Abstract
The last decade of acute heart failure (HF) research is characterized by disappointments in large phase 2 and 3 pharmacologic studies of therapeutics including calcium-sensitizing agents and antagonists of endothelin, vasopressin, and adenosine. As a result, pharmacologic management for acute HF has changed little in recent years, and adverse event rates remain higher than in chronic HF. Despite neutral results in many acute HF trials, recent studies including RELAX-AHF, ASTRONAUT, and PRONTO have highlighted the role of appropriate timing of patient enrollment, targeting the “right” patients, and selecting appropriate end points and sites. We describe lessons learned from recent trials in acute HF and outline strategies to improve the potential for success in future trials. This review is based on discussions between scientists, clinical trialists, and regulatory representatives at the 9th Global Cardio Vascular Clinical Trialists Forum in Paris, France, from November 30 to December 1, 2012.
The last decade of acute heart failure (HF) research is characterized by disappointments in large phase 2 and 3 pharmacologic studies of therapeutics including B-type natriuretic peptide (BNP) agonists and antagonists of endothelin, vasopressin, and adenosine.1 As a result, management for acute HF has changed little in the recent years,2,3 and most guidelines are based on consensus rather than evidence. Hospitalization for HF represents a critical juncture for patients because their event rates are more than twice those of individuals without recent hospitalization.4 Greater than one-third of patients hospitalized with HF will be rehospitalized or dead within 90 days after discharge.5 Although recent progress in characterizing patients with acute HF has occurred, evidence for strategies to reduce their postdischarge event rate is sparse.6 However, several recent studies have highlighted the importance of appropriate timing of patient enrollment, targeting the “right” patients, and selecting appropriate end points and sites. We describe lessons learned from recent acute HF trials and outline strategies to improve the chance for success in future trials. We discuss the large-scale randomized trials of RELAXin in Acute HF (RELAX-AHF) and the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) and the pilot study of clevidipine (PRONTO). These trials were selected based on recent publication or presentation status and their use in illustrating issues related to the design of acute HF trials. We included the PRONTO trial to discuss emergency department enrollment rather than specifics related to the investigational therapy. These discussions can be placed in the context of other reviews of neutral acute HF trials including studies of nesiritide, rolofylline, and ultrafiltration.7 This review is based on discussions between scientists, clinical trialists, and regulatory representatives at the 9th Global Cardio Vascular Clinical Trialists Forum in Paris, France, from November 30 to December 1, 2012.
Recent acute HF trials: RELAX-AHF, ASTRONAUT, and PRONTO
The RELAX-AHF trial investigated the use of the vasoactive peptide hormone serelaxin in a placebo-controlled, randomized trial with coprimary end points of dyspnea improvement via 2 dyspnea instruments8 (Table I). A 48-hour intravenous (IV) infusion of serelaxin resulted in an improvement in the visual analog scale (VAS) area under the curve from baseline to 5 days, but not dyspnea improvement by Likert scale. There were no between-group differences for the secondary composite end points including death or hospitalization, but all-cause mortality at 180 days (a prespecified end point) was lower with serelaxin. Benefits with serelaxin were also observed for rates of worsening HF, length of stay, and prognostic biomarkers.
Table I.
