant shortening of the colon in treated animals as compared to the placebo group (Fig. 1C). In addition, mice treated with RO28-2653 benefitted more from selective gelatinase blockage as compared to animals treated with the nonselective MMP blocker doxycycline as indicated by significantly less distinct colon shrinkage following DSS (p < 0.05). To further characterize the beneficial effects of the respective compounds during DSS colitis, we studied the effects on colitis-severity-related histopathological changes in the colon. Treatment with either MMP-blocking compound resulted in significantly less pronounced colonic histopathology as compared to placebo controls at day 8 p.i. as indicated by lower histopathological scores (Fig. 2A): doxycycline-treated mice exhibited erosions or focal ulcerations of the colon mucosa with inflammatory cells extending into the submucosa (Fig. 2B). Discrete epithelial lesions and increased numbers of inflammatory cells within the colonic lamina propria could be detected in mice following selective gelatinase treatment (Fig. 2B). However, severe mucosal damage with extended ulcerations affecting the entire colon length and complete loss of the epithelium as well as a thickened submucosa with distinct transmural leukocyte infiltrates were present in the large intestine of the placebo group at day 8 p.i. (Fig. 2B). Taken together, following oral treatment with either doxycycline or RO28-2653, mice exhibited less macroscopic, clinical as well as histopathological signs of colonic inflammation following induction of acute DSS colitis. Furthermore, selective gelatinase blockage resulted in a better clinical outcome and less inflammation-induced colon shortening when compared to nonselective MMP blockage by doxycycline.
Fig. 2.
Less colonic histopathology after treatment with doxyxycline or RO28-2653 in acute DSS colitis. (A) Histopathology scores of the colon were determined at day 8 after oral treatment with placebo (PLC; black bars), doxycycline (DOX; gray bars), or RO28-2653 (RO28; white bars) from day 0 until day 8 in mice treated with DSS for 7 days. Numbers of analyzed animals are given in parentheses. Mean values, standard errors of the mean (SEM), and significance levels as indicated were determined by the Student’s t-test. Data are pooled from at least four independent experiments. (B) Paraffin sections of colon samples were obtained from mice with DSS colitis at day 8 after oral treatment with placebo (PLC; upper left), doxycycline (DOX; upper right), RO28-2653 (RO28; lower left) as well as from naïve controls (naïve; lower right) and HE-stained as described (see Methods). Open arrow head indicates lymph follicle; solid arrow heads indicate mucosal ulcerations. Solid and dotted arrows point towards mucosal and submucosal leukocyte infiltrates, respectively. Representative photomicrographs (magnification 100×) from three independent experiments are shown
