Abstract
Tacrolimus impairs allo- and viral-specific T cell responses. Belatacept, a costimulation-based alterative to tacrolimus, has emerged with a paradoxical picture of less complete control of alloimmunity with concomitant impaired viral immunity limited to viral-naïve patients. To reconcile these signatures, bulk population and purified memory and naïve lymphocytes from Cytomegalovirus (CMV) seropositive (n=10) and seronegative (n=10) volunteers were studied using flow cytometry, interrogating proliferation (CFSE dilution) and function (intracellular cytokine staining) in response to alloantigens or CMV-pp-65 peptides. As anticipated, T cells from CMV-experienced, but not naïve, individuals responded to pp-65 with a small percentage of their repertoire (<2.5%) consisting predominantly of mature, polyfunctional (expressing IFN-γ, TNF-α, and IL-2) T effector memory cells. Both CMV naïve and experienced individuals responded similarly to alloantigen with a substantially larger percentage of the repertoire (up to 48.2%) containing proportionately fewer polyfunctional cells. Tacrolimus completely inhibited responses of CMV- and allo-specific T cells regardless of their maturation. However, belatacept’s effects were decreasingly evident in increasingly matured cells, with minimal effect on viral-specific triple cytokine producers and CD28 negative allospecific cells. These data indicate that belatacept’s immunosuppressive effect, unlike tacrolimus’s, wanes on progressively developed effector responses, and may explain the observed clinical effects of belatacept.
Keywords: Belatacept, Calcineurin inhibitor, Costimulation blockade, Cytomegalovirus, Memory T cells, T cell maturation
Introduction
Calcineurin inhibitors (CNIs) such as tacrolimus inhibit naïve and memory T cell activation by blocking downstream signaling of the T cell receptor (TCR), and in doing so effectively suppress alloimmunity in transplant recipients(1–2). Unfortunately, CNIs similarly impair established host protective immunity, and result in lifelong increased risks of viral infection, and in particular, reactivation of latent herpesviruses including cytomegalovirus (CMV)(3–4). Therefore, patients treated with tacrolimus present with a consistent phenotype of immunosuppression, enjoying relatively low rejection rates at the expense of impaired viral immunity. CNIs also have numerous off-target side effects that have impelled a quest for agents with greater allospecificity(5–7).
Belatacept, a B7-specific fusion protein, has recently been approved as a CNI alternative in kidney transplantation. Belatacept is highly specific for its target molecules and thus lacks off-target side effects. However, early clinical experience with belatacept has presented a paradoxical and variable phenotype of immunosuppression. Specifically, belatacept patients have had substantially higher rejection rates than patients treated with CNIs, but at the same time have retained a clinical picture of impaired viral immunity. Interestingly, the risk for viral illness appears most evident in viral-naïve individuals subsequently exposed to viral pathogens, with viral experienced individuals being relatively spared(8–10). This has suggested to some that belatacept could have differential effects based on responses to different antigens, e.g. be more suppressive to viral specific responses and less suppressive to allo-specific responses. Alternatively, belatacept’s influence could vary as a function of unique and distinguishing properties that differentiate the allo- and viral-specific repertoires in general.
We therefore sought to better understand a mechanism by which CNIs and belatacept, both immunosuppressive medications, could present with starkly different immune effects versus viral- or allo-specific targets. We hypothesized that the maturation state characteristic of ones typical viral- or allo-specific T cell repertoire could explain the clinically observed difference. Specifically, the evolutionarily conserved response to a virus is highly focused, and comprised of T cells with defined specificity and matured functionality against a small number of cognate antigens derived from tight T cell receptor interactions with viral peptides expressed by self MHC. This differs substantially from the more stochastic response to alloantigen, which is comprised of cross reactive cells that vary from person to person with regards to the degree of functional maturity, and emerge through TCR-MHC interactions of more variable, and generally lower, affinity. As such, if belatacept’s capacity to inhibit a T cell was limited to cells in early stages of effector development whereas CNIs had inhibitor capacity regardless of a cell’s stage of maturation, a situation of allo-inhibitory efficacy and viral immune impairment varying depending on the immune history of the patient would emerge for belatacept.
