Abstract
The Saccharomyces cerevisiae gene YPK9 encodes a putative integral membrane protein which is 58% similar and 38% identical in amino acid sequence to the human lysosomal P5B ATPase ATP13A2. Mutations in ATP13A2 have been found in patients with Kufor-Rakeb syndrome, a form of juvenile Parkinsonism. We report that Ypk9p localizes to the yeast vacuole and that deletion of YPK9 confers sensitivity for growth for cadmium, manganese, nickel or selenium. These results suggest that Ypk9p may play a role in sequestration of divalent heavy metal ions. Further studies on the function of Ypk9p/ATP13A2 may help to define the molecular basis of Kufor-Rakeb syndrome and provide a potential link to environmental factors such as heavy metals contributing to some forms of Parkinsonism.
Introduction
Kufor Rakeb syndrome (KRS) is a rare form of juvenile Parkinsonism that follows autosomal recessive inheritance, first described in 1994 by Al-Din et al. Manifesting between seven and 24 years of age, these patients present with juvenile-onset parkinsonian symptoms attributed to pallido-pyrimidal syndrome (PPS), including bradykinesia, paraparesis, stooped posture and hyperreflexia (Davison, 1954; Jankovic, 1989; Nisipeanu, et al., 1994). Kufor Rakeb patients also have distinct symptoms including widespread neurodegeneration resulting in dementia and upgaze paresis, yet lack the intention tremor typical to parkinsonism disorders (al-Din, et al., 1994; Davison, 1954; Hunt, 1917; Nisipeanu, et al., 1994; Williams, et al., 2005). The causative gene associated with KRS is ATP13A2. ATP13A2 encodes an 1180 amino acid P-type ATPase, specifically of the P5 subfamily, that localizes to the lysosome (Axelsen and Palmgren, 1998; Kuhlbrandt, 2004; Ramirez, et al., 2006). There are several mutations identified with various forms of KRS (Di Fonzo, et al., 2007; Lin, et al., 2008; Ramirez, et al., 2006).
Ypk9p (YOR291w) is the Saccharomyces cerevisiae homologue to human ATP13A2 based on amino acid sequence alignment. Ypk9p is a 1472 amino acid P5 ATPase and has 58% similarity and 38% identity to ATP13A2. Recently, Ypk9p was shown to suppress a-synuclein and manganese toxicity in yeast, revealing a connection between the yeast gene and PD genetic and environmental risk factors (Gitler, et al., 2009). We demonstrate that deletion of YPK9, ypk9-Δ, results in sensitivity to cadmium, manganese, nickel and selenium. Further studies of the yeast protein may help to elucidate the function of ATP13A2 and uncover underlying defects of Kufor Rakeb syndrome.
Materials and Methods
Strains and plasmid construction
The parental (MATαhis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and single deletion strains used in this study were purchased from Open BioSystems. YPK9-GST in pEGH was purchased from Open Biosystems and transformed into ypk9-Δ using standard lithium acetate transformation protocol (Ito, et al., 1983; Schiestl and Gietz, 1989). ATP13A2 was amplified from human cDNA and subcloned into pcDNA3.1 (Invitrogen) and ligated into pYeura3 (Clontech) and transformed into YPK9+ and ypk9-Δ strains. YPK9-GFP was purchased from Invitrogen (Huh, et al., 2003).
Media and growth conditions
Rich media (YPD) contained 1% yeast extract, 2% peptone and 2% dextrose. Synthetic complete (SC) media contains 6.7 mg/ml yeast nitrogen base without amino acids, 5 mg/ml ammonium sulfate, 2% dextrose and all essential amino acids. Strains transformed with plasmids were selected and maintained on synthetic complete media lacking uracil (SC-ura). Galactose inducible plasmids were induced in SC-ura media containing 2% galactose and 0.1% raffinose in place of dextrose. Filter sterilized metal solutions were added to the appropriate concentration to either YPD or SC media after autoclaving.
