Abstract
Enterocolitis caused by Campylobacter jejuni-infections represents an important socioeconomic burden worldwide. Recent results from novel murine infection models reveal that the intestinal microbiota is essential for maintaining colonization resistance against C. jejuni. We extended these studies to investigate the role of nutrition and obesity in susceptibility to C. jejuni-infection. Gnotobiotic (GB) mice generated by antibiotic treatment, which were fed with a human cafeteria diet (CAF), as well as obese (ob/ob) mice with a conventional microbiota harbored higher Escherichia coli loads in their colon as compared to respective controls. Following oral infection, C. jejuni 43431 ATCC readily colonized the intestines of CAF and ob/ob mice, whereas GB mice fed with a standard chow (MUD) eradicated the pathogen within days. Furthermore, live C. jejuni translocated into mesenteric lymph nodes of CAF, but not MUD mice. Strikingly, stably infected animals developed enterocolitis as indicated by increased numbers of immune and apoptotic cells in the colon in situ.
We conclude that a specific human diet and obesity render mice susceptible to C. jejuni infection. The corresponding murine models are excellently suited for the study of C. jejuni pathogenesis and will help to get further insights into interplays between C. jejuni, microbiota, diet, obesity and immunity.
Keywords: cafeteria diet, Campylobacter jejuni, colonization resistance, gnotobiotic mice, host–pathogen interaction, human microbiota, innate immunity, nutrition, ob/ob mice, obesity
Introduction
The Gram-negative bacterial pathogen Campylobacter jejuni is transmitted via the food chain from farm animals to humans [1, 2]. Infected patients present with abdominal pain, fever, myalgia, and watery or bloody diarrhea. The acute stage of C. jejuni enteritis is characterized histologically by crypt abscesses, ulcerations, and proliferation of immune cells in the colon in situ [1, 3–5]. The vast majority of infections is self-limited and not followed by further complications. However, in rare cases C. jejuni infection triggers chronic sequelae such as Reiter’s syndrome, reactive polyarthropathy, and Guillain–Barré syndrome [1, 6].
Virulence factors, pathogenicity, and epidemiology of C. jejuni have been intensively investigated since the discovery of the disease in the 1970s [7]. Mechanisms mediating intestinal colonization resistance and the role of nutrition in susceptibility to C. jejuni infection, however, are not well understood. In addition, immunopathological responses within the vertebrate colon following C. jejuni infection are still poorly investigated. These limitations are mainly due to the scarcity of suitable experimental in vivo models [8]. Whereas mice are highly convenient for the study of colonization capacity of bacterial pathogens and can help to overcome the limitations in studying immunopathology, murine infection models have the disadvantage of sporadic colonization and/or absence of disease manifestations [1, 8, 9]. This is due to the fact that conventional mice with a normal intestinal microbiota are highly resistant to C. jejuni colonization. Very recent work from our group revealed that gnotobiotic (GB) mice and GB mice harboring a modified microflora are well suited for the study of C. jejuni infection [9]. GB mice generated by quintuple antibiotic treatment, GB mice reconstituted with a human flora, and IL-10-deficient mice exerting chronic colitis could be readily infected by C. jejuni, and they developed immune as well as inflammatory responses against the pathogen [9–11]. Furthermore, C. jejuni was shown to colonize the entire gastrointestinal (GI) tract of isolator-raised germfree (GF) mice and to induce clinical signs of disease including granulocyte infiltrates, bloody diarrhea, and humoral immune responses, reproducibly occurring after infection [12–16]. Given that GF mice, however, are immuno-compromised due to the lack of an intact immune system [17, 18], they do not represent a suitable experimental model of C. jejuni infections in humans [9].
In order to further optimize murine models for C. jejuni enteritis by mimicking the patho-physiological conditions in humans, we used in this study our GB mouse model in which the intestinal flora was completely abrogated by quintuple antibiotic treatment for 6–8 weeks [9, 19–21]. Given that the physiological intraluminal milieu within the GI tract including the gut bacteria composition is mainly determined by host-specific nutrition, GB mice were fed with a human cafeteria diet (CAF) [22–24]. The results obtained after C. jejuni infection prove that the host-specific diet plays a crucial role in colonization resistance against or susceptibility to C. jejuni infection. Furthermore, obese (ob/ob) mice with a conventional murine microbiota were highly susceptible to C. jejuni infection. The fact that C. jejuni-infected animals developed characteristic immune responses similar to those seen in humans indicates that GB mice fed with a human diet as well as obese mice with a conventional microbiota represent valuable models for studying C. jejuni infection. Thus, the study presented here indicates for the first time that the quadrangle relationship between diet, obesity, intestinal microbiota, and infection is crucial for the establishment of campylobacteriosis in a vertebrate host.
Materials and methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, Germany). Animal welfare was monitored twice daily by assessment of clinical conditions. Fresh human fecal samples for recolonizing GB mice (hfa) were collected from healthy volunteers. Before sample collection, written informed consent was obtained from all volunteers. Since fecal samples were obtained from coworkers of our laboratory and thus outside a clinical environment and used for recolonization of mice only, experiments were exempted from approval by the Charité – Universitätsmedizin ethical committee according to German legacy (§15, Legal Basis for Clinical Trials).
Mice
All animals were bred and maintained in the facilities of the “Forschungsinstitut für Experimentelle Medizin” (FEM, Charité – Universitätsmedizin, Berlin, Germany), under specific-pathogen-free (SPF) conditions. Mice homozygous for the obese spontaneous mutation (Lepob commonly referred to as ob or ob/ob; in the KS background) and C57BL/6 (C6) mice were used in the experiments. Age- and sex-matched mice between 10 and 12 weeks of age were used.
Generation of gnotobiotic mice
To eradicate the commensal gut flora, mice were transferred to sterile cages and treated by adding ampicillin (1 g/L; Ratiopharm), vancomycin (500 mg/L; Cell Pharm), ciprofloxacin (200 mg/L; Bayer Vital), imipenem (250 mg/L; MSD), and metronidazole (1 g/L; Fresenius) to the drinking water ad libitum for 6–8 weeks as described earlier in detail [9, 19–21].