Herein, we have examined the T cell response to alloantigen and CMV, defining their breadth (as defined by percent of the repertoire), functional character (specifically their capacity for polyfunctional cytokine production and division), maturation state (assessed by surface phenotype), and susceptibility to inhibition with either tacrolimus or belatacept. We find that the differential response observed between tacrolimus and belatacept relates predominantly to the degree to which the response in question is composed of highly differentiated effectors, with tacrolimus being effective regardless of the differentiation state of the response in both CMV- and allo-responding cells, and belatacept controlling alloreactive T cells in a dose dependent manner that wanes with maturation, and is influenced highly by maturation dependent loss of CD28. In contrast, CMV-specific memory T cells, all of which are highly differentiated, are resistant to costimulation blockade with belatacept. These data may be relevant in assessing the appropriateness of belatacept therapy for an individual patient, assigning belatacept to individuals with low risk of belatacept resistant rejection or viral disease, and relegating patients at higher risk of rejection to a more toxic but consistent CNI alternative.
Methods
Reagents and monoclonal antibodies
The fluorochrome labeled monoclonal antibodies (mAbs) anti-CD3-Alexa 700, anti-CD4-V450, anti-CD4-PEcy7, anti-CD8-APC, anti-CD8-V450, anti-CD2-APC, anti-CD28-FITC, anti-TNF-α-PE, anti-IFN-γ-PerCPcy5.5, and anti-IL-2-PEcy7 were purchased from BD Biosciences (Franklin Lakes, NJ). Anti-CD45RA-QDOT655 mAb and carboxyfluorescein succinimidyl ester (CFSE) were obtained from Invitrogen (Carlsbad, CA). Anti-CD197-APC anti-CD8-Alexa780 mAb was obtained from Ebioscience (San Diego, CA). Human CMV peptide pool of pp65 sequence consisting of 138 peptides (15 mers with 11 amino acid overlaps) was purchased from JPT Peptide Technologies (Berlin, Germany). Perm/Wash buffer, Cytofix/Cytoperm solution, and GolgiPlug containing brefeldin A were obtained from BD Biosciences.
CMV-pp65 peptide stimulation and allo-stimulation
Human peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood of CMV-seropositive (n=10) and CMV-seronegative (n=10) healthy volunteers who were consented under an Institutional Review Board-approved tissue acquisition protocol. The PBMCs were obtained through Ficoll density gradient centrifugation according to the manufacture’s protocol (BD Biosciences). Seropositivity was established based on CMV-IgG reactivity as determined by the Emory University clinical pathology lab. PBMCs were washed with phosphate-buffered saline (PBS), and diluted to 106 cells/ml with RPMI-1640 medium (HyClone Laboratories Inc. Logan, UT) containing 10% fetal bovine serum (FBS). 2.5 × 105 cells were stimulated by 1.75 µg/ml of CMV pp65 peptides with 1 µl/ml of GolgiPlug at 37°C for 12 hours.
T cell-mediated responses to allo-donors were also evaluated in comparison with CMV-specific T cell responses using the method described previously(11–12). Briefly, allo-stimulator PBMCs were depleted of CD3+ cells with magnetic CD3 MicroBeads and LS magnetic columns according to manufacturer's instruction (Miltenyi Biotec, Auburn, CA). Stimulators were irradiated and then diluted with RPMI-1640 medium supplemented with 10% FBS. 2.5 × 105 responder PBMCs were incubated with 2.5 × 105 irradiated stimulators containing 1 µl/ml GolgiPlug at 37°C for 12 hours. To determine the effects of belatacept (Bristol-Myers Squibb, New York, NY) in inhibiting responses of CMV- and allo-specific T cells, belatacept was tested at various concentrations. Cells treated with tacrolimus (Astellas Pharma US, Northbrook, IL), were used as controls.
Viral peptide- and allo-stimulation of purified naïve and memory T cells
Human CD3+ T cells were purified using negative selection methods according to manufacture’s protocol (Miltenyi Biotec, Auburn, CA). Briefly, PBMCs were incubated with the Miltenyi pan T cell purification biotin-antibody cocktail and then with the pan T cell purification microbead cocktail. Non-T cells were removed with magnetic columns. Both naïve and memory T cells were sorted using positive selection methods based on expression of anti-CD45RA-APC and anti-CD197-APC Alexa780 mAb using a BD FACSAria™ Cell Sorter (BD Biosciences, Franklin Lakes, NJ). Purified naïve and memory cells were verified to be greater than 90% by FACSAria™ Cell Sorter following cell purification. Autologous dendritic cells were generated from CD14+ monocytes. Briefly CD14+ cells were isolated by positive selection according to the manufacture’s protocol (Miltenyi Biotec, Auburn, CA). Purified CD14+ monocytes were re-suspended in RPMI-1640 culture medium containing IL-4 (50 ng/ml), granulocyte-macrophage colony stimulating factor (100 ng/ml), and 10% FCS after final wash, and incubated for 5 days to generate monocyte-derived dendritic cells. Dendritic cells were verified with polychromatic flow cytometry for positive staining of CD40, CD80, CD86, CD11c, HLA-DR, and HLA-ABC.