Less pronounced immune cell responses in colonic mucosa in situ following treatment with MMP-blocking compounds in acute DSS colitis
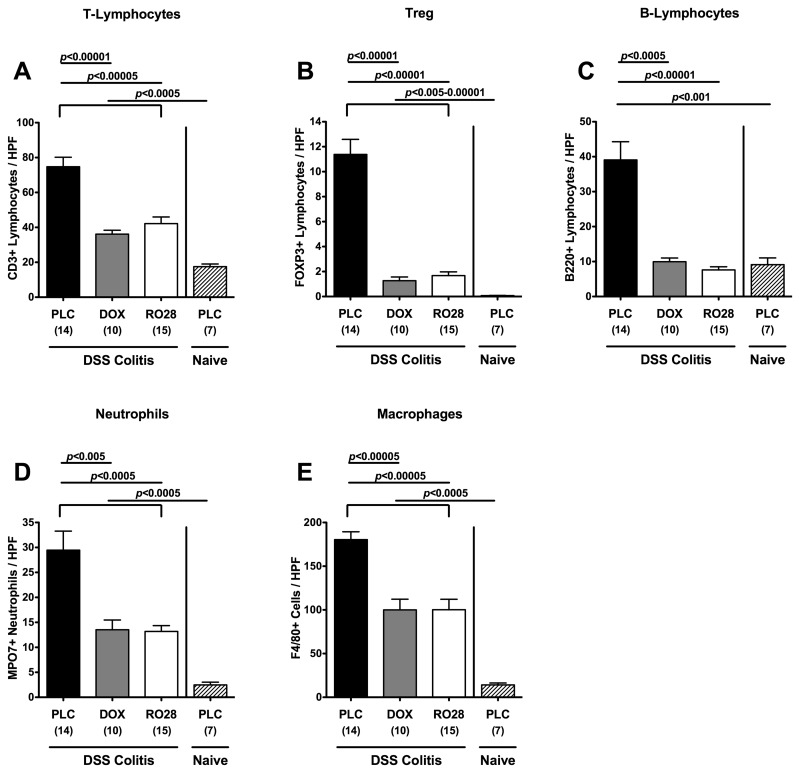
Given that human colitis is accompanied by the recruitment of pro-inflammatory immune cell populations at sites of inflammation in the large intestine [33], we next quantitated inflammatory cells as well as immune cell recruitment by immunohistochemical staining of colon paraffin sections of animals with antibodies against CD3 (T-lymphocytes), FOXP3 (regulatory T-cells, Treg), B220 (B-lymphocytes), MPO7 (neutrophils), and F4/80 (macrophages). At day 8 following colitis induction, placebo control mice displayed a substantial increase in T- and B-lymphocytes, Tregs, neutrophils, and macrophages within the colonic mucosa as compared to naïve mice (Fig. 3). This influx of immune cells, however, was significantly less pronounced in mice treated with either doxycycline or RO28-2653, indicating that amelioration of colonic inflammation following MMP blockage was accompanied by decreased immune cell responses within the colonic mucosa (Fig. 3).
Fig. 3.
Quantification of defined cell population in the colon of mice in situ after treatment with doxyxycline or RO28-2653 in acute DSS colitis. The average number of cells positive for (A) CD3 (T-lymphocytes), (B) FOXP3 (regulatory T cells, Treg), (C) B220 (B-lymphocytes), (D) MPO7 (neutrophils), and (E) F4/80 (macrophages) from at least six high-power fields (HPF, 400× magnification) per animal were determined microscopically in immunostained colon sections isolated at day 8 after oral treatment with placebo (PLC; black bars), doxycycline (DOX; gray bars), or RO28-2653 (RO28; white bars) from day 0 until day 8 from mice treated with DSS for 7 days and placebo-treated mice without colitis (naïve; hatched bars). Numbers of analyzed animals are given in parentheses. Mean values, standard errors of the mean, and significance levels as indicated were determined by the Student’s t-test. Data are pooled from at least three independent experiments
Less pro-inflammatory cytokine secretion in ex vivo colon cultures following synthetic MMP inhibition
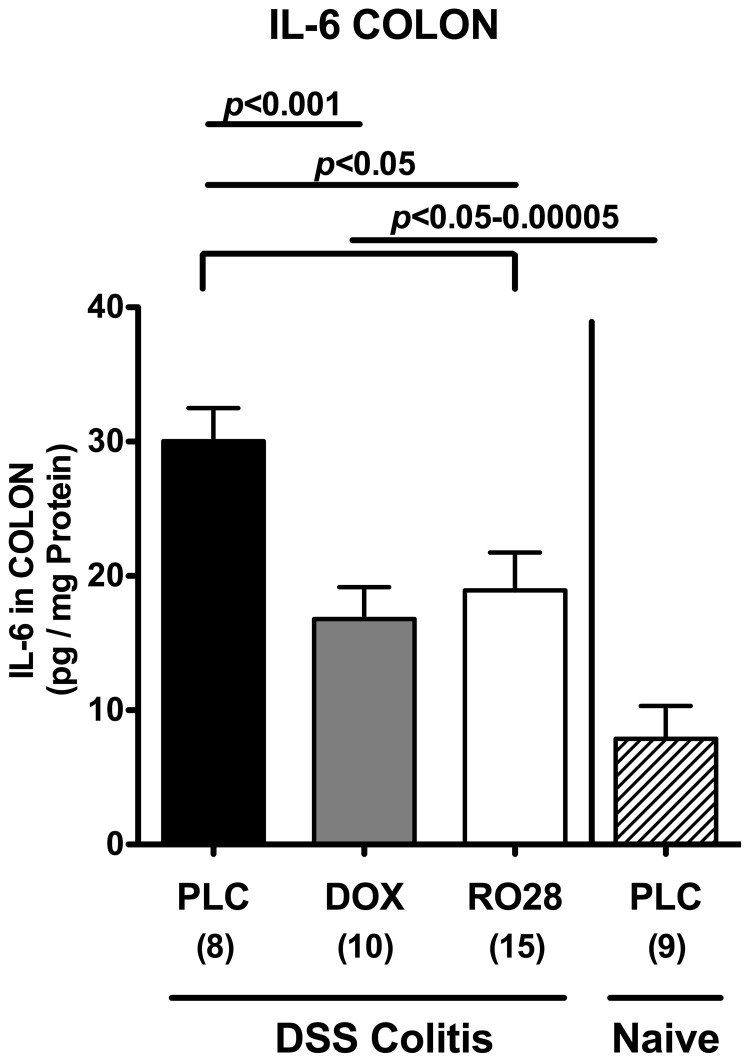
To further underline the anti-inflammatory properties of synthetic MMP blockage in acute DSS colitis, we next determined protein levels of the pro-inflammatory cytokine IL-6 in ex vivo colon cultures by ELISA. At day 8 after colitis induction, IL-6 levels increased more than threefold in placebo control animals with severe colitis as compared to healthy mice (Fig. 4). This effect was not as prominent in doxycycline- and RO28-2653-treated animals as indicated by IL-6 levels reaching approximately 50% of levels in the placebo group (Fig. 4). Thus, amelioration of colitis severity following MMP blockage is paralleled by less IL-6 secretion from the inflamed colon epithelium.