Generation of gnotobiotic mice with a defined microbiota
For recolonization experiments, E. coli, Lactobacillus johnsonii, and a mix of Lactobacillus spp. (L. johnsonii, L. acidophilus, L. delbrückii, L. murinus, L. reuteri, and other taxonomically uncharacterized Lactobacillus spp.) were isolated from naïve mice, grown, and characterized as described in detail earlier [19]. Four days prior to the recolonization experiments, the antibiotic cocktail was replaced by sterile drinking water. For recolonization, GB mice received the respective strain(s) in a volume of 0.3 mL on three consecutive days by gavage with a load of approximately 109 CFU/mL. After further 7 days (to assure proper colonization of the entire GI tract), and right before C. jejuni infection, fecal samples from individual animals were taken to quantitate the bacterial loads in serial dilutions on appropriate solid culture media and to rule out secondary contamination.
Fresh human and murine fecal samples free of enteropathogenic bacteria, parasites, and viruses were collected from five individual healthy volunteers and animals, respectively, pooled and dissolved in an equal volume of sterile PBS, aliquoted, and stored at –80 °C until use. For recolonization experiments, aliquots were thawed and bacterial communities quantified by cultural methods [9] before gavage of mice with 0.3 mL of the respective suspension. Between independent experiments, bacterial counts of groups varied by <0.5 log orders of magnitude.
Cafeteria diet
Mice were given free access to human food (cafeteria diet, CAF; [22–24]) ad libitum for 10 weeks. The CAF consisted of typical local specialties from Berlin such as meat balls (“Buletten”), curry sausages (“Currywurst”), Döner Kebab, fried chicken nuggets, pizza, ham, salami, cheese, spaghetti, French fries, walnuts, apple, carrots, paprica, salad, chocolate cookies, crackers, bread, toast bread, and many others. The food was handled under regular, common human hygiene conditions (thus not in a sterile environment, no use of gloves, regular hand hygiene while handling food). In addition, mice could choose between tap water and alcohol-free beer (mostly preferring the latter). Control mice were fed with regular chow (murine diet, MUD) and tap water ad libitum.
C. jejuni infection of mice
Mice were infected with approximately 109 viable CFU of C. jejuni strains ATCC 43431 by gavage in a total volume of 0.3 mL PBS (phosphate buffered saline) on three consecutive days as described earlier in detail [9].
Sampling procedures
Mice were sacrificed by isofluran treatment (Abbott, Germany). Cardiac blood and tissue samples from spleen, liver, abdominal fat, mesenteric lymph nodes (MLNs), and GI tract (stomach, duodenum, ileum, and colon) were removed under sterile conditions. GI samples from each mouse were collected in parallel for microbiological and immunohistochemical analyses. For immunohistochemical stainings, colon samples were immediately fixed in 5% formalin and embedded in paraffin, and sections (5 µm) were stained with the respective antibodies as described below.
Immunohistochemistry
In situ immunohistochemical analysis of colon paraffin sections was performed as described previously [9, 25]. Primary antibodies against CD3 (#N1580, Dako, Denmark, dilution 1:10), myeloperoxidase-7 (MPO-7, # A0398, Dako, 1:10000), FOXP-3 (FJK-16s, eBioscience, 1:100), B220 (eBioscience, San Diego, CA, USA, 1:200), and cleaved caspase-3 (Asp175, Cell Signaling, USA, 1:200) were used. For each animal, the average number of positively stained cells within at least six high power fields (HPF, 400× magnification) were determined light-microscopically by three independent investigators (MMH, RP, AAK).
Quantitative analysis of C. jejuni translocation
Live C. jejuni were detected in MLNs, spleen, liver, abdominal fat tissue, and cardiac blood by culture as described earlier [9]. In brief, tissues were homogenized in sterile PBS and analyzed in dilution series on karmali agar (Oxoid, Wesel, Germany) in a microaerophilic atmosphere at 37 °C for at least 48 h. Cardiac blood (0.2 mL) was immediately streaked out on karmali agar plates.
Analysis of the intestinal microflora
Cultural analyses and biochemical identification of luminal bacterial communities from stomach, duodenum, ileum, and colon were performed as previously described [19, 20, 26].
Statistical analysis
Mean values, medians, standard deviations, and levels of significance were determined using appropriate tests as indicated (two-tailed Student’s t-test, Mann–Whitney-U test). Two-sided probability (P) values ≤0.05 were considered significant. All experiments were repeated at least twice.