The anti- allo and -CMV activity of purified memory and naïve cells were studied by ICCS. Briefly, allo-stimulator PBMCs were depleted of CD3+ cells with magnetic CD3 MicroBeads and LS magnetic columns, and then irradiated. 0.25 × 105 memory cells or 5 × 104 naïve cells were stimulated by 1.75 µg/ml of CMV-pp65 peptides in the presence of autologous dendritic cells with 1 µl/ml of GolgiPlug at 37°C for 12 hours followed by ICCS. 0.25 × 105 responder memory cells with autologous dendritic cells were incubated with 0.25 × 105 irradiated stimulators containing 1 µl/ml GolgiPlug at 37°C for 12 hours. 5 × 104 naïve cells and autologous dendritic cells were incubated with 5 × 104 irradiated stimulators in the presence of 1 µl/ml GolgiPlug at 37°C for 12 hours. Belatacept was tested at various concentrations. All studies performed on sorted cells were repeated three times in three different individuals.
Intracellular cytokine staining (ICCS)
Cells were collected after 12-hour stimulation with CMV-pp65 peptides and allogeneic stimulators, and were surface stained with mAb at room temperature for 15 minutes followed by permeabilization and fixation with Cytofix/Cytoperm for 45 minutes on ice. ICCS with mAb directed to TNF-α, IFN-γ, and IL-2 was performed at 4°C for 30 minutes after final wash with Perm/Wash buffer. Cells were washed and analyzed using polychromatic flow cytometry (BD Biosciences LSR II), and the analysis of data was performed using FlowJo software (Tree Star, San Carlos, CA).
CFSE-based lymphocyte proliferation assay
The proliferative response of CMV-specific T cells to CMV-pp65 peptide stimulation was assessed with CFSE-based lymphocyte proliferation assay. Briefly, PBMCs isolated from CMV-seropositive healthy volunteers were labeled with CFSE at 37°C for 10 minutes according to the manufacture’s instruction (Invitrogen, Carlsbad, CA). After final wash with RPMI-1640 medium containing 10% FBS, cells were diluted to 1 × 106 cells/ml with RPMI-1640 medium containing 10% FBS. 2.5 × 105 cells were incubated with 1.75 µg/ml of CMV-pp65 peptides for 5 days.
To assess the allo-specific T cell proliferative responses of these CMV-seropositive healthy volunteers in comparison with CMV-specific T cell proliferation, a one-way mixed lymphocyte reaction (MLR) was performed. 2.5 × 105 CFSE-labeled responder PBMCs were incubated with 2.5 × 105 irradiated allo-stimulators in RPMI-1640 medium containing 10% FBS at 37°C for 5 days. After stimulation for 5 days, cells were washed and surface stained with mAb directed to CD3, CD4, and CD8. Cells were analyzed acquisition with polychromatic flow cytometry.
Statistical analysis
To determine the statistic difference of cytokine producers, we used the nonparametric Wilcoxon signed rank test to compare CMV-seropositive and seronegative healthy volunteers. An unpaired t test with Welch’s correction was utilized to determine the statistic difference of cytokine producers between CMV-reactive and allo-responding cells. The trends in decrease of frequency of producers with increase in belatacept concentration were tested using one-way ANOVA with post-test for linear trend and linear regression models that accounted for repeated measures for each subject (mixed linear models with random intercept term). All calculated p values were two-tailed analysis, and a p value of less than 0.05 was considered as statistically significant.
Results
The allo-specific response is larger, more heterogeneous, and less dominated by polyfunctional cytokine producing T cells than the viral-specific response
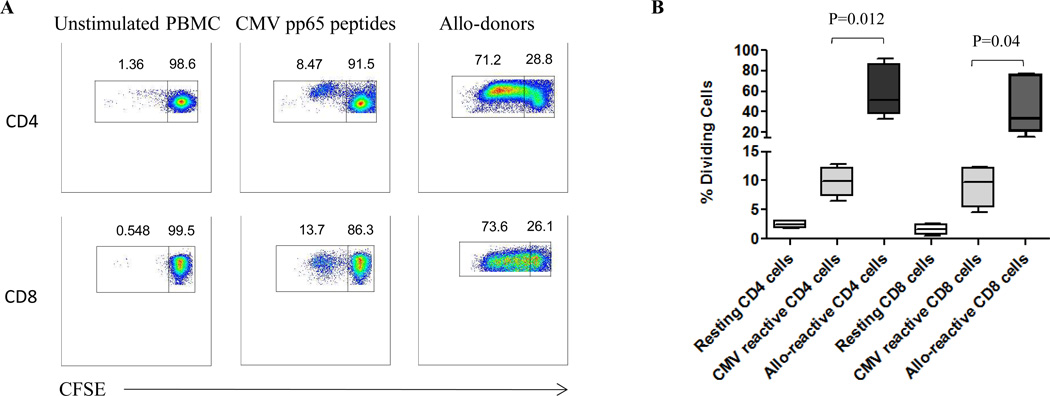
We first tested the proliferative responses of CMV- and allo-specific T cells by a CFSE-based proliferation assay (Figure 1). As anticipated, proliferative responses to CMV-peptides and alloantigen targets were seen in CMV-seropositive individuals. Proliferative responses to alloantigen were also seen in CMV seronegative individuals, while CMV-specific responses were not (not shown). In all individuals, the allo-specific proliferative response was of substantially greater magnitude than the CMV-specific proliferative response (CD4+ cells p=0.012, CD8+ cells p=0.04). As this was a 5-day assay, it reflected the effects both of reactivated antigen-specific memory T cells and de novo priming of naïve T cells.