Fig. 4.
Less colonic IL-6 secretion after treatment with doxyxycline or RO28-2653 in acute DSS colitis. IL-6 concentrations were determined by ELISA in supernatants of colonic ex vivo cultures isolated at day 8 after oral treatment with placebo (PLC; black bars), doxycycline (DOX; gray bars), or RO28-2653 (RO28; white bars) from day 0 until day 8 from mice treated with DSS for 7 days and healthy placebo-treated mice (naïve; hatched bars). Numbers of analyzed animals are given in parentheses. Mean values, standard errors of the mean (SEM), and significance levels as indicated were determined by the Student’s t-test. Data are pooled from three independent experiments
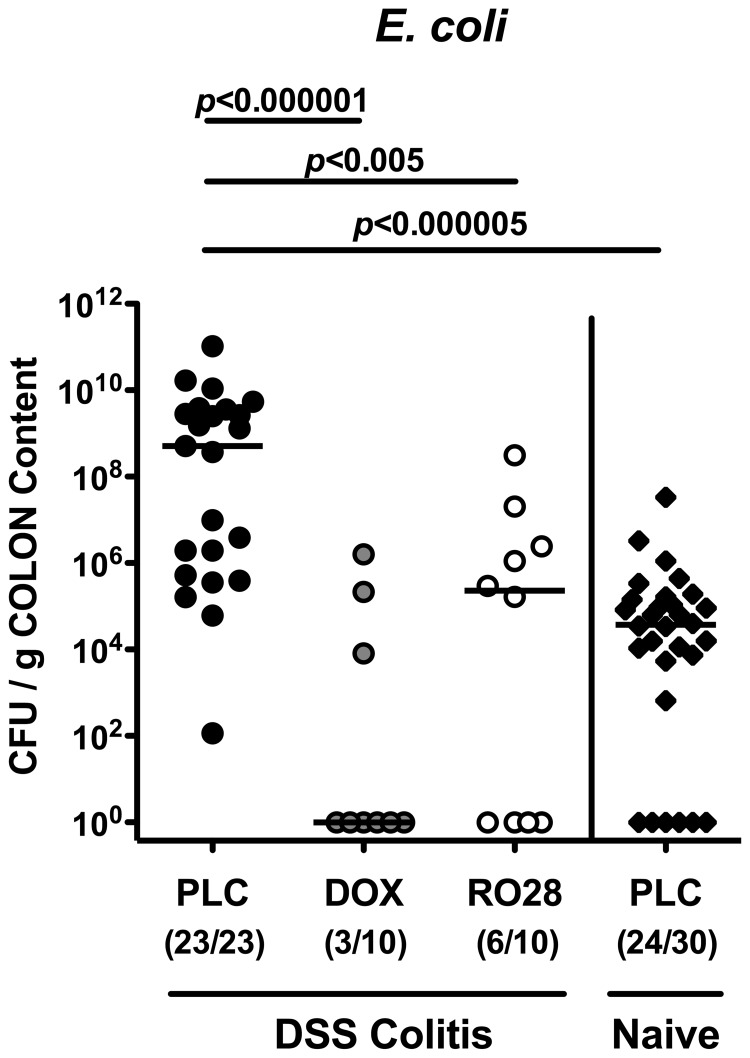
Lower E. coli loads in colon lumen following MMP blockage in acute DSS colitis
Given that our group has recently shown that acute small-intestinal inflammation is accompanied by a tremendous overgrowth of the ileum lumen by commensal enterobacteria such as E. coli with pro-inflammatory properties (up to 6–8 orders of magnitude [28]), we next determined the E. coli numbers in the colon lumen of mice with acute DSS colitis by culture. Until day 8 after colitis induction, luminal E. coli loads increased by approximately 4 orders of magnitude in placebo control mice with severe colitis (Fig. 5). The number of E. coli cultured from the large intestines at day 8 following doxycycline or RO28-2653 treatment, however, did not differ from that in the colons of naïve mice without colitis (Fig. 5). It is of note that RO28-2653 was lacking any antimicrobial effect in vitro (data not shown). However, a bacteriostatic effect also directed against E. coli needs to be taken into consideration when discussing the anti-inflammatory role of doxycycline in acute colitis.
Fig. 5.
Lack of overgrowth of the colon lumen with commensal E. coli after treatment with doxycycline or RO28-2653 in acute DSS colitis. Quantitative analysis of E. coli loads of the colon lumen was performed at day 8 by culture (see Methods). Individual bacterial counts (CFU, colony forming units) were determined in colonic content of mice treated with placebo (PLC; black bars), doxycycline (DOX; gray bars), or RO28-2653 (RO28; white bars) from day 0 until day 8 and DSS for 7 days and healthy placebo controls (naïve; hatched bars). Numbers of mice harboring E. coli out of the total number of analyzed animals are given in parentheses. Medians and levels of significance determined by Mann–Whitney-U test are indicated. Data are pooled from at least three independent experiments