Summary of trials
| Trial | Study arms | Acuity of treatment | Patient population | Primary end point | Results |
|---|---|---|---|---|---|
| RELAX-AHF (n = 1161) | Seralaxin 30 μg kg−1 d−1 vs placebo for 48 h | Randomization within 16 h of hospital arrival | Patients with dyspnea at rest or minimal exertion, congestion on chest x-ray, BNP ≥350 ng/L, eGFR 30–75 mL/min per 1.73 m2, and SBP >125 mm Hg | Change in patient-reported dyspnea from baseline to day 5 (VAS AUC) Moderately or markedly improved patient-reported dyspnea at 6, 12, and 24 h (7-point Likert scale) |
Seralaxin significantly improved dyspnea compared with placebo by VAS AUC (P = .007) No difference between dyspnea improvement by Likert (P = .70) |
| ASTRONAUT (n = 1639 randomized; 1615 in final efficacy analysis) | Aliskiren 150 mg daily (increased to 300 mg as tolerated) vs placebo | After a period of stabilization (median 5 d after admission) | Hemodynamically stable patients hospitalized for HF with EF ≤40%, BNP ≥400 pg/mL or NT-proBNP ≥1600 pg/mL, and signs/symptoms of fluid overload | CV death or HF rehospitalization at 6 and 12 mo | No difference in CV death or HF hospitalization at 6 (P = .41) or 12 mo (P = .36) |
| PRONTO (n=104) | Clevidipine vs standard-of-care IV antihypertensives | Emergency department: door-to-treatment time of 149 mo (104, 219) | Dyspneic patients with acute HF with SBP ≥160 mm Hg | Median time and percent of patients that attain the initial prespecified SBP target range and a 15% reduction in SBP from baseline within the first 30 minutes | More patients reached the target SBP range and a 15% SBP reduction with clevidipine vs usual care (P = .0006) |
Abbreviations: eGFR, Estimated glomerular filtration rate; AUC, area under the curve; CV, cardiovascular.
Several lessons can be highlighted from the RELAX-AHF trial design and results (Table II).9 First, the trial enrolled patients relatively early after presentation (ie, randomization within 16 hours of hospital arrival). The therapeutic window to achieve improvement in an efficacy end point, such as dyspnea improvement, benefits from patient enrollment earlier in the clinical course.10 The first several days of an acute HF episode appear to be a critical window because it is during this time that organ damage and lack of decongestion may exert long-term adverse effects.11 A recent exploratory analysis of the mortality benefit in RELAX-AHF demonstrated that serelaxin improved markers of cardiac, renal, and hepatic function early during acute HF hospitalization consistent with the prevention of organ dysfunction and improved decongestion.11 Hence, improved dyspnea relief, reduced organ damage, and less use of concomitant IV therapies may support improved long-term outcomes with novel agents in acute HF. To conduct similar studies in the future, a shift from typical trial entry criteria may be necessary to allow for streamlined enrollment. The RELAX-AHF trial did not have an ejection fraction (EF) entry criterion, which could delay enrollment time and limit overall recruitment.
Table II.
Lessons from recent acute HF trials
|
Second, by enrolling those with systolic blood pressure (SBP) >125 mm Hg, vascular congestion, and renal impairment, the entry criteria targeted those felt most likely to derive clinical benefit, while avoiding potential harm related to study drug (eg, hypotension). The desire to demonstrate broad efficacy of a novel agent should be tempered by the perspective that success is dependent on the drug being matched to the correct patient.12 Because the acute HF population is characterized by marked heterogeneity, a one-size-fits-all approach to drug testing is less likely to succeed.
Third, the interpretation of the long-term mortality benefit seen with a 48-hour infusion is illustrative. The results confirm the importance of investigating long-term outcomes with a short-term therapy. Importantly, the relatively few events for the 180-day end point (placebo, 65 deaths; serelaxin, 42 deaths) with a reduced number at risk over time (444 placebo patients and 463 serelaxin patients at 180 days from 580 and 581 at randomization, respectively) highlights the hypothesis-generating nature of this observation. However, the comprehensive assessment of worsening HF, biomarker data and adverse events in RELAX-AHF provides a convincing argument for future investigation in an appropriately powered trial. The lack of a reduction in rehospitalizations with serelaxin despite a reduction in mortality requires further investigation. Although rehospitalization has historically been regarded as a risk factor for mortality, recent data have shown a poor correlation between the two.13
The ASTRONAUT study evaluated the effect of the direct renin inhibitor aliskiren.14 The ASTRONAUT study enrolled 1,639 patients hospitalized with worsening HF, EF ≤40%, and elevated BNP. For the primary end point of cardiovascular death or HF rehospitalization, aliskiren did not result in a reduction in events. Adverse events including hyperkalemia, hypotension, and renal impairment were more frequent in the aliskiren group.