Figure 1. Proliferative responses of CMV- and allo-specific T cells following stimulation.
(A) CFSE-labeled responder PBMCs were stimulated by CMV-pp65 peptides or allo-donor cells. CD3+CD4+ and CD3+CD8+ cells were analyzed for proliferative responses after 5 days through assessment of CFSE dilution. Representative results from one individual are shown in panel A. (B) The percentages of divided CMV- and allo-reactive CD4+ and CD8+ cells from all tested individuals after stimulation are shown demonstrating that the magnitude of the proliferative response to alloantigen substantially exceeds that to viral antigen (CD4+ cells p=0.012, CD8+ cells p=0.04).
To further characterize and contrast the phenotype, functional capacity, and size of CMV- and allo-reactive T cell repertoires, and in particular to assess the relative size of the memory response to allo- and CMV-antigens, PBMCs from CMV-seropositive and seronegative volunteers were stimulated with CMV-pp65 peptides or allo-donor cells for 12 hours and interrogated by ICCS. Both CD4+ and CD8+ T cells from CMV-seronegative individuals failed to produce cytokine after stimulation with CMV-pp65 peptides (confirming CMV naiveté), while both CD4+ and CD8+ cells from CMV-seropositive individuals demonstrated TNF-α/IFN-γ expression after stimulation (Figure 2A & B; p=0.002). In keeping with the proliferation results, TNF-α/IFN-γ production were detected in both allo-responding CD4+ and CD8+ cells of both CMV-seronegative and CMV-seropositive subjects following allo-stimulation; there was no significant difference in alloresponsiveness that segregated based on viral seropositivity (Figure 2C & D).
Figure 2. Activation of CMV-specific T cells.
(A) PBMCs, isolated from CMV-seropositive and seronegative normal individuals, were stimulated with or without CMV-pp65 peptides followed by ICCS to detect TNF-α and IFN-γ. CD3+ T cells were analyzed based on CD4+ and CD8+ expression, and activation of CMV-specific T cells were identified based on expression of intracellular cytokines. (B) The percentage of TNF-α/IFN-γ dual producers detected after CMV-pp65 peptide stimulation in CMV-seropositive individuals is significantly higher than seronegative individuals with lack of dual cytokine producers (p=0.002). (C) Responder PBMCs were stimulated with irradiated CD3 depleted allo-stimulators for 12 hours followed by ICCS to detect TNF-α and IFN-γ within CD3+CD4+ and CD3+CD8+ populations. (D) The percent of cells expressing both TNF-α and IFN-γ was determined after allo-stimulation in CMV-seropositive and CMV-seronegative individuals. While all individuals had a substantial allospecific response, there was no difference in alloresponsiveness based on CMV sero-status.
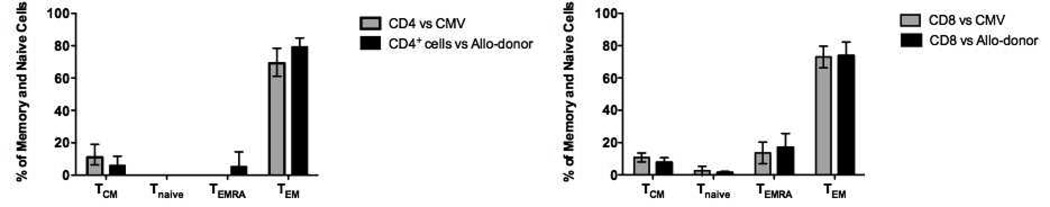
Human T cells can be segregated into four distinct subsets based on their surface expression of CD197 (CCR7) and CD45RA(13): naïve (CD197+CD45RA+), terminally differential effector memory (TEMRA; CD197−CD45RA+), effector memory (TEM; CD197−CD45RA−), and central memory (TCM; CD197+CD45RA−). To characterize the memory phenotype of T cells most responsive to viral and alloantigen, we analyzed the phenotype of T cells producing both TNF-α and IFN-γ in response to CMV- and allo-antigen. The vast majority of CMV- and allo-reactive CD4+ and CD8+ TNF-α/IFN-γ producers were phenotypically defined as TEM cells (CD197−CD45RA−) (Figure 3). Thus, differential sensitivity to belatacept and tacrolimus could not be determined solely on memory phenotypic grounds.