Results
Association of gnotobiotic mice with distinct bacterial species alone does not affect susceptibility to C. jejuni infection
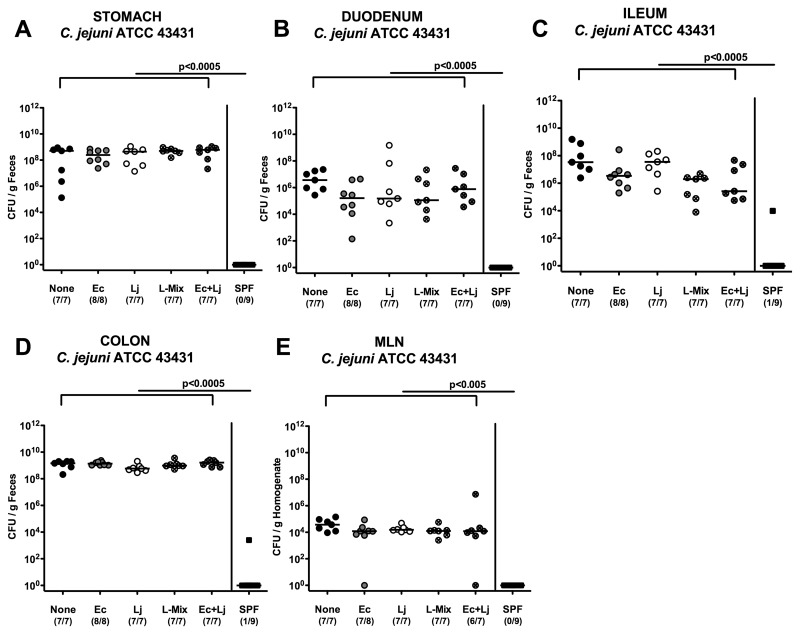
We have recently shown that the host-specific intestinal microbiota plays a key role in maintaining colonization resistance against C. jejuni. Conventional mice and GB mice reconstituted with a specific murine intestinal flora were resistant to C. jejuni colonization and eradicated the pathogen within two days after oral infection [9]. In contrast, GB animals colonized with a complex human gut microbiota were highly susceptible to C. jejuni infection and developed significant immunopathology in the colon. When compared to the murine gut microbiota, the intestinal human flora harbored higher enterobacteria but lower lactobacilli numbers. Thus, we aimed at investigating whether these respective bacterial species might influence colonization resistance against C. jejuni. We hypothesized that lactobacilli might be causatively involved in colonization resistance against C. jejuni, whereas E. coli might be beneficial for establishment of C. jejuni in the GI tract and thus counteract colonization resistance. GB mice (generated by quintuple antibiotic treatment for 8 weeks, see methods) were perorally recolonized with approximately 109 CFU of E. coli, L. johnsonii, a lactobacilli mix (consisting of L. johnsonii, L. acidophilus, L. delbrückii, L. murinus, L. reuteri, and other taxonomically uncharacterized Lactobacillus spp. [19]), or a combination of E. coli and L. johnsonii on three consecutive days (data not shown). Mice recolonized with a conventional murine SPF flora served as negative controls. One week following reconstitution, mice harboring comparable loads of the respective species within the ileum and colon were then orally infected with C. jejuni ATCC 43431 on three consecutive days. Twelve days following infection (post infection, p.i.), C. jejuni ATCC 43431 was found at comparable levels within the entire GI tract (stomach, duodenum, ileum, and colon), with the highest numbers in the colon, irrespective of the bacterial species established in the GB mice (Fig. 1A–D). Control animals with a conventional flora serving as negative controls had eradicated C. jejuni ATCC 43431 from their intestines until day 12 (Fig. 1A–D). In addition, C. jejuni ATCC 43431 translocation into local lymphoid organs was not prevented by lactobacilli colonization of the GI tract alone, as live C. jejuni ATCC 43431 could be detected at comparable levels in MLNs of all recolonized GB mice. Conventional mice with a normal microbiota, however, did not display any translocated C. jejuni ATCC 43431 in their MLNs (Fig. 1E).
Fig. 1.
C. jejuni colonization along the gastrointestinal tract and translocation into MLNs of defined recolonized gnotobiotic mice. Gnotobiotic mice were generated by antibiotic gut decontamination, recolonized with E. coli (Ec, gray circles), Lactobacillus johnsonii (Lj, white circles), a Lactobacilli-mix (L-mix, crossed circles), a mix of E. coli and L. johnsonii (Ec+Lj, crossed gray circles), sterile PBS (none, solid circles), or a complex conventional murine SPF microbiota (solid squares), and orally infected with C. jejuni strain ATCC 43431 as described in the section titled Materials and methods. The pathogen densities in distinct compartments of the gastrointestinal tract (A–D) and mesenteric lymph nodes (MLNs, E) were determined by quantification of live C. jejuni in luminal samples taken from (A) stomach, (B) duodenum, (C) ileum, and (D) colon at day 12 p.i. by cultural analysis (CFU, colony forming units). Numbers of animals harboring C. jejuni out of the total number of analyzed animals are given in parentheses. Medians (black bars) and levels of significance (P-values) determined by Mann–Whitney-U test are indicated. Data shown are representative for three independent experiments
Taken together, colonization resistance against C. jejuni could not be attributed to murine lactobacilli alone and is rather mediated by the complex interplay of bacterial communities in the gut microbiota and/or by distinct physiological conditions of the intraluminal milieu, which need to be further investigated in detail.
Nutrition affects susceptibility to C. jejuni infection, composition of the gut microbiota, and the intraluminal milieu
The intraluminal milieu of the GI tract and the composition of the gut microbiota are predominantly influenced by nutrition. Therefore, we investigated whether feeding GB mice with a human “Western-style” diet would induce a human microbiota composition, which renders GB mice susceptible to C. jejuni infection. In order to mimick the intraluminal milieu of the GI tract following human nutrition, GB mice were fed with a cafeteria diet (CAF) whereas controls received a standard murine diet (MUD). CAF mice with free access to “human food” ad libitum for 10 weeks (for details and handling conditions refer to methods) gained between 40% and 50% of their individual body weights whereas MUD mice did not (<5% weight gain, data not shown).
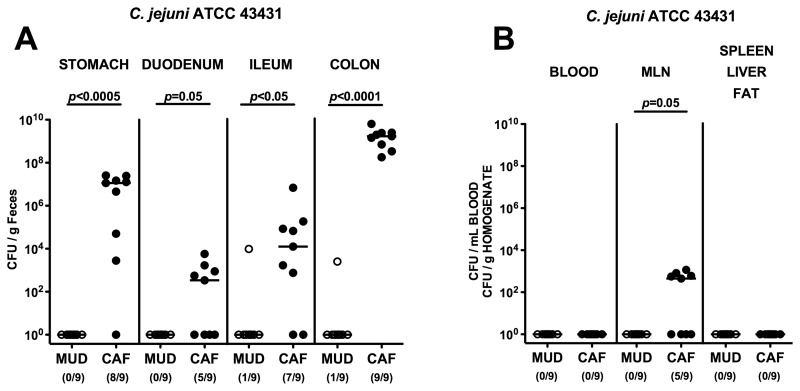
After 10 weeks of feeding, CAF and MUD mice were orally infected with C. jejuni ATCC 43431. At day 12 p.i., CAF mice harbored C. jejuni ATCC 43431 throughout the entire GI tract at high numbers, with the highest counts of 109 CFU/g luminal content in the colon, whereas the MUD animals had expelled the pathogen until then (Fig. 2A). In addition, in more than half of the infected mice fed with CAF, but none of MUD animals, live C. jejuni ATCC 43431 could be detected in MLNs at day 12 p.i. (Fig. 2B). C. jejuni ATCC 43431, however, did not translocate into other organs such as blood, spleen, liver, or abdominal fat of mice, irrespective of the dietary regimen (Fig. 2B).