The ASTRONAUT design may inform future acute HF trials. First, patients were enrolled during acute HF hospitalization, but after a stabilization period. Because of a longer preenrollment period, this study design may serve to homogenize the enrolled candidate population and mitigate increased risk with a novel therapy early during HF hospitalization, thus targeting the high-risk postdischarge period. Some have suggested that chronic HF can be subdivided into ambulatory patients (ie, those with a low event rate) and patients hospitalized with worsening chronic HF who, after stabilization (as in ASTRONAUT), are considered to have chronic HF but continue to have unacceptably high postdischarge event rates. Therefore, depending on the mechanism of action and therapeutic window for a particular agent, the ideal time for investigation could be earlier in the clinical course (eg, RELAX-AHF) or after a period of stabilization (eg, ASTRONAUT). For instance, agents that target reversible muscle injury through early decongestion can be contrasted with agents designed to reduce adverse remodeling after hospital discharge.
Second, patients in ASTRONAUT were continued on study drug postdischarge. This strategy (ie, Stage C trial) is in contrast to the traditional model involving a short duration of IV therapy during hospitalization. Although 48 hours of serelaxin resulted in reduced 180-day mortality in the RELAX-AHF, the backdrop of multiple previous neutral studies of short durations of therapy7 suggests that routine use of this approach may need reevaluation. Short-term administration of a therapy should not routinely be expected to improve long-term outcomes without a clear mechanism for changing the underlying substrate (eg, thrombolytics for ST-elevation myocardial infarction). However, it also remains to be determined whether Stage C trials will translate into improved postdischarge outcomes.
Third, subgroup analysis demonstrated an interaction between diabetes status and outcome with increased all-cause mortality at 12 months in diabetic patients. Although the findings in the diabetic patients should be interpreted with caution because of statistical limitations (eg, subgroup analysis of a nonprimary end point), they illustrate the heterogeneity of the acute HF population and suggest that further work is needed to explore the optimal method to stratify patients in future studies.
Despite evidence of a significant reduction in BNP with aliskiren, the ASTRONAUT trial failed to show a benefit with respect to major clinical outcomes. This example highlights how surrogate markers may help to establish biological plausibility but should not replace hard clinical outcomes. Furthermore, it may challenge whether a global rank end point (death, HF hospitalization, and BNP) is appropriate because this type of end point would have been positive in ASTRONAUT despite no benefit on clinical outcomes. In some cases, it may be that biomarkers are sensitive but not specific for outcomes. For instance, the lack of changes in biomarkers may strongly predict a lack of efficacy, but favorable changes in biomarkers may not guarantee long-term efficacy. Furthermore, HF biomarkers may be less predictive when there is a mechanism of harm or off-target effect that is not directly HF related (eg, hyperkalemia). Ongoing investigations may further clarify the BNP observations from ASTRONAUT.
The PRONTO study was a randomized trial of the short-acting arteriospecific calcium antagonist clevidipine versus standard-of-care IV antihypertensives in patients with acute HF.15 Although PRONTO was a small, open-label, early-phase study not comparable with RELAX-AHF and ASTRONAUT, the study may inform future trial design. The emergency department study enrolled 104 dyspneic patients with acute HF and hypertension and set a 30-minute target SBP range. Significantly more patients reached the target SBP range and a 15% SBP reduction with clevidipine versus usual care. At 45 minutes after treatment, the dyspnea VAS decrease from baseline was greater with clevidipine than usual care, an effect maintained at 3 hours. Although not statistically significant, exploratory analyses found that clevipidine was associated with numerically lower procedural rates and intensive care unit admissions and reduced length of stay without an increase in drug-related adverse events compared with usual care.