Serial Dilutions
Strains were grown overnight in SC-ura media, harvested and washed twice in sterile water, re-inoculated and induced in 2% galactose media. Uninduced control strains were grown overnight in either YPD or SC-ura media. Strains were then harvested, washed twice, and resuspended at 3 × 108 cells/ml. Strains were serially diluted 10-fold. Cells were transferred to media with a 36-pin replicator, and plates were incubated at 30°C or 37°C for 3-5 days.
Colocalization of YPK9 with FM4-64
YPK9 tagged with green fluorescent protein (Invitrogen) was grown to log-phase in YPD, harvested, washed twice in 1 ml SC media, and resuspended in 100μls of SC media. FM4-64 staining was performed as previously described by Vida and Emr (Vida and Emr, 1995). Photomicrographs were obtained using an Axioplan2 Epifluorescent microscope. Cells were visualized under the 100× objective using an Epifluorescent microscope (Olympus BX61, Melville, NY), a CoolSNAP HQ CCD camera (Photometrics, Tucson, AR), and IPLab 4.0 acquisition software (BD Bioscience, Rockville, MD). Post imaging processing was performed using Autoquant X2 (Media Cybernetics). Image deconvolution was performed using the Autodeblur software package (Media Cybernetics, Bethesda, MD) and overlays of fluorescent images were performed using ImageJ software (NIH).
Results
Identification of YPK9
YOR291w is the yeast homolog to human ATP13A2 (58% similarity and 38% identity). ATP13A2 and Ypk9p both contain a PPALP sequence at the proposed ion binding site, classifying them as P5 ATPases of the P5B subfamily (Moller, et al., 2008). Spf1p, the other yeast P-type ATPase, is classified as a P5A ATPase and is presumed to have different ion specificities from Ypk9p based on the presence of 2 negative charges in place of the hydrophobic residue in P5B ATPases (Moller, et al., 2008). There appears to be some functional overlap between the two yeast P-type ATPases, however, as overexpression of YPK9 is able to rescue the a-syn toxicity seen in spf1-Δ (Gitler, et al., 2009).
Cadmium, manganese, nickel, and selenium are toxic to ypk9-Δ cells
P-type ATPases hydrolyze ATP to maintain an ion gradient across a membrane. We therefore compared growth for serial dilutions of YPK9+ and ypk9-Δ cells on media containing various dibasic metals. Sublethal metal concentrations were used or modified from a previous study (Table 1) (Pearce and Sherman, 1999). All strains were plated on unsupplemented YPD to determine a standard level of growth (Figure 1). ypk9-Δ exhibited growth defects when grown at 30° on YPD containing 8μM cadmium, 3mM manganese, 2.5mM nickel or 0.7mM selenium (Figure 1 and 2). Growth defects were also evident on YPD supplemented with these metals at 37° and SC media at both 30° and 37° (data not shown). Plasmid borne expression of YPK9 complemented these phenotypes (Figure 2). The function of a GFP tagged Ypk9p was also tested. There was a small growth defect of the GFP tagged Ypk9p on all 4 of the metal supplemented media, indicating that the construct is only partially functional (Figure 3). Human ATP13A2 does not appear to complement the ypk9-Δ phenotype (Figure 2).
Table I. Metal concentrations used for the screening of growth defects in ypk9-Δ.
Metal salts were added to YPD and SC media at the corresponding sublethal level. All metal salt solutions were filter sterilized prior to use.
| Metal | Final Concentration |
|---|---|
| Aluminum chloride | 4.5 mM |
| Cadmium chloride | 8 μM |
| Calcium chloride | 100 mM |
| Chromium chloride | 8 mM |
| Cobalt chloride | 1.2 mM |
| Copper sulfate | 2.4 mM |
| Ferric chloride | 5.5 mM |
| Ferrous chloride | 7 mM |
| Lead chloride | 2.5 mM |
| Lithium chloride | 40 mM |
| Magnesium chloride | 150 mM |
| Manganese chloride | 3 mM |
| Nickel chloride | 2.5 mM |
| Potassium chloride | 540 mM |
| Rubidium chloride | 5 mM |
| Selenium chloride | 0.7 mM |
| Silver nitrate | 0.35 mM |
| Sodium arsenic | 2.5 mM |
| Sodium chloride | 360 mM |
| Strontium chloride | 2.25 mM |
| Vanadium oxide | 13 mM |
| Zinc chloride | 4.4 mM |
Figure 1. The deletion of YPK9 confers sensitivity to cadmium, manganese, nickel and selenium.