Fig. 2.
C. jejuni colonization along the gastrointestinal (GI) tract and translocation in mice with cafeteria diet. Gnotobiotic mice were generated by antibiotic gut decontamination, fed either a cafeteria diet (CAF, solid circles) or conventional murine diet (MUD, open circles) for 10 weeks, and orally infected with C. jejuni strain ATCC 43431 as described in methods. The pathogen densities in distinct compartments of the GI tract (stomach, duodenum, ileum, and colon; A) or cardiac blood and organs such as mesenteric lymph nodes (MLNs), spleen, liver, and abdominal fat (B) were determined by quantification of live C. jejuni in luminal GI samples, blood and organ homogenates, respectively, taken at day 12 p.i. by cultural analysis (CFU, colony forming units). Numbers of animals harboring C. jejuni out of the total number of analyzed animals are given in parentheses. Medians (black bars) and levels of significance (P-values) determined by Mann–Whitney-U test are indicated. Data shown are representative for three independent experiments
Impact of nutrition on intestinal microbiota composition
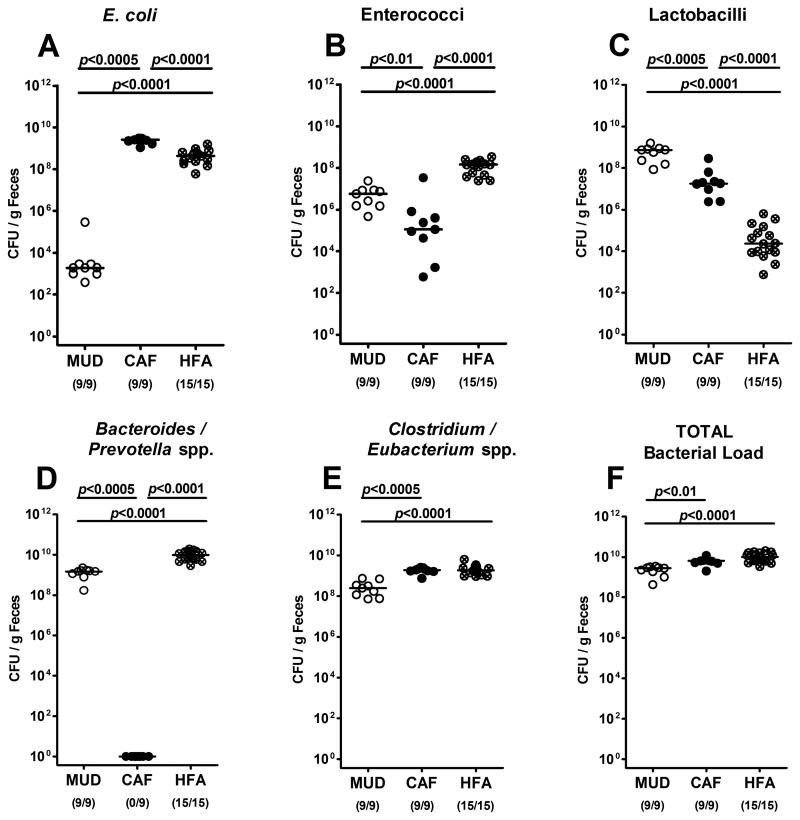
Given that nutrition influences the gut microbiota composition, and the gut microbiota determines the susceptibility to and resistance against C. jejuni infection, we next performed a quantitative survey of the intraluminal bacterial species composition of the colon of mice on CAF or MUD by culture and compared the respective loads with those obtained in initially GB mice which had been reconstituted with a complete human microbiota (i.e. human-flora-associated mice, HFA).
Interestingly, CAF mice harbored significantly higher E. coli loads (of more than 6 orders of magnitude) in their large intestines as compared to MUD animals, whereas the difference to HFA animals was rather subtle (<1 order of magnitude; Fig. 3A). The enterococci counts in the CAF group, however, were approximately 2 and 3 log orders lower than those in MUD and HFA mice, respectively (Fig. 3B). As expected, MUD mice harbored approximately 2 and 5 orders of magnitude higher Lactobacillus spp. loads as compared to CAF and HFA mice (Fig. 3C). Surprisingly, no Bacteroides/Prevotella spp. at all could be detected in the colons of mice following CAF, whereas HFA mice displayed higher loads of these obligate anaerobic Gram-negative rods (1 order of magnitude) than mice on MUD (Fig. 3D). Finally, CAF and HFA mice displayed approximately 1.5 orders and <1 order of magnitude higher counts of Clostridium/Eubacterium spp. and total bacterial loads as compared to mice from the MUD group (Figs. 3E, F), respectively. Taken together, 10 weeks following CAF, initially GB mice harbored higher total bacteria loads as well as higher numbers of anaerobic Gram-positive rods and, most pronounced, E. coli, but lower Lactobacillus spp. counts when compared to SPF mice on MUD. Most importantly, the gut microbiota composition following CAF was more comparable to the flora of “humanized” animals than to the one displayed by SPF mice on MUD.
Fig. 3.