Although the interpretation of its results is challenged by its open-label design, PRONTO highlights the potential role for initiating acute HF trials in the emergency department. The door-to-treatment time of 149 minutes (104, 219) was substantially shorter than previous trials. This supports a new model of intervening within the first several hours after presentation. The trial encouraged enrollment based only on physician clinical diagnosis. Patients were required to have a pretreatment chest radiography and natriuretic peptide level, but the results were not required before dosing. Rather, the results from these tests were used to confirm acute HF poststudy drug infusion. Innovative study designs capitalizing on increased collaboration between emergency department and inpatient clinicians may improve patient enrollment and increase the potential of a novel agent demonstrating a benefit. Importantly, the lack of blinding demonstrates the importance of subsequent confirmatory trials with additional clinically relevant outcomes. Thus, the PRONTO trial highlights the challenge of capturing the right patients for a trial while optimizing the speed of enrollment.
Trial design and execution
New approaches to pharmacologic therapies in acute HF
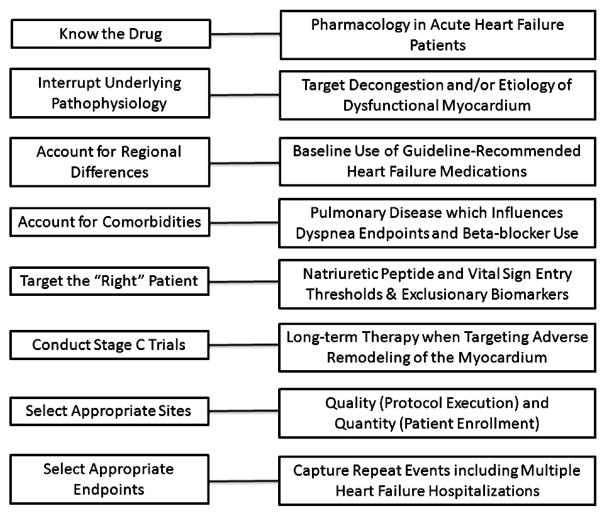
Contemporary strategies to improve outcomes in acute HF should take into account past experiences and recent advances (Figure). First, the pharmacologic properties of the study drug must be understood in the acute HF population.16 Acute HF trials may be limited by investigating novel molecules with poorly described pharmacology in congested patients with end-organ dysfunction. There is a critical need to distinguish between acute HF and chronic HF. One of the Achilles heels of acute HF drug development is that phase I studies are commonly conducted in healthy volunteers with phase II studies in patients with chronic HF and phase III in patients with acute HF. This sequence may misinform development programs. Consideration should be given to the possibility that drugs targeting patients with acute HF may not be as effective or display a similar dose-response curve in chronic HF. That efficacy and dose selection in the Pre-RELAX phase II trial were studied in the same population as the phase III trial may have been a key to the success of the program.
Figure.
Considerations for the investigation of acute HF therapies.
Second, the study drug must be matched to the correct patient. Patient selection criteria should target the population most likely to benefit from the agent while excluding those more likely to experience adverse effects. Selection criteria could include elevated BNP to obtain a higher-risk population and potentially also renal marker thresholds (eg, Neutrophil Gelatinase-Associated Lipocalin and Kidney Injury Molecule-1) to avoid inclusion of those more likely to experience renal toxicity. Given the heterogeneity in patients with acute HF around the world,17 geographical differences in management should be recognized and incorporated into entry criteria to improve standardization (eg, baseline medication use). Beyond these regional differences, significant heterogeneity exists within patients enrolled at a single institution with respect to baseline characteristics, symptoms, and response to therapy. Determination of the target population should account for comorbid diseases. Patients with HF commonly exhibit a significant comorbidity burden that can lead to hospitalization because of comorbidity exacerbation, worsening HF, or both.18 A focus on HF therapies without attention to comorbid diseases may be less likely to improve patient outcomes.
Finally, because a drug given for hours to days over the course of an HF hospitalization is less likely to impact long-term outcomes, initiation of a therapy during HF hospitalization and continuation of the therapy postdischarge represents a more logical and promising approach for many acute HF trials (ie, Stage C trials).12 Regardless of the trial design used, it will be necessary to select the appropriate enrolling sites with a focus on both quality (protocol execution) and quantity (patient enrollment). Improving the alignment between academia, industry, regulatory agencies, and health care payers may enhance the ability to conduct acute HF trials. Collaboration between the academic leadership and the pharmaceutical sponsor represents a critical step in trial design.