Figure 3. Memory phenotype of CMV- and allo-specific T cells.
Lymphocytes from PBMCs, stimulated for 12 hours by CMV-pp65 peptides or allo-donor cells, were identified using forward and side scatter followed by gating on singlet populations, and the CD3+ cells were then subdivided to CD4+ and CD8+ populations. Both CD4+ and CD8+ cells were then segregate into naïve (CD197+CD45RA+), TEMRA (CD197−CD45RA+), TEM (CD197−CD45RA−), and TCM (CD197+CD45RA−) subsets. The majority of both CMV-specific and allospecific CD4+ and CD8+ cells producing TNF-α and IFN-γ were characterized as TEM cells. TNF-α and IFN-γ producing cells responding to either stimulus were phenotypically indistinguishable.
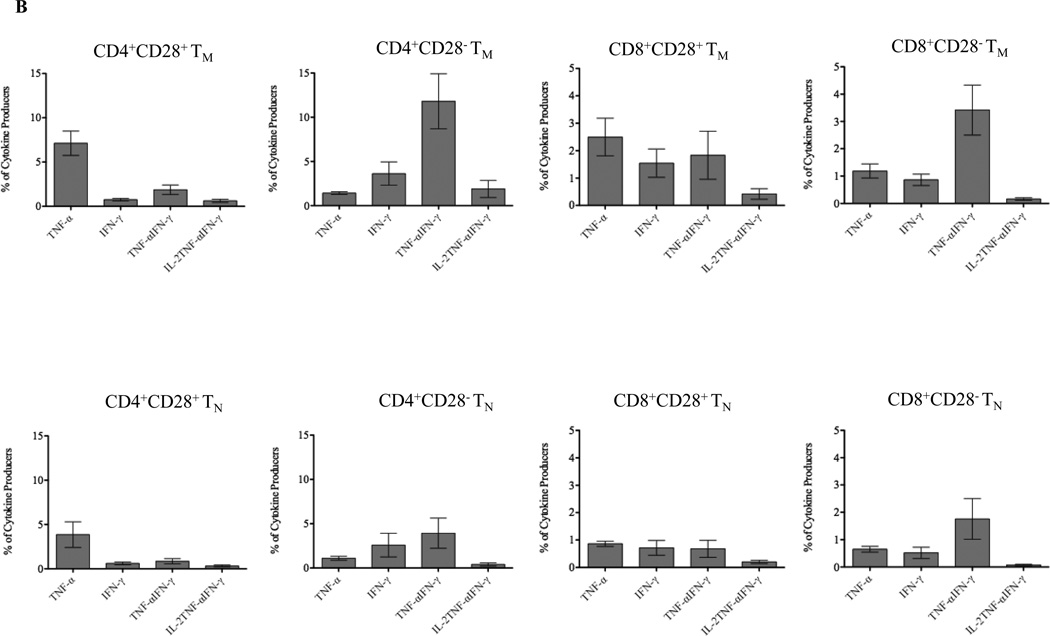
Previous studies have demonstrated the vigorous and superior function within multi-cytokine producing T cells when compared with single cytokine producers(12, 14–15). To further define composition of the CMV- and allo-responsive T cell repertoire, the reactivity of CMV- and allo-responsive T cells was evaluated with regard to their single, dual or triple cytokine expression (TNF-α, IFN-γ, and IL-2; Figure-4A). Allo-responding T cells (both CD4+ and CD8+) demonstrated greater frequency of single (TNF-α or IFN-γ), dual (TNF-α and IFN-γ) and total cytokine producers (both single and dual producers) when compared with cytokine producers of CMV-responding CD4+ and CD8+ cells (Figure-4B; see figure for all statistical values). As shown in Figure 4C, the frequency of IL-2 producers within TNF-α and IFN-γ dual producers (triple producers) of allo-responding T cells was significantly lower than CMV-reactive cells (CD4+ cells p=0.0005, CD8+ cells p=0.0003), i.e. alloresponders were less likely to be fully functional T cells with regard to cytokine production. However, IL-2/TNF-α/IFN-γ triple producers as a percentage of the entire repertoire was not significantly different between CMV- and allo-responding cells, indicating that the number of fully matured cells responding to allo- or viral-antigen were similar despite the markedly larger total allo-responsive repertoire.