Bacterial colonization of intestines in mice following different dietary regimens. Gnotobiotic mice were generated by antibiotic gut decontamination, fed either a cafeteria diet (CAF) or conventional murine diet (MUD) for 10 weeks, and compared to gnotobiotic mice harboring a human gut microbiota (HFA) following oral recolonization as described in methods. Numbers of (A) E. coli, (B) Enterococcus spp., (C) Lactobacillus spp., (D) Bacteroides/Prevotella spp., (E) Clostridium/Eubacterium spp., and (F) total bacterial loads were determined in feces samples of mice right before C. jejuni infection (day 0) by detection of colony forming units (CFU) per gram feces on appropriate culture media (see methods). Bacterial species were identified by biochemical analysis and reconfirmed by comparative sequence analyses of 16S rRNA genes. Numbers of animals harboring the respective bacterial species are given in parentheses. Medians and significance levels (P-values) determined by Mann–Whitney-U test are indicated. Data shown were pooled from three independent experiments
C. jejuni induced intestinal immunopathology in mice following Cafeteria diet
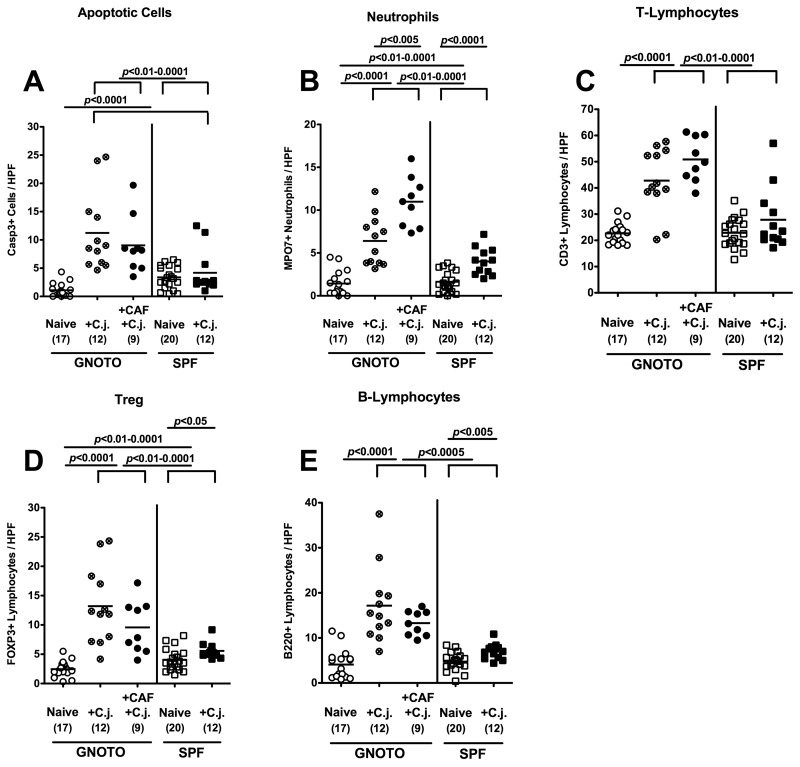
Given that in humans C. jejuni induces the recruitment of pro-inflammatory immune cell populations at sites of inflammation in the large intestine, we next quantitated inflammatory cells as well as immune cell recruitment by immunohistochemical staining of colon paraffin sections of C. jejuni ATCC 43431-infected animals with antibodies against caspase-3 (apoptotic cells), MPO7 (neutrophils), CD3 (T-lymphocytes), FOXP3 (regulatory T-cells, Treg), and B220 (B-lymphocytes).
At day 12 following oral infection with C. jejuni ATCC 43431, GB mice as well as mice fed with CAF displayed significantly higher numbers of apoptotic cells as well as neutrophils, T- and B-lymphocytes, and Tregs in their large intestines as compared to infected SPF mice (Fig. 4). In addition, CAF mice displayed twice as many neutrophils in the colon as GB mice at day 12 p.i. (Fig. 4B).
Fig. 4.
Immunopathology in the colons of C. jejuni-infected mice following different dietary regimens. Gnotobiotic mice (GNOTO, circles) were generated by antibiotic gut decontamination and fed either cafeteria diet (CAF, solid circles) or conventional murine diet (sterile pellets, open and crossed circles), orally infected with C. jejuni strain ATCC 43431 (C.j.), and compared to noninfected gnotobiotic controls (open circles) and to naïve (open squares) or C. jejuni strain ATCC 43431 (C.j., solid squares)-infected animals harboring a conventional murine microbiota (SPF). The average numbers of apoptotic cells (positive for caspase-3, panel A), neutrophilic granulocytes (neutrophils, positive for MPO-7, panel B), T-lymphocytes (positive for CD3, panel C), regulatory T-cells (Treg, positive for FOXP3, panel D), and B-lymphocytes (positive for B220, panel E) from at least six high power fields (HPF, 400× magnification) per animal were determined microscopically in immunohistochemically stained colon sections. Numbers of animals of the respective genotype analyzed are given in parentheses. Means (black bars) and levels of significance (P-values) as compared to the respective groups (determined by the Student’s t-test) are indicated. Data shown were pooled from at least three independent experiments
Taken together, mice fed with CAF and as a consequence “suffering” from obesity not only harbored a gut flora which rendered them susceptible to C. jejuni infection but also displayed pro-infammatory immune responses in their colon following C. jejuni ATCC 43431 infection.
Impact of obesity on susceptibility to C. jejuni infection
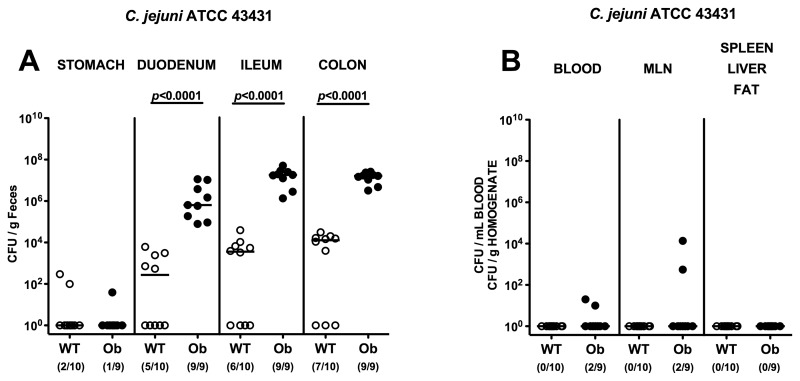
To confirm our results obtained in CAF mice (with obesity) and further investigate the quadrangle relationship between nutrition, obesity, gut flora changes, and susceptibility to Campylobacter infection, another model was employed to assess the role of obesity in our findings. Mice homozygous for the obese spontaneous mutation Lepob, referred to as ob/ob, and wild-type (WT) control animals (both in KS background), all of them harboring a conventional gut flora, were orally infected with C. jejuni ATCC 43431 on three consecutive days. Ob/ob mice harbored up to 4 orders of magnitude higher C. jejuni ATCC 43431 loads in their GI tract (duodenum, ileum, and colon), with the highest counts of >107 CFU/g luminal content in ileum and colon as compared to WT controls (Fig. 5A). Furthermore, live C. jejuni ATCC 43431 could be cultured from cardiac blood and MLNs from 22.2% of ob/ob, but none of WT, mice, whereas no translocation of C. jejuni ATCC 43431 into spleen, liver, or abdominal fat could be detected (Fig. 5B).