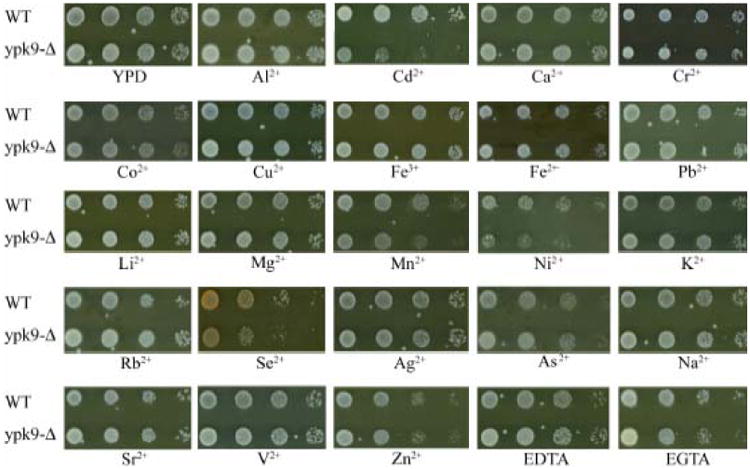
Ten fold serial dilutions of wild-type and ypk9-Δ cells were plated on YPD media and media supplemented with metals at concentrations listed in Table I.
Figure 2. Expression of YPK9 rescues cadmium, manganese, nickel and selenium sensitivity.
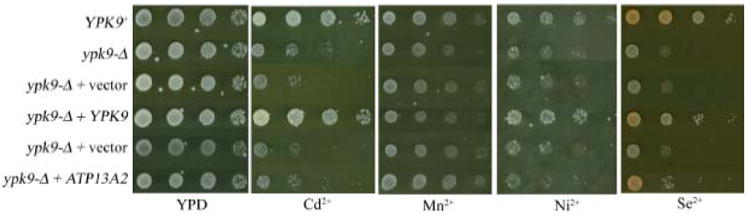
Ten-fold serial dilutions of the listed strains were plated ontoYPD media and media supplemented with cadmium, manganese, nickel and selenium at concentrations listed in Table I. YPK9 expression, but not ATP13A2 expression, restored growth in ypk9-Δ cells.
Figure 3. YPK9p localizes to the vacuole.
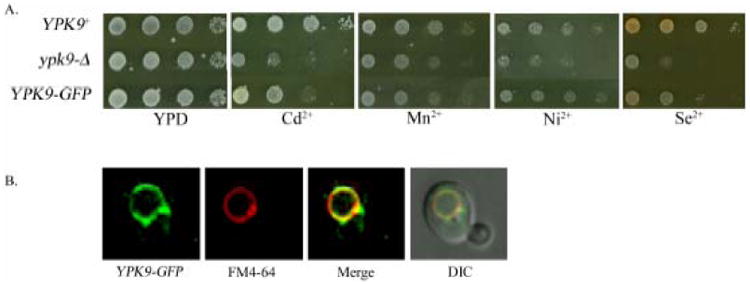
The sterol dye FM4-64 was used to stain the vacuolar membrane. Colocalization of Ypk9-GFP with FM4-64 indicates vacuolar localization of Ypk9p. The GFP tagged protein maintains slight functionality in the cell, indicated by a resistance to cadmium, manganese, nickel and selenium compared to ypk9-Δ.