Taken together, treatment with MMP-blocking agents ameliorates inflammation in acute DSS colitis as indicated by a better clinical, macroscopic as well as histopathological outcome, paralleled by less influx of pro-inflammatory immune cells, less IL-6 secretion by inflamed epithelial cells, and missing overgrowth of the colon lumen by potentially pro-inflammatory enterobacteria of the commensal colon microbiota.
Discussion
MMPs play essential roles in mediating inflammatory episodes in human IBD. Clinical studies investigating beneficial effects of compounds nonselectively blocking MMPs in inflammation or cancer have been hampered by the lack of effectiveness or by serious side effects [23–27] mainly due to blockage of collagenases (e.g. MMP-1) or matrilysin (MMP-7) exerting important functions in physiological homeostatic repair properties in cartilage and gut [18, 38]. Thus, as potential pharmacological treatment options, more selective and effective MMP-blocking agents without unwanted side effects would be highly appreciable.
The gelatinases A (MMP-2) and B (MMP-9) have been shown to be highly upregulated in intestinal inflammation in mice and men, in vitro and in vivo [11–16]. We have recently reported that selective blockage of gelatinases by the synthetic compound RO28-2653 was effective in the prevention and treatment of acute small-intestinal inflammation in a hyper-acute Th1-driven immunopathology model following T. gondii infection [28]. Surprisingly, selective gelatinase blockage was more effective than nonselective MMP inhibition by doxycycline. Therefore, we studied the potential beneficial, anti-inflammatory effects of the respective compounds in acute inflammation of the large intestine using the acute DSS colitis model. As in the small-intestinal model, nonselective MMP blockage by doxycycline treatment starting upon colitis induction resulted in significantly better clinical and macroscopic outcomes as well as in significantly less histopathology of the colon mucosa at day 8. These effects were paralleled by less infiltration of the colon mucosa and lamina propria by immune cells such as T- and B-lymphocytes, Tregs, neutrophils, and macrophages as compared to placebo control mice. Furthermore, less IL-6 secretion from the inflamed colon mucosa could be detected. Surprisingly, selective gelatinase blockage by RO28-2653 was as effective as nonselective MMP inhibition. Strikingly, with RO28-2653 treated animals exhibited better clinical conditions as well as less colon shortening at day 8 after colitis induction, indicating less macroscopic disease when compared to doxycycline treatment. Thus, the clinical efficacy of RO28-2653 in acute colitis could be clearly demonstrated in the study presented here. Given that RO28-2653 lacks the anti-MMP-1 and -7 properties [29] which had been made responsible for serious side effects in clinical studies with nonselective MMP compounds, the chance of unwanted effects following gelatinase blockage is rather low [39].