Fig. 5.
C. jejuni colonization along the gastrointestinal (GI) tract and translocation in obese mice. Mice homozygous for the obese spontaneous mutation (ob/ob, solid circles) and wild-type controls (WT, KS background; open circles) were orally infected with C. jejuni strain ATCC 43431 as described in methods. The pathogen densities in distinct compartments of the GI tract (stomach, duodenum, ileum, and colon; A), or cardiac blood and organs such as mesenteric lymph nodes (MLNs), spleen, liver, and abdominal fat (B) were determined by quantification of live C. jejuni in luminal GI samples, blood, and organ homogenates, respectively, taken at day 12 p.i. by cultural analysis (CFU, colony forming units). Numbers of animals harboring C. jejuni out of the total number of analyzed animals are given in parentheses. Medians (black bars) and levels of significance (P-values) determined by Mann–Whitney-U test are indicated. Data shown were pooled from three independent experiments
These findings independently demonstrate in another experimental model that obese mice kept under standard conditions and harboring a conventional microbiota were more susceptible to C. jejuni infection as compared to lean WT controls with the same diet.
Obese mice harbor higher E. coli and enterococci loads in the colon lumen
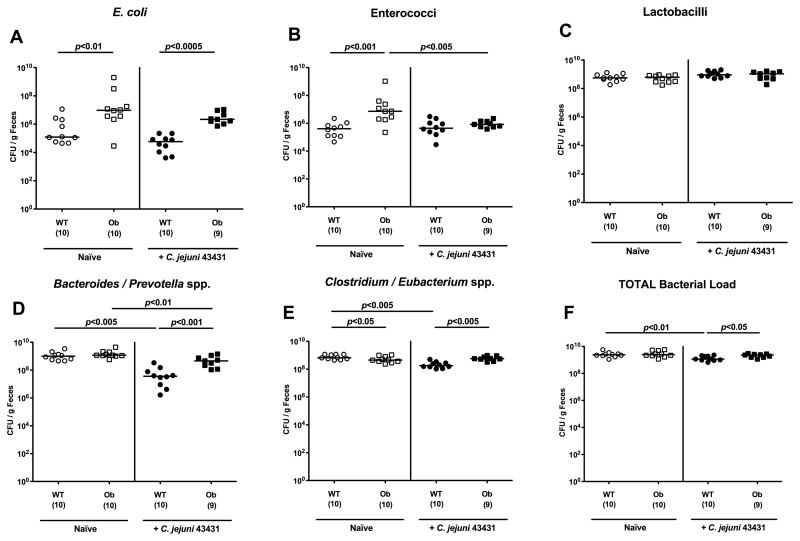
Next, we were interested in differences in the gut microflora composition of obese as compared to lean animals, which might render ob/ob mice susceptible to C. jejuni infection. Most interestingly, naive ob/ob mice harbored significantly higher E. coli and enterococci loads (approximately 2 orders of magnitude) in the colons as compared to naive WT controls, whereas Clostridium/Eubacterium spp. numbers were slightly lower (Fig. 6). At day 12 following C. jejuni ATCC 43431 infection, ob/ob mice harbored higher total bacterial numbers as well as higher loads of E. coli, Bacteroides/Prevotella spp., Clostridium/Eubacterium spp. in the colon (Fig. 6). Taken together, higher E. coli counts could uniquely be detected in mice prone to C. jejuni infection – irrespective of whether “humanized” mice, CAF-fed animals, or ob/ob mice with conventional flora had been examined.
Fig. 6.
Bacterial colonization status of intestines in obese mice. Mice homozygous for the obese spontaneous mutation (ob/ob, squares) and wild-type controls (WT, KS background; circles), both harboring a conventional gut microbiota, were orally infected with C. jejuni strain ATCC 43431 (open symbols) as described in methods. Numbers of (A) E. coli, (B) Enterococcus spp., (C) Lactobacillus spp., (D) Bacteroides/Prevotella spp., (E) Clostridium/Eubacterium spp., and (F) total bacterial loads were determined in feces samples of mice at day 12 p.i. (filled symbols) and compared to the respective uninfected control animals (open symbols) by detection of colony forming units (CFU) per gram feces on appropriate culture media (see methods). Bacterial species were identified by biochemical analysis and reconfirmed by comparative sequence analyses of 16S rRNA genes. Numbers of animals harboring the respective bacterial species are given in parentheses. Medians and significance levels (P-values) determined by Mann–Whitney-U test are indicated. Data shown were pooled from three independent experiments
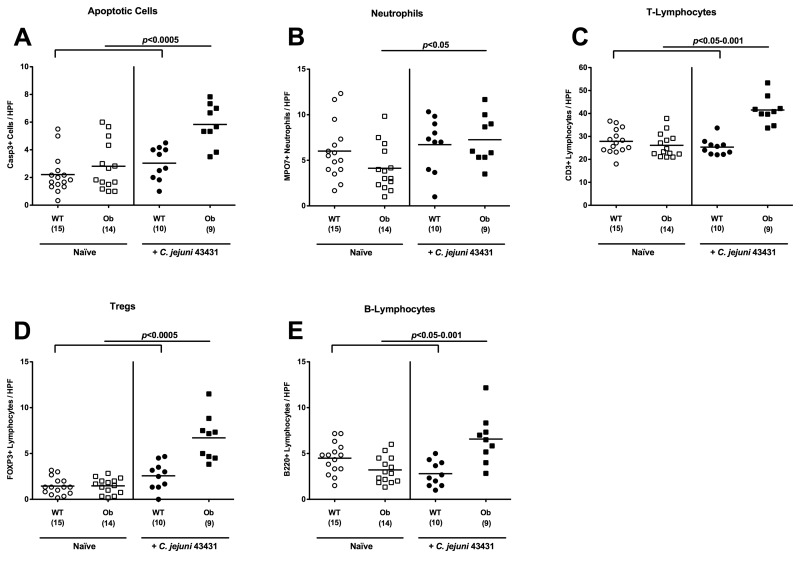
C. jejuni-induced intestinal inflammation in obese mice
In the naïve, uninfected condition, Casp3+, MPO7+, CD3+, B220+, and FOXP3+ cell numbers in the colons in situ did not differ when comparing ob/ob and WT control mice (Fig. 7). Twelve days following oral C. jejuni ATCC 43431 infection, however, a substantial increase (more than or equal to twofold) of apoptotic cells, neutrophilic granulocytes, T- and B-lymphocytes as well as Tregs in colon sections of ob/ob, but not WT, animals could be detected (Fig. 7). Thus, obese mice displayed a gut flora composition rendering them susceptible to C. jejuni infection; also, inflammatory changes in the colon as a consequence of high C. jejuni loads could be determined. Taken together, we could extend the murine models for campylobacteriosis by the use of obese mice and mice fed with a human diet, indicating that both diet and obesity are sufficient to render mice susceptible to C. jejuni infection.