Selecting appropriate sites
A key component of study conduct relates to the selection of study sites. Heart failure trials are increasingly global ventures to reduce costs and shorten timelines. Potential problems with trial globalization include regional differences in patient characteristics and practice patterns, which may influence study outcomes.19 Patients from sites with low recruitment may have worse outcomes compared with sites with higher enrollment patterns.20 These observations may be related to study reimbursement rates, multiple competing studies, inadequate training of site monitors, higher dropout rates, and more protocol violations. There remains an unmet need to develop strategies to identify trial sites that have acceptable patient quantity and high-quality protocol execution. Pretrial registries, global HF networks, and the development of site performance metrics have been proposed.21 Pretrial registries may offer an improved understanding of the number and characteristics of eligible patients as well as the average clinical course and event rates at specific sites. The current benchmark for enrollment (~0.4 patients/site per month in EVEREST) may not be conducive to proper execution in future trials. Regional differences in enrollment exist and are notable for low enrollment in Western Europe and North America.20 Increasing regulatory issues leading to more detailed monitoring may be reasons for lower enrollment and site motivation in certain regions. Protocols, case-report forms, and recruitment strategies should be designed so that sites can recruit more than 1 patient per month.
Appropriate end points
Possible explanations for the disappointments from previous acute HF trials include the heterogeneity of the patient population and lack of appropriate trial end points.1,22 We suggest that future acute HF studies focus on patient outcomes in addition to symptomatic relief. Dyspnea relief has been a primary end point in many acute HF trials,23 owing, in part, to it being an approvable end point from a regulatory perspective, yet the relevance of this end point has been questioned.24 Dyspnea relief occurs early in the course of hospitalization with current standard therapy, dyspnea measurements are not standardized, and the relationship between dyspnea relief and clinical outcomes is not completely clear.10 Improved standardization of dyspnea measurements has been proposed25 and has recently been incorporated in clinical trials.26
The time between presentation and receipt of an investigational agent is critical. As demonstrated in the standard-of-care arm of PRONTO, dyspnea resolved in as little as 12 hours, leaving little remaining impact for any investigational agent if enrollment occurred later. Regardless, the dyspnea target has led to acute HF investigations largely confined to the use of higher doses of IV medications targeting the peripheral hemo-dynamics to rapidly lower pulmonary capillary wedge pressure to achieve rapid symptom improvement. However, to truly improve outcomes, it may be necessary to target the dysfunctional myocardium and use meaningful end points that include clinical outcomes in addition to surrogates or transient relief of symptoms. For instance, the prevention of worsening HF may be another appropriate end point as supported by multiple studies.11,27 Formal cost-effectiveness analyses are needed that demonstrate incremental benefits of newer therapies over the usual care. Importantly, defining “accurate” end points is only one component of optimizing trial design because an effective treatment should be able to improve outcomes in multiple different areas including quality of life, symptoms, biomarkers, and outcomes.
The value of capturing repeat events in postdischarge acute HF trials
The conventional end points in HF trials have emphasized time to the first HF hospitalization or cardiovascular death. However, these end points ignore repeat HF hospitalizations as well as death after hospitalization. For instance, an analysis of the EMPHASIS trial demonstrated that the primary end point of time to the first event of cardiovascular death or HF hospitalization ignored 285 repeat HF hospitalizations (41% of the total) and 144 cardiovascular deaths that occurred after HF hospitalization (43% of the total).28 Similarly, an analysis of CHARM-Preserved showed that the primary end point of cardiovascular mortality or HF hospitalization used only 53% of all HF hospitalizations and 57% of all cardiovascular deaths.29,30
There remains a need for agreed-upon methods that capture the importance of all HF hospitalizations. Potential options to analyze repeat hospitalizations include the following methods: Andersen-Gill, Poisson, Negative Binomial, win ratio,31 and a method that jointly models hospitalizations and mortality.30 Such end points may provide benefits related to increased statistical power and smaller sample sizes. However, there would also a need to be an accepted method to take into account cardiovascular mortality. One option would be to include cardiovascular death as the “final hospitalization.” Arguments against this approach are that more weight could be given to repeat hospitalizations than mortality. Future research is needed to clarify the use of these novel end points and would require validation and acceptance from regulatory agencies.