Furthermore, recent studies using mice lacking genes for MMP-2 and/or MMP-9 in experimental colitis models revealed that epithelial-derived MMP-9 is an important mediator in colitis, whereas MMP-2 exerts protective function preserving intestinal epithelial barrier integrity [40, 41]. Because pro-inflammatory functions of MMP-9 were shown to override MMP-2’s protective role during colitis [41], negative side effects of blocking both gelatinases seem to be rather negligible.
Given that MMPs are involved in the recruitment of immune cells into the gut [18, 42] and shed biologically active IL-1, IL-6, and TNF-α molecules from the surfaces of macrophages, which, in turn, are able to induce MMP expression from immune, epithelial, and parenchymal cells [17, 18], it would be utmost desirable to pharmacologically cut this vicious cycle all in one. In our study, MMP inhibition by doxycycline or RO28-2653 treatment resulted in less influx of macrophages, as indicated by significantly lower F4/80+ cells in the colon at day 8 and reduced IL-6 expression in ex vivo colon biopsies. Furthermore, we and others [43, 44] could show that in acute experimental colitis, following MMP blockage, the influx of neutrophilic granulocytes into the colon could be diminished, which reduced oxidative stress for the colon epithelium.
Experimental studies on acute ileitis [28, 30–32] and colitis models [33, 34] revealed that disease development was accompanied by a marked overgrowth of the intestinal lumen with commensal enterobacteria such as E. coli with potentially pro-inflammatory properties. Overgrowing E. coli easily translocate through a disrupted epithelial-cell barrier into the intestinal lamina propria and, subsequently, come in contact with immune cells, thereby exacerbating the inflammatory scenario [30, 32]. After colitis induction by DSS, luminal E. coli loads increased by more than 4 orders of magnitude in the colons of placebo control mice at day 8, whereas following doxycycline or RO28-2653 treatment, E. coli numbers did not differ from those quantified in naïve, healthy animals. One might argue that this result might be solely due to anti-bacterial action exerted by the used compounds, which might, in a positive point of view, affect the enterobacteria but, as a malum, compromise the remaining bacterial species of the commensal gut microbiota. This is, in part, true in the case of doxycycline treatment. Surprisingly, RO28-2653 did not display any anti-bacterial effect in vitro. Thus, it is highly appreciable to have an MMP blocker available, which (secondarily) prevents from E. coli overgrowth without anti-bacterial properties. Thus, these very promising results further underline the anti-inflammatory potency of the used MMP-blocking compounds.
Taken together, the data presented here demonstrate for the first time that selective gelatinase blockage by the synthetic compound RO28-2653 ameliorates acute murine DSS colitis. RO28-2653 not only exerted its beneficial effect by disrupting the vicious cycle of the positive feedback loop between “immune cell stimulation and MMP induction” but also prevented overgrowth of the colon lumen by pro-inflammatory E. coli despite a lack of anti-bacterial properties. These features put RO28-2653 in a pole position for future intervention strategies in human IBD.
Acknowledgments
This work was supported by grants from the German Research Foundation (DFG) to SB and AF (SFB633, TP A7), AK and CL (SFB633, TP Z1), AB, and BS (SFB633, TP A12), MMH (SFB633, TP B6), and from the German Federal Ministry of Education and Research (BMBF) to SB (“Lab in a hanky” projects TP 1.1 and TP 8.2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Michaela Wattrodt, Ursula Rüschendorf, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance, animal breeding, and genotyping of mice. We are grateful to Simone Spieckermann for immunohistochemistry staining of colon sections. We further gratefully acknowledge critical discussions with Prof. Dr. Dr. h.c. Helmut Hahn, Prof. Dr. Rajan Somasundaram, and Dr. Martin Rühl.