YPK9p localizes to the vacuole
ATP13A2 localizes to the lysosome. Ypk9p-GFP co-localizes with the steryl dye FM4-64, indicating that Ypk9p is a vacuolar membrane protein, which is analogous to the mammalian lysosomal membrane (Figure 3). This confirms vacuolar localization of Ypk9-GFP (Gitler, et al., 2009).
Histidine metabolism affects toxicity of nickel and selenium in ypk9-Δ cells
In yeast the ability to synthesize histidine increases resistance to copper, cobalt and nickel salts (Farcasanu, et al., 2005; Pearce and Sherman, 1999). As our strains are histidine auxotrophs, due to the presence of his3Δ1, we tested if complementation with the HIS3 gene on the centromeric plasmid, pRS313, conferred tolerance to cadmium, manganese, nickel or selenium in ypk9-Δ. Toxicity of nickel and selenium in ypk9-Δ was partially suppressed by expression of HIS3 (Figure 4), as well as YPK9 (Figure 2).
Figure 4. Histidine metabolism affects nickel and selenium toxicity in ypk9-Δ.
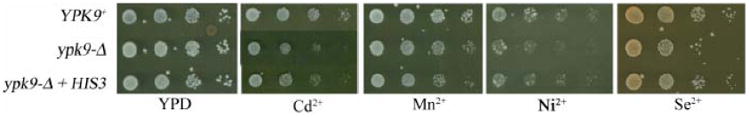
The introduction of HIS3 on a centromeric plasmid confers a complete histidine biosynthetic pathway in the strain used in this study and decreases the toxicity of nickel and selenium to ypk9-Δ cells.
Discussion
YPK9 is the yeast homolog of human gene ATP13A2, which is mutated in Kufor Rakeb syndrome patients. Our results suggest that Ypk9p could play a role in resistance to cadmium, manganese, nickel and selenium in the vacuole. However, nickel and selenium resistance may be influenced by the status of histidine metabolism. The sensitivity of ypk9-Δ to these metals may result from the inability of the cells to sequester the surplus of metals in the vacuole, thereby increasing cytosolic concentrations to toxic levels.
Cadmium and nickel are known cellular toxicants that increase reactive oxygen species (ROS) in cells. Both metals bind to sulfhydryl groups, common in antioxidants and other enzymes which reduce ROS. The binding of cadmium or nickel results in the inactivation of these enzymes and a consequent increase in ROS (Das, et al., 2008; Ercal, et al., 2001; Jarup, et al., 1998). Cadmium and nickel also inhibit yeast glutathione/reductase, the enzyme responsible for the reduction of oxidized glutathione (GSSH) to reduced glutathione (GSH) (Tandogan and Ulusu, 2007). GSH is the required substrate for glutathione peroxidase, which couples the reduction of H2O2 to H2O with the oxidation of GSH to GSSH. Cadmium and nickel therefore pose a double threat to antioxidant mechanisms in the cell, as they can either directly inhibit critical antioxidants or deplete glutathione levels necessary for the function of these enzymes. Additionally, cadmium has an inhibitory effect on complex III of the mitochondrial electron transport chain (Miccadei and Floridi, 1993; Wang, et al., 2004). Inhibition of this complex specifically stops electron transport to cytochrome C, resulting in the formation of ROS.
Since cadmium and nickel are not essential ions, specific importers for these metals are not reported and they most likely enter cells nonspecifically through other essential metal ion transporters. For example, cadmium has been shown to enter cells through divalent metal transporter (DMT1), iron transporters and zinc transporters, as well as through calcium channels (Bressler, et al., 2004; Dalton, et al., 2005; Gomes, et al., 2002; Leslie, et al., 2006; Perfus-Barbeoch, et al., 2002). The cadmium-transporting P1B-type ATPase in Saccharomyces cerevisiae, Pca1p, has been shown to be crucial for cadmium efflux. Interestingly, the gene has been shown to contain a mutation in several common laboratory strains, including the strain used in this study, which renders the protein nonfunctional (Adle and Lee, 2008). If Pca1p works in tandem with Ypk9p, the deletion of ypk9 from a strain already containing a nonfunctional cadmium efflux transporter would render the cells unable to purge cadmium through either ATPase, resulting in the toxicity we see here.