Figure 4. Cytokine production by activated CMV- and allo-specific T cells.
(A) Activation of CMV- and allo-specific CD4+ and CD8+ T cells were identified as TNF-α, IFN-γ and TNF-α/IFN-γ producers. The triple IL-2+TNF-α+IFN-γ+ producers were determined by gating on TNF-α/IFN-γ dual producers. (B) Single, dual, and total cytokine producers were detected during activation of CMV- and allo-responding T cells, in all cases the number of allo-responsive cells exceeded the number of CMV responsive cells. (C) The frequency of IL-2+ cells within TNF-α/IFN-γ dual producers (top) and IL-2/TNF-α/IFN-γ triple producers (bottom) after activation of CMV- and allo-responding T cells are shown. T cells responding to CMV are more likely to be triple producers that T cells responding to alloantigen, however, owing to the much larger number of cells responding to alloantigen, the size of the fully matured (triple producer) repertoire responding to CMV or alloantigen is similar.
CD28 loss is typical of both CMV- and allo-specific CD8+ T cells, but more common amongst allospecific CD4+ T cells
We have demonstrated previously that a large fraction of alloreactive T cells are CD2hiCD28− cells with reduced susceptibility to belatacept(12, 16). Given that CD28 is a critical receptor for the B7 ligands targeted by belatacept, we assessed the CD28 expression on CD4+ and CD8+ TNF-α/IFN-γ dual producers following stimulation by CMV-pp65 peptides and allo-donor cells. Activated TNF-α/IFN-γ expressing T cells were subdivided into CD28+ and CD28− subsets. As shown in Figure 5, CMV-reactive CD4+ cells were evenly divided between CD28+ and CD28−, while alloreactive CD4+ T cells were markedly more likely to be CD28−. Both CMV reactive and alloreactive CD8+ effectors were dominated by CD28− cells. Thus, CD28 loss did not distinguish allo- from viral-responsive CD8+ effector cells, but was more common amongst alloresponsive CD4+ cells, a difference that could distinguish belatacept’s effect on alloantigen from its effect on viral antigen.
Figure 5. CD28 expression on activated CMV- and allo-specific T cells.
(A) Activation of CMV- and allo-specific CD4+ and CD8+ cells were identified as TNF-α/IFN-γ dual producers, and the CD4+ and CD8+ TNF-α/IFN-γ dual producers were then sub-segregated into CD28− and CD28+ subsets based on the surface expression of CD28. Representative allo-responding T cells from one individual are shown. (B) CD28− and CD28+ subsets of TNF-α/IFN-γ dual producers of CMV-specific and allo-responding CD4+ and CD8+ cells after stimulation with CMV-pp65 peptides or allo-donors. CD28 loss is typical of both allo- and viral-specific CD8+ cells, but is more likely to be seen in allo-specific CD4+ cells compared to viral-specific CD4+ cells.
The inhibitory effects of belatacept and tacrolimus on CMV- and allo-specific T cells vary by maturation status
Given that a large proportion of both activated CMV-specific and allo-specific T cells were CD28− TEM cells, we surmised that CD28− TEM cells would be resistant to belatacept, a CD28/B7 inhibitor, regardless of specificity, and that neither would be resistant to CNIs. Bulk PBMCs isolated from CMV-seropositive individuals were stimulated with CMV-pp65 peptides or allo-donor cells in the presence of belatacept or tacrolimus followed by the detection of cytokine producers and T cell proliferation. Tacrolimus completely inhibited both CMV- and allo-specific T cell responses as confirmed by the lack of cytokine production (Figure 6A) and proliferation (Figure 6B). The effects of belatacept, however, were less striking on proliferation, and varied by the degree of effector maturation as measured by cytokine production. Specifically, the proliferation of bulk alloreactive T cells, cells with a broad spectrum of functionality, were significantly more inhibited by belatacept compared to CMV-specific T cells (Figure 6C; vs. allo-CD4+ cells p=0.006; vs. allo-CD8+ cells p=0.004) suggesting that the de novo priming of naïve T cells responding in the 5 day proliferation assay and/or the reactivity of incompletely matured T cells responding to allo-stimulation was more susceptible to the B7 inhibitor belatacept. Indeed, belatacept demonstrated minimal inhibitory effects in preventing proliferative responses of CMV-specific cells in CMV-seropositive individuals, those whose cells that were exclusively multifunctional in nature.