Fig. 7.
Induction of immunopathology in the colon of obese mice following C. jejuni infection. Mice homozygous for the obese spontaneous mutation (ob/ob, squares) and wild-type controls (WT, KS background; circles) were orally infected with C. jejuni strain ATCC 43431 (filled symbols) as described in methods and compared to noninfected control animals (open symbols). The average numbers of apoptotic cells (positive for caspase-3, panel A), neutrophilic granulocytes (Neutrophils, positive for MPO-7, panel B), T-lymphocytes (positive for CD3, panel C), regulatory T-cells (Treg, positive for FOXP3, panel D), and B-lymphocytes (positive for B220, panel E) from at least six high power fields (HPF, 400× magnification) per animal were determined microscopically in immunohistochemically stained colon sections. Numbers of animals of the respective genotype analyzed are given in parentheses. Means (black bars) and levels of significance (P-values) as compared to the respective groups (determined by the Student’s t-test) are indicated. Data shown were pooled from three independent experiments
Discussion
Valid experimental data concerning intestinal colonization capacities of C. jejuni, mechanisms of its host interactions, and immunopathological responses in the course of C. jejuni infections of vertebrates are scarce. This is mainly due to the fact that mice harboring a conventional gut microbiota display a strong colonization resistance against C. jejuni and are thus not suitable as standardized vertebrate model for the study of C. jejuni infection in vivo. We have successfully overcome these limitations and developed novel murine C. jejuni infection models by modification of the commensal gut microbiota [9]. GB mice in which the gut flora had been completely eradicated by antibiotic treatment as well as mice colonized with a complex human intestinal flora were highly susceptible to C. jejuni infection, harbored high C. jejuni loads within their intestines, displayed significant host immune and inflammatory responses, and can thus excellently serve as novel experimental models for C. jejuni-infection and -mediated immunopathology (summarized in Ref. [9]).
In the study presented here, comparable and stable C. jejuni infection of intestines in GB mice which had been mono- and co-colonized with defined bacterial species from the commensal gut microbiota further confirms the GB mouse model as a valuable tool for the study of pathogen–bacteria interactions in vivo.
Given that the host-specific gut microbiota composition determines colonization resistance against C. jejuni infection and that the murine differed from the human intestinal microbiota by higher lactobacilli but lower enterobacteria numbers [9], we had raised the simple hypothesis that colonization resistance might be due to relatively high lactobacilli (and lower E. coli loads), whereas susceptibility to C. jejuni infection might be facilitated by higher E. coli (and lower lactobacilli) counts. Recolonization experiments of GB mice, however, indicate that colonization resistance against and susceptibility to C. jejuni infection are rather due to the complex microflora composition, physiological intraluminal milieu of the intestine, or so-far unknown factors and cannot be simply attributed to absolute loads or relative distribution of single bacterial species alone. Thus, we can only conclude that mechanisms underlying murine colonization resistance against C. jejuni are multifactorial.
Given that the gut microbiota composition and, in turn, the intestinal intraluminal milieu are mainly determined by dietary habits, we were interested in knowing whether nutrition can predispose and render a host susceptible to or resistant against C. jejuni infection. Interestingly, GB mice fed with a human CAF exhibited a gut microbiota composition which was comparable to the one seen in GB mice following reconstitution with a complex human flora (HFA): As compared to mice with a conventional gut microbiota, which was fed with a regular chow (MUD), CAF and HFA mice exhibited higher total bacterial, enterobacterial (E. coli), and Clostridium/Eubacterium spp. loads but lower Lactobacillus spp. numbers in their colon, indicating that the human diet favors the establishment of a human-like microflora in mice. Furthermore, CAF and HFA, but not MUD, mice were highly susceptible to C. jejuni infection, as indicated by high pathogen densities throughout the entire GI tract at day 12 p.i., subsequent immune and inflammatory host responses within the colon, and translocation of live C. jejuni into MLNs. Thus, we show for the first time that nutritional modification plays a crucial role in murine colonization resistance against and susceptibility to C. jejuni infection. In this context, it is interesting to note that results from a recent study in dogs revealed that dogs fed with a human diet were more susceptible to C. jejuni infection as compared to animals fed with a standard diet [27].