Selenium binds in the active site of glutathione peroxidase (GPx), the enzyme responsible for the reduction of H2O2 to H2O (Li, et al., 1990; Rotruck, et al., 1973). A positive correlation is seen between GPx activity and selenium uptake, resulting in an increase in resistance to oxidative damage (Aguilar, et al., 1998; Castano, et al., 1993; Huang, et al., 1994; Zafar, et al., 2003). Yeast do not encode a classical GPx; rather they contain 3 phospholipid hydroperoxide glutathione peroxidase-like enzymes, denoted Gpx1, 2 and 3, which are selenium independent. Gpx3p acts as a redox-transducer in the presence of hydroperoxide and signals to transcription factor Yap1p, which can also be activated by cadmium and selenium independently of GPx3 (Azevedo, et al., 2003; Delaunay, et al., 2002). A selenium transporter has not been identified in S. cerevisiae; however, selenium accumulates primarily in the vacuole, suggesting the presence of such a pump (Gharieb and Gadd, 1998). It is important to note that the deletion of either yap1 or gpx3 results in sensitivity to cadmium and selenium, indicating that both of these play a role in protecting the cell against the toxicity of these metals. This also implies the existence of a functional link between Yap1p, Gpx3, and Ypk9p.
Manganese enters S. cerevisiae through the divalent metal ion transporter Smf2p and is required as a cofactor for the mitochondrial superoxide dismutase SOD2, a key protectant against mitochondrial oxidative stress (Ravindranath and Fridovich, 1975; Weisiger and Fridovich, 1973). It has been shown that binding of the metal ion to the enzyme occurs in the mitochondrial, presumably because cytosolic concentrations of manganese are too low to activate Sod2p (Luk, et al., 2005). The low cytosolic concentration indicates that either the majority of the ion accumulates in the mitochondria or else other organelles have sequestered the remaining manganese. The protein Ccc1p may sequester manganese to the golgi (Lapinskas, et al., 1996). Currently there is no identified vacuolar manganese transporter, although sequestration here remains likely.
Oxidative stress has been implicated as a pathogenic pathway in many neurodegenerative diseases, including Parkinsons Disease (PD), via its contribution to programmed cell death through the disruption of protein and mitochondrial function (Andersen, 2004). Moreover, evidence suggests that elevated levels of metal ions could lead to oxidative stress, with cadmium, manganese, and selenium being cited as possible examples (Barnham, et al., 2004; Zecca, et al., 2004).
The sequence similarity between the human ATP13A2 and Ypk9p suggest that the two proteins may be functionally related. Mutations in ATP13A2 result in Kufor-Rakeb syndrome. While the function of ATP13A2 still needs to be determined, it is plausible to suggest that it is a transporter for an unidentified cation. Compromised function of ATP13A2 may disrupt the balance of this essential divalent cation. In addition, competition with this divalent cation from other metal ions may provide additional disease burden. Moreover, normal ATP13A2 activity could be compromised upon exposure to certain metal ions providing a potential link to the environment and PD.