Figure 6. Percent inhibition of CMV- and allo-specific T cells by CNIs and Belatacept.
(A) Tacrolimus completely inhibits activation of CMV- and allo-specific T cells as determined by the lack of cytokine production. (B) Tacrolimus completely inhibits proliferation of CMV- and allo-specific T cells. (C) Belatacept inhibits the proliferation of alloreactive T cells (CD4+ cells p=0.006, CD8+ cells p=0.004), but is less effective in preventing the proliferation of CMV-specific T cells (CD4+ cells p=0.185, CD8+ cells p=0.019).
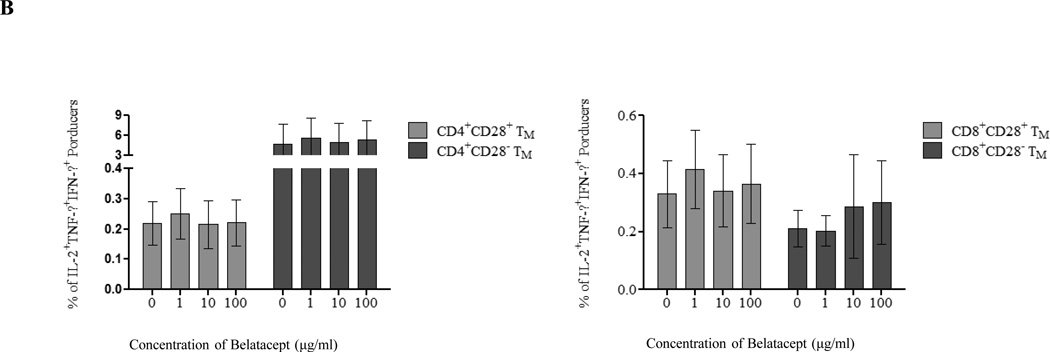
To determine whether the differential effect of belatacept in inhibiting activation of CMV- and allo-responding cells segregated based on the maturation state of the responding memory repertoire, we stimulated PBMCs with CMV-pp65 peptides or allo-donor cells in the presence or absence of belatacept and interrogated the results using ICCS. As shown in Figure 7A–C, belatacept’s effect was substantial and dose-dependent on incompletely matured T cells, those expressing single or dual cytokine production. This was only assayable in allospecific populations, as essentially all CMV-responsive cells were multi-cytokine producers. However, although belatacept had a measurable effect on fully matured allospecific effectors (triple producers) that was evident only at the highest dose, these were a small minority of the responding population; belatacept had essentially no effect on CMV-specific cells, and as these were essentially all triple producers, the appearance was one of viral sparing (Figure 7b). As shown in Figure 7c, the dose responsiveness to belatacept, as determined by linear regression modeling, was trivial in triple producers compared to that seen in less matured cells, and there were significantly greater inhibitory effects of belatacept on single and dual producers when compared with CMV and allo-responding triple producers.
Figure 7. Inhibitory effects of belatacept on CMV- and allo-specific T cells by cytokine production status.
PBMCs were stimulated by CMV peptides or allo-donor cells in the presence of increasing concentrations of belatacept followed by ICCS. (A) The inhibitory effects of belatacept on IFN-γ single producers and dual producers are shown demonstrating a dose dependent inhibitory effect. (B) The frequency of IL-2/TNF-α/IFN-γ triple producers during allo-specific (left) and CMV-specific (right) T cell responses in the presence of increasing concentrations of belatacept is shown. Belatacept has limited effect on cells of either specificity, with a measurable effect at its highest dose versus alloresponsive cells and no effect on CMV-specific cells. The size of the triple producer response is higher versus CMV than versus alloantigen. (C) Slopes of dose responsiveness for all cells tested by ICCS demonstrating that belatacept mostly influences cells with incomplete cytokine maturation, and the effects to suppress cells with a fully developed response to alloantigen are significantly limited when compared with single and dual producers.
To further define the characteristics of matured cells that rendered them resistant to belatacept, T cells were sorted into memory and naive populations and then stimulated with CMV-pp65 peptides or allo-stimulators and assessed by ICCS. As shown in Figure 8A, naïve T cells from CMV-seropositive individuals were unresponsive to CMV-pp65 peptide stimulation (a short term assay specific for memory responses), while substantial CMV reactivity was identified within the memory population. This responsive population included both CD4+ and CD8+ cells and within both of these subsets, CD28− and CD28+ cells. Belatacept was ineffective in inhibiting CMV-specific cells regardless of CD28 expression (Figure 8B) indicating that the failure of belatacept to block established anti-CMV immunity was not solely dictated by CD28 dependence, but rather also was related to the T cells’ maturation states.