Glossary
Abbreviations
- DSS:
dextrane sulfate sodium
- ELISA:
enzyme linked immunosorbent assay
- HE:
hematoxylin eosin
- PBS:
phosphate buffered saline
- TNF:
tumor necrosis factor
Contributor Information
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
I. R. Dunay, 2Department of Microbiology and Hygiene, University of Magdeburg, Magdeburg, Germany.
D. Fuchs, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
D. Trautmann, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. Fischer, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. A. Kühl, 3Department of Pathology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
C. Loddenkemper, 3Department of Pathology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
A. Batra, 4Department of Internal Medicine, Charité – University Medicine Berlin, Berlin, Germany.
B. Siegmund, 4Department of Internal Medicine, Charité – University Medicine Berlin, Berlin, Germany.
H.-W. Krell, 5MAB Discovery GmbH, Neuried, Germany.
S. Bereswill, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
O. Liesenfeld, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
References
- 1.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002 Mar;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 2.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 3.Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996 Jan 1;156(1):1–4. [PubMed] [Google Scholar]
- 4.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000 Mar;18(5):1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 5.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996;49(1-3):20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- 6.Sarén P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996 Nov 1;157(9):4159–4165. [PubMed] [Google Scholar]
- 7.Cawston TE, Billington C. Metalloproteinases in the rheumatic diseases. J Pathol. 1996 Oct;180(2):115–117. doi: 10.1002/(SICI)1096-9896(199610)180:2<115::AID-PATH674>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002 Sep 1;169(5):2643–2467. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 9.Uzui H, Harpf A, Liu M, Doherty TM, Shukla A, Chai NN, Tripathi PV, Jovinge S, Wilkin DJ, Asotra K, Shah PK, Rajavashisth TB. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation. 2002 Dec 10;106(24):3024–3030. doi: 10.1161/01.cir.0000041433.94868.12. [DOI] [PubMed] [Google Scholar]
- 10.Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL. Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut. 2002 Oct;51(4):540–547. doi: 10.1136/gut.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994 Feb;47(2):113–116. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999 Oct;117(4):814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 13.Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut. 2000 Jul;47(1):57–62. doi: 10.1136/gut.47.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000 Jul;47(1):63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis E, Ribbens C, Godon A, Franchimont D, De Groote D, Hardy N, Boniver J, Belaiche J, Malaise M. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol. 2000 May;120(2):241–246. doi: 10.1046/j.1365-2249.2000.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stallmach A, Chan CC, Ecker KW, Feifel G, Herbst H, Schuppan D, Zeitz M. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000 Sep;47(3):415–422. doi: 10.1136/gut.47.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG. Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci U S A. 1999 Jun 8;96(12):6885–6889. doi: 10.1073/pnas.96.12.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naito Y, Takagi T, Kuroda M, Katada K, Ichikawa H, Kokura S, Yoshida N, Okanoue T, Yoshikawa T. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflamm Res. 2004 Sep;53(9):462–468. doi: 10.1007/s00011-004-1281-1. [DOI] [PubMed] [Google Scholar]
- 19.Talbot DC, Brown PD. Experimental and clinical studies on the use of matrix metalloproteinase inhibitors for the treatment of cancer. Eur J Cancer. 1996 Dec;32A(14):2528–2533. doi: 10.1016/s0959-8049(96)00398-x. [DOI] [PubMed] [Google Scholar]
- 20.Di Sebastiano P, di Mola FF, Artese L, Rossi C, Mascetta G, Pernthaler H, Innocenti P. Beneficial effects of Batimastat (BB-94), a matrix metalloproteinase inhibitor, in rat experimental colitis. Digestion. 2001;63(4):234–239. doi: 10.1159/000051895. [DOI] [PubMed] [Google Scholar]
- 21.Medina C, Videla S, Radomski A, Radomski M, Antolín M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Therapeutic effect of phenantroline in two rat models of inflammatory bowel disease. Scand J Gastroenterol. 2001 Dec;36(12):1314–1319. doi: 10.1080/003655201317097182. [DOI] [PubMed] [Google Scholar]