Conclusion
Acute HF is a growing public health problem worldwide, and there have been few positive acute HF trials. We described lessons learned from recent trials including RELAX-AHF, ASTRONAUT, and PRONTO and outlined potential strategies for success in future trials. Specifically, we discussed appropriate timing of patient enrollment, understanding the pharmacology of the investigational drug, targeting the right patients, and selecting appropriate end points and clinical sites. As trials are increasingly conducted globally, there remains an unmet need to better define which patients to enroll in clinical trials and from which clinical sites.
Footnotes
Disclosures
No extramural funding was used to support this work. G.M.F. has received consulting fees from Novartis, Medpace, Amgen, Otsuka, Trevena, Roche Diagnostics, Merck, BG Medicine, Medtronic, and St Jude, and grant funding from Amgen, Otsuka, Roche Diagnostics, and NHLBI. W.F.P. has received research grants from Abbott, Alere, Brahms, Novartis, Roche, and The Medicine’s Company; is a consultant for Abbott, Alere, BG, Cardiorentis, GE, Jannsen, Lily, The Medicine’s Company, Singulex, and Verathon; and served on the speaker’s bureaus of Abbott, Alere, Astra Zeneca, and Daichi-Sankyo; and has received ownership interest from Comprehensive Research Associates LLC, Vital Sensors, and Emergencies in Medicine LLC. B.P. has received consulting fees from Pfizer, Merck, Novartis, Takeda, Astra Zeneca, Bayer, Lilly, BMS, Cytopherx, Amorcyte, Relypsa, BG Medicine, Aurasense, and GE-Health Care; stocks options from Relypsa, BG Medicine, and Aurasense; and grant support from Novartis, Forrest Laboratories, and Medtronic. A.P.M. has received consulting fees from Bayer, Amgen, Cardiorentis, and J&J; grants support from Novartis, Medtronic, and Abbott; and consulting fees/travel support from Novartis Pharma. M.G. has received consulting fees from Abbott Laboratories, Astellas, AstraZeneca, Bayer Schering Pharma, Bayer HealthCare, Cardiorentis, CorThera, Cytokinetics, CytoPherx, DebioPharm, Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Intersection Medical, INC, J&J, Medtronic, Merck, Novartis Pharma, Ono Pharma USA, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, sanofi-aventis, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT, Takeda Pharmaceuticals, and Trevena Therapeutics. S.P. has received grant support from Astra Zeneca and Pfizer. F.Z. has received grants support from Novartis, BG Medicine, and Roche Diagnostics; has served on the speakers’ bureaus from Pfizer and AstraZeneca; is a board member of Boston Scientific; and has received consulting fees from Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and ResMed. The other authors report no relevant conflicts. The authors are solely responsible for the drafting and editing of the manuscript and its final contents.