Figure 8. Cytokine production by sorted naïve and memory T cells and the effects of belatacept on cytokine producers following CMV stimulation.
T cells were sorted into memory and naïve populations, then stimulated by CMV-pp65 peptides, analyzed using ICCS to detect TNF-α and IFN-γ, and further characterized with regard to their expression of CD4, CD8 and CD28. (A) CMV-specific activation was evident in the memory but not naïve populations. (B) The frequency of IL-2/TNF-α/IFN-γ triple producers within the memory population in the presence of increasing concentrations of belatacept is shown, demonstrating that belatacept does not inhibit cytokine production by CMV-specific memory cells.
Allo-specific activation was more diverse and involved some degree of activation in both memory and naïve populations, although the maturation of the allo-responders in the memory populations was more advanced, including a higher percentage of dual producers (Figure 9A). Activity was detected in both CD28+ and CD28− cells, but trended higher and more polyfunctional in cells lacking CD28.
Figure 9. Cytokine production by sorted naïve and memory T cells and the effects of belatacept on cytokine producers following Allostimulation.
T cells were sorted into memory and naïve populations, then stimulated by allo-stimulators, analyzed using ICCS to detect TNF-α and IFN-γ, and further characterized with regard to their expression of CD4, CD8 and CD28. (A) Allospecific activation was evident in both the memory and the naïve populations, but the memory responses are more developed with regard to cytokine production. (B) The distribution of alloreactivity is shown in memory populations (upper panels), and naïve populations (lower panel) over three independent experiments. Alloreactivity is seen across the entire repertoire, but is more prominent in memory cells and includes relatively few triple producers. (C) Belatacept has an inhibitory influence on alloresponsive cells that express CD28, and have no effect on those cells that lack CD28.
Consistent with the bulk population analysis, alloreactive cells were seen to be generally distributed throughout the entire repertoire, including mostly single and dual producers with relatively few matured triple producers (Figure 9B). Responsiveness was more evident amongst memory cells (in this short term assay that favors memory responses), but included a mixture of CD28 expressing cells and those lacking CD28 expression. Thus, consistent with the stochastic nature of allo-cross-reactivity, the alloimmune response was broad, less well developed, and evident across all populations to some extent.
We then looked at belatacept sensitivity as it related to CD28 expression and cytokine production (Figure 9C). Belatacept was found to have dose dependent effects in both single and triple producers that expressed CD28, although the absolute numbers of triple producers responding to alloantigen, was shown above, a minority of the responding population. In stark contrast, belatacept had no inhibitory effect on CD28− T cells regardless of effector status. Thus, considering allo-responsive cells, the predominant feature limiting belatacept’s effectiveness was a loss of CD28 with effector maturation.
Thus, although belatacept had a substantial inhibitory effect on alloresponsiveness, this was due mainly to its impact on non-terminally matured effectors, with the magnitude of the effect derived from the fact that these cells compose the bulk of the allospecific repertoire. The differential impact of belatacept on viral-versus allo-specific populations was attributable not to the antigen specificity, but rather the composition of the responding population.
Discussion
Transplant recipients remain dependent on immunosuppressive drug therapies, the most prevalent of which are CNI-based. In general, immunosuppressive therapies are used with the expectation that their T cell suppressive effects will provide low rates of rejection, but for that benefit, patients accept an increased risk of infection and latent viral reactivation proportional to the intensity of the therapy used. Belatacept has been developed with similar expectations, but its clinical use has presented an unusual pattern of less complete control of alloimmunity, and at the same time, perhaps disproportionate concerns with viral control, particularly in viral naïve individuals(8–10). In this study, we have directly compared the relative influences of tacrolimus and belatacept on T cell responses to viral- and allo-antigen, asking whether the different patterns of efficacy noted clinically can be explained by properties of the T cell repertoire used for these two target types. Indeed, we find that while tacrolimus provides substantial inhibition to T cells regardless of their maturation state, belatacept’s influence is largely limited to immature effectors. Further, we have shown that the maturation state characteristic of an evolutionarily conserved response to a virus differs from the more stochastic response to an allograft, and that this difference is relevant in understanding the clinical application of these two distinct immunosuppressive approaches. Specifically, to the extent that immature (or at least incompletely responsive) effectors make up the responding repertoire (large in alloresponses and trivial in viral-experienced responses) belatacept’s impact is more evident. Several points from this study are worthy of discussion.
It is generally accepted that alloimmune responses are substantially larger than viral-specific responses, encompassing log-fold greater numbers of cells and a broader percentage of the repertoire. Indeed, we find this to be true, but note that the number of fully matured allospecific cells, as defined