- 22.Sykes AP, Bhogal R, Brampton C, Chander C, Whelan C, Parsons ME, Bird J. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment Pharmacol Ther. 1999 Nov;13(11):1535–1542. doi: 10.1046/j.1365-2036.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 23.Rudek MA, Venitz J, Figg WD. Matrix metalloproteinase inhibitors: do they have a place in anticancer therapy? Pharmacotherapy. 2002 Jun;22(6):705–720. doi: 10.1592/phco.22.9.705.34062. [DOI] [PubMed] [Google Scholar]
- 24.Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006 Jan 1;11:479–491. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- 25.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002 Mar 29;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 26.Raffo D, Pontiggia O, Simian M. Role of MMPs in metastatic dissemination: implications for therapeutic advances. Curr Pharm Biotechnol. 2011 Nov;12(11):1937–1947. doi: 10.2174/138920111798377085. [DOI] [PubMed] [Google Scholar]
- 27.Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: protective roles in cancer. J Cell Mol Med. 2011 Jun;15(6):1254–1265. doi: 10.1111/j.1582-4934.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009 Dec 21;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lein M, Jung K, Ortel B, Stephan C, Rothaug W, Juchem R, Johannsen M, Deger S, Schnorr D, Loening S, Krell HW. The new synthetic matrix metalloproteinase inhibitor (Roche 28-2653) reduces tumor growth and prolongs survival in a prostate cancer standard rat model. Oncogene. 2002 Mar 27;21(13):2089–2096. doi: 10.1038/sj.onc.1205267. [DOI] [PubMed] [Google Scholar]
- 30.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006 Dec 15;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 31.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Göbel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007 Jul;56(7):941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bereswill S, Muñoz M, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Loddenkemper C, Göbel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010 Dec 3;5(12):e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A, Lehr HA, Liesenfeld O, Blaut M, Göbel UB, Schumann RR, Bereswill S. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007 Jul 25;2(7):e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS One. 2010 Feb 9;5(2):e9125. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegmund B, Rieder F, Albrich S, Wolf K, Bidlingmaier C, Firestein GS, Boyle D, Lehr HA, Loher F, Hartmann G, Endres S, Eigler A. Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J Pharmacol Exp Ther. 2001 Jan;296(1):99–105. [PubMed] [Google Scholar]
- 36.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Göbel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010 Aug;59(8):1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 37.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "ménage à trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parks WC, López-Boado YS, Wilson CL. Matrilysin in epithelial repair and defense. Chest. 2001 Jul;120(1 Suppl):36S–41S. doi: 10.1378/chest.120.1_suppl.s36. [DOI] [PubMed] [Google Scholar]
- 39.Bernardo MM, Brown S, Li ZH, Fridman R, Mobashery S. Design, synthesis, and characterization of potent, slow-binding inhibitors that are selective for gelatinases. J Biol Chem. 2002 Mar 29;277(13):11201–11207. doi: 10.1074/jbc.M111021200. [DOI] [PubMed] [Google Scholar]
- 40.Garg P, Rojas M, Ravi A, Bockbrader K, Epstein S, Vijay-Kumar M, Gewirtz AT, Merlin D, Sitaraman SV. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol. 2006 Sep 15;177(6):4103–4112. doi: 10.4049/jimmunol.177.6.4103. [DOI] [PubMed] [Google Scholar]
- 41.Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009 Feb;296(2):G175–G184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinesi M, Treves C, Bonanomi AG, Milla M, Bagnoli S, Zuegel U, Steinmeyer A, Stio M. Down-regulation of adhesion molecules and matrix metalloproteinases by ZK 156979 in inflammatory bowel diseases. Clin Immunol. 2010 Jul;136(1):51–60. doi: 10.1016/j.clim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Medina C, Videla S, Radomski A, Radomski MW, Antolín M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol. 2003 Jan;284(1):G116–G122. doi: 10.1152/ajpheart.00036.2002. [DOI] [PubMed] [Google Scholar]
- 44.Huang TY, Chu HC, Lin YL, Lin CK, Hsieh TY, Chang WK, Chao YC, Liao CL. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicol Appl Pharmacol. 2009 May 15;237(1):69–82. doi: 10.1016/j.taap.2009.02.026. [DOI] [PubMed] [Google Scholar]