Acknowledgments
This work was supported in part by NIH R01 NS36610 and P30 ES01247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adle DJ, Lee J. Expressional control of a cadmium-transporting P1B-type ATPase by a metal sensing degradation signal. J Biol Chem. 2008;283:31460–8. doi: 10.1074/jbc.M806054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar MV, Jimenez-Jimenez FJ, Molina JA, Meseguer I, Mateos-Vega CJ, Gonzalez-Munoz MJ, de Bustos F, Gomez-Escalonilla C, Ort-Pareja M, Zurdo M, Martinez-Para MC. Cerebrospinal fluid selenium and chromium levels in patients with Parkinson's disease. J Neural Transm. 1998;105:1245–51. doi: 10.1007/s007020050127. [DOI] [PubMed] [Google Scholar]
- al-Din SN, Anderson M, Eeg-Olofsson O, Trontelj JV. Neuro- ophthalmic manifestations of the syndrome of ophthalmoplegia, ataxia and areflexia: a review. Acta Neurol Scand. 1994;89:157–63. doi: 10.1111/j.1600-0404.1994.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10 Suppl:S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- Azevedo D, Tacnet F, Delaunay A, Rodrigues-Pousada C, Toledano MB. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicalssignaling. Free Radic Biol Med. 2003;35:889–900. doi: 10.1016/s0891-5849(03)00434-9. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases andoxidative stress. Nat Rev Drug Discov. 2004;3:205–14. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D. Divalent metaltransporter 1 in lead and cadmium transport. Ann N Y Acad Sci. 2004;1012:142–52. doi: 10.1196/annals.1306.011. [DOI] [PubMed] [Google Scholar]
- Castano A, Cano J, Machado A. Low selenium diet affects monoamineturnover differentially in substantia nigra and striatum. J Neurochem. 1993;61:1302–7. doi: 10.1111/j.1471-4159.1993.tb13622.x. [DOI] [PubMed] [Google Scholar]
- Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as thetransporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci U S A. 2005;102:3401–6. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KK, Das SN, Dhundasi SA. Nickel, its adverse health effects &oxidative stress. Indian J Med Res. 2008;128:412–25. [PubMed] [Google Scholar]
- Davison C. Pallido-pyramidal disease. J Neuropathol Exp Neurol. 1954;13:50–9. doi: 10.1097/00005072-195401000-00007. [DOI] [PubMed] [Google Scholar]
- Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. A thiolperoxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–81. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Chien HF, Socal M, Giraudo S, Tassorelli C, Iliceto G, Fabbrini G, Marconi R, Fincati E, Abbruzzese G, Marini P, Squitieri F, Horstink MW, Montagna P, Libera AD, Stocchi F, Goldwurm S, Ferreira JJ, Meco G, Martignoni E, Lopiano L, Jardim LB, Oostra BA, Barbosa ER, Bonifati V. ATP13A2 missense mutations in juvenileparkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–62. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidativestress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–39. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Farcasanu IC, Mizunuma M, Nishiyama F, Miyakawa T. Role of L-histidine in conferring tolerance to Ni2+ in Sacchromyces cerevisiae cells. Biosci Biotechnol Biochem. 2005;69:2343–8. doi: 10.1271/bbb.69.2343. [DOI] [PubMed] [Google Scholar]
- Gharieb MM, Gadd GM. Evidence for the involvement of vacuolaractivity in metal(loid) tolerance: vacuolar-lacking and -defective mutants of Saccharomyces cerevisiae display higher sensitivity to chromate, tellurite andselenite. Biometals. 1998;11:101–6. doi: 10.1023/a:1009221810760. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S. alpha-Synuclein is part of a diverse and highly conserved interactionnetwork that includes PARK9 and manganese toxicity. Nat Genet. 2009 doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes DS, Fragoso LC, Riger CJ, Panek AD, Eleutherio EC. Regulation of cadmium uptake by Saccharomyces cerevisiae. Biochim Biophys Acta. 2002;1573:21–5. doi: 10.1016/s0304-4165(02)00324-0. [DOI] [PubMed] [Google Scholar]
- Huang K, Lauridsen E, Clausen J. The uptake of Na-selenite in rat brain. Localization of new glutathione peroxidases in the rat brain. Biol Trace Elem Res. 1994;46:91–102. doi: 10.1007/BF02790070. [DOI] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Hunt RH. Correspondence. Cal State J Med. 1917;15:474–5. [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeastcells treated with alkali cations. J Bacteriol. 1983;153:163–8. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinsonism-plus syndromes. Mov Disord. 1989;4 Suppl 1:S95–119. doi: 10.1002/mds.870040512. [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24 Suppl 1:1–51. [PubMed] [Google Scholar]
- Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5:282–95. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- Lapinskas PJ, Lin SJ, Culotta VC. The role of the Saccharomycescerevisiae CCC1 gene in the homeostasis of manganese ions. Mol Microbiol. 1996;21:519–28. doi: 10.1111/j.1365-2958.1996.tb02561.x. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Liu J, Klaassen CD, Waalkes MP. Acquired cadmiumresistance in metallothionein-I/II(-/-) knockout cells: role of the T-type calciumchannel Cacnalpha1G in cadmium uptake. Mol Pharmacol. 2006;69:629–39. doi: 10.1124/mol.105.014241. [DOI] [PubMed] [Google Scholar]
- Li NQ, Reddy PS, Thyagaraju K, Reddy AP, Hsu BL, Scholz RW, Tu CP, Reddy CC. Elevation of rat liver mRNA for selenium-dependentglutathione peroxidase by selenium deficiency. J Biol Chem. 1990;265:108–13. [PubMed] [Google Scholar]
- Lin CH, Tan EK, Chen ML, Tan LC, Lim HQ, Chen GS, Wu RM. Novel ATP13A2 variant associated with Parkinson disease in Taiwan and Singapore. Neurology. 2008;71:1727–32. doi: 10.1212/01.wnl.0000335167.72412.68. [DOI] [PubMed] [Google Scholar]
- Luk E, Yang M, Jensen LT, Bourbonnais Y, Culotta VC. Manganeseactivation of superoxide dismutase 2 in the mitochondria of Saccharomycescerevisiae. J Biol Chem. 2005;280:22715–20. doi: 10.1074/jbc.M504257200. [DOI] [PubMed] [Google Scholar]
- Miccadei S, Floridi A. Sites of inhibition of mitochondrial electron transportby cadmium. Chem Biol Interact. 1993;89:159–67. doi: 10.1016/0009-2797(93)90006-k. [DOI] [PubMed] [Google Scholar]
- Moller AB, Asp T, Holm PB, Palmgren MG. Phylogenetic analysis of P5 P-type ATPases, a eukaryotic lineage of secretory pathway pumps. Mol Phylogenet Evol. 2008;46:619–34. doi: 10.1016/j.ympev.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Nisipeanu P, Kuritzky A, Korczyn AD. Familial levodopa-responsiveparkinsonian-pyramidal syndrome. Mov Disord. 1994;9:673–5. doi: 10.1002/mds.870090614. [DOI] [PubMed] [Google Scholar]
- Pearce DA, Sherman F. Toxicity of copper, cobalt, and nickel salts isdependent on histidine metabolism in the yeast Saccharomyces cerevisiae. J Bacteriol. 1999;181:4774–9. doi: 10.1128/jb.181.16.4774-4779.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. Heavy metaltoxicity: cadmium permeates through calcium channels and disturbs the plantwater status. Plant J. 2002;32:539–48. doi: 10.1046/j.1365-313x.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–91. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Ravindranath SD, Fridovich I. Isolation and characterization of amanganese-containing superoxide dismutase from yeast. J Biol Chem. 1975;250:6107–12. [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component ofglutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeastcells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–46. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Tandogan B, Ulusu NN. The inhibition kinetics of yeast glutathionereductase by some metal ions. J Enzyme Inhib Med Chem. 2007;22:489–95. doi: 10.1080/14756360601162147. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membranedynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–92. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electrontransfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–43. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Weisiger RA, Fridovich I. Mitochondrial superoxide simutase. Site ofsynthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–6. [PubMed] [Google Scholar]
- Williams DR, Hadeed A, al-Din AS, Wreikat AL, Lees AJ. Kufor Rakeb disease: autosomal recessive, levodopa-responsive parkinsonism withpyramidal degeneration, supranuclear gaze palsy, and dementia. Mov Disord. 2005;20:1264–71. doi: 10.1002/mds.20511. [DOI] [PubMed] [Google Scholar]
- Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F. Dose-dependent protective effect of selenium in rat model of Parkinson's disease: neurobehavioral and neurochemical evidences. J Neurochem. 2003;84:438–46. doi: 10.1046/j.1471-4159.2003.01531.x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–73. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]