References
- 1.Allen LA, Hernandez AF, O’Connor CM, et al. End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol. 2009;53(24):2248–58. doi: 10.1016/j.jacc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez A, Abelmann WH. Cardiac decompensation. N Engl J Med. 1974;290(9):499–501. doi: 10.1056/NEJM197402282900906. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Pang PS, Ambrosy AP, et al. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev. 2012;17(3):485–509. doi: 10.1007/s10741-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 6.Butler J, Kalogeropoulos A. Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat! J Am Coll Cardiol. 2008;52(6):435–7. doi: 10.1016/j.jacc.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Givertz MM, Teerlink JR, Albert NM, et al. Acute decompensated heart failure: update on new and emerging evidence and directions for future research. J Card Fail. 2013;19(6):371–89. doi: 10.1016/j.cardfail.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 9.Konstam MA. RELAX-AHF: rising from the doldrums in acute heart failure. Lancet. 2013;381(9860):5–6. doi: 10.1016/S0140-6736(12)61896-0. [DOI] [PubMed] [Google Scholar]
- 10.Mentz RJ, Hernandez AF, Stebbins A, et al. Predictors of early dyspnoea relief in acute heart failure and the association with 30-day outcomes: findings from ASCEND-HF. Eur J Heart Fail. 2013;15(4):456–64. doi: 10.1093/eurjhf/hfs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61(2):196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112(25):3958–68. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 13.Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–93. doi: 10.1001/jama.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gheorghiade M, Bohm M, Greene SJ, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309(11):1125–35. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 15.Peacock WF, Chandra A, Collins S, et al. A. Clevidipine improves dyspnea in emergency department acute heart failure: a randomized, open label study. Presentation (abstract 15606), presented November 5 at the American Heart Association Scientific Sessions; 2012; Los Angeles, California. [Google Scholar]
- 16.Gheorghiade M, Pang PS, O’Connor CM, et al. Clinical development of pharmacologic agents for acute heart failure syndromes: a proposal for a mechanistic translational phase. Am Heart J. 2011;161(2):224–32. doi: 10.1016/j.ahj.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Atherton JJ, Hayward CS, Wan Ahmad WA, et al. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific) J Card Fail. 2012;18(1):82–8. doi: 10.1016/j.cardfail.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–33. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 19.Mentz RJ, Kaski JC, Dan GA, et al. Implications of geographical variation on clinical outcomes of cardiovascular trials. Am Heart J. 2012;164(3):303–12. doi: 10.1016/j.ahj.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, Subacius H, Vaduganathan M, et al. Relationship between clinical trial site enrollment with participant characteristics, protocol completion, and outcomes: insights from the EVEREST trial. J Am Coll Cardiol. 2013;61(5):571–9. doi: 10.1016/j.jacc.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Gheorghiade M, Vaduganathan M, Greene SJ, et al. Site selection in global clinical trials in patients hospitalized for heart failure: perceived problems and potential solutions. Heart Fail Rev. 2012 doi: 10.1007/s10741-012-9361-8. http://dx.doi.org/10.1007/s10741-012-9361-8. [DOI] [PMC free article] [PubMed]
- 22.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557–73. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 23.Teerlink JR. Dyspnea as an end point in clinical trials of therapies for acute decompensated heart failure. Am Heart J. 2003;145(2 Suppl):S26–33. doi: 10.1067/mhj.2003.151. [DOI] [PubMed] [Google Scholar]
- 24.West RL, Hernandez AF, O’Connor CM, et al. A review of dyspnea in acute heart failure syndromes. Am Heart J. 2010;160(2):209–14. doi: 10.1016/j.ahj.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Gheorghiade M, Adams KF, Cleland JG, et al. Phase III clinical trial end points in acute heart failure syndromes: a virtual roundtable with the Acute Heart Failure Syndromes International Working Group. Am Heart J. 2009;157(6):957–70. doi: 10.1016/j.ahj.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Mebazaa A, Pang PS, Tavares M, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31(7):832–41. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 27.Cotter G, Metra M, Weatherley BD, et al. Physician-determined worsening heart failure: a novel definition for early worsening heart failure in patients hospitalized for acute heart failure—association with signs and symptoms, hospitalization duration, and 60-day outcomes. Cardiology. 2010;115(1):29–36. doi: 10.1159/000249280. [DOI] [PubMed] [Google Scholar]
- 28.Rogers JK, McMurray JJ, Pocock SJ, et al. Eplerenone in patients with systolic heart failure and mild symptoms: analysis of repeat hospitalizations. Circulation. 2012;126(19):2317–23. doi: 10.1161/CIRCULATIONAHA.112.110536. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 30.Rogers JK, Pocock SJ, McMurray JJV, et al. Analysing recurrent hospitalisations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2013 doi: 10.1002/ejhf.29. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pocock SJ, Ariti CA, Collier TJ, et al. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33(2):176–82. doi: 10.1093/eurheartj/ehr352. [DOI] [PubMed] [Google Scholar]