To confirm our results obtained in CAF mice subjected to 10 weeks of human “Western” diet that resulted in a significant gain of >40% body weight and thus obesity, we used another well-established model of obesity. Interestingly, ob/ob mice fed with a regular chow harbored a “normal” flora with higher E. coli and enterococci counts as compared to the conventional microbiota of lean WT controls on MUD. Following oral infection, ob/ob mice could be readily colonized by C. jejuni, and they exhibited significant immune and inflammatory responses in the intestine, as indicated by increased numbers of T- and B-lymphocytes, Tregs, neutrophils, and apoptotic cells in the colon in situ. Thus, similar to human campylobacteriosis, C. jejuni triggers both innate and adaptive immune responses in HFA, CAF, and ob/ob mice.
In summary, the study presented here demonstrates that mono-colonized mice, CAF mice (with obesity), and ob/ob mice can serve as suitable vertebrate models for the study of C. jejuni infection. The obtained results further hint at a quadrangle relationship between nutrition, gut microbiota composition, obesity, susceptibility to C. jejuni infection, and – as a consequence – intestinal inflammation. It is tempting to speculate that dietary habits have a pivotal effect on pathogen susceptibility and changes therein might lower the risk of human campylobacteriosis.
Acknowledgments
This work was supported by grants from the German Research Foundation (DFG) to UBG (GO363/12–1, CampyGerm; SFB633, TP A7), SB and AF (SFB633, TP A7), AK and CL (SFB633, TP Z1), AB and BS (SFB633, TP A12), MMH (SFB633, TP B6), and from the German Federal Ministry of Education and Research (BMBF) to SB (“Lab in a hanky” projects TP 1.1 and TP 8.2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Michaela Wattrodt, Ursula Rüschendorf, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance, animal breeding, and genotyping of mice. We are grateful to Simone Spieckermann for immunohistochemistry staining of colon sections.
Contributor Information
S. Bereswill, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
R. Plickert, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. Fischer, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. A. Kühl, 2Department of Pathology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
C. Loddenkemper, 2Department of Pathology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
A. Batra, 3Department of Internal Medicine, Charité – University Medicine Berlin, Berlin, Germany.
B. Siegmund, 3Department of Internal Medicine, Charité – University Medicine Berlin, Berlin, Germany.
U. B. Göbel, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
References
- 1.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 2.Bereswill S, Kist M. Recent developments in Campylobacter pathogenesis. Curr Opin Infect Dis. 2003 Oct;16(5):487–491. doi: 10.1097/00001432-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 3.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985 Sep;26(9):945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, Ruiz-Palacios GM. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ketley JM. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997 Jan;143(Pt 1):5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 6.Tsang RS. The relationship of Campylobacter jejuni infection and the development of Guillain-Barré syndrome. Curr Opin Infect Dis. 2002 Jun;15(3):221–228. doi: 10.1097/00001432-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010 Apr;300(4):205–211. doi: 10.1016/j.ijmm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Dorrell N, Wren BW. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr Opin Infect Dis. 2007 Oct;20(5):514–518. doi: 10.1097/QCO.0b013e3282a56b15. [DOI] [PubMed] [Google Scholar]
- 9.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "ménage à trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, Rathinam VA, Fierro BR, Linz JE, Young VB. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun. 2007 Mar;75(3):1099–1115. doi: 10.1128/IAI.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansfield LS, Patterson JS, Fierro BR, Murphy AJ, Rathinam VA, Kopper JJ, Barbu NI, Onifade TJ, Bell JA. Genetic background of IL-10(-/-) mice alters host-pathogen interactions with Campylobacter jejuni and influences disease phenotype. Microb Pathog. 2008 Oct;45(4):241–257. doi: 10.1016/j.micpath.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yrios JW, Balish E. Immune response of athymic and euthymic germfree mice to Campylobacter spp. Infect Immun. 1986 Nov;54(2):339–346. doi: 10.1128/iai.54.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yrios JW, Balish E. Colonization and infection of athymic and euthymic germfree mice by Campylobacter jejuni and Campylobacter fetus subsp. fetus. Infect Immun. 1986 Aug;53(2):378–383. doi: 10.1128/iai.53.2.378-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yrios JW, Balish E. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect Immun. 1986 Aug;53(2):384–392. doi: 10.1128/iai.53.2.384-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesudason MV, Hentges DJ, Pongpech P. Colonization of mice by Campylobacter jejuni. Infect Immun. 1989 Aug;57(8):2279–2282. doi: 10.1128/iai.57.8.2279-2282.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youssef M, Corthier G, Goossens H, Tancrede C, Henry-Amar M, Andremont A. Comparative translocation of enteropathogenic Campylobacter spp. and Escherichia coli from the intestinal tract of gnotobiotic mice. Infect Immun. 1987 Apr;55(4):1019–1021. doi: 10.1128/iai.55.4.1019-1021.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savidge TC, Smith MW, James PS, Aldred P. Salmonella-induced M-cell formation in germ-free mouse Peyer's patch tissue. Am J Pathol. 1991 Jul;139(1):177–184. [PMC free article] [PubMed] [Google Scholar]
- 18.Shroff KE, Cebra JJ. Development of mucosal humoral immune responses in germ-free (GF) mice. Adv Exp Med Biol. 1995;371A:441–446. doi: 10.1007/978-1-4615-1941-6_92. [DOI] [PubMed] [Google Scholar]
- 19.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006 Dec 15;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 20.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Göbel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007 Jul;56(7):941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009 Dec 21;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sclafani A, Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976 Sep;17(3):461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell NJ, Stock MJ. Combined effects of cafeteria and tube-feeding on energy balance in the rat. Proc Nutr Soc. 1979 May 1;38(1):5A. doi: 10.1079/pns19790026. [DOI] [PubMed] [Google Scholar]
- 24.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011 Jun;19(6):1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Göbel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010 Aug;59(8):1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 26.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A, Lehr HA, Liesenfeld O, Blaut M, Göbel UB, Schumann RR, Bereswill S. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007 Jul 25;2(7):e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stavisky J, Radford AD, Gaskell R, Dawson S, German A, Parsons B, Clegg S, Newman J, Pinchbeck G. A case-control study of pathogen and lifestyle risk factors for diarrhoea in dogs. Prev Vet Med. 2011 May 1;99(2-4):185–192. doi: 10.1016/j.prevetmed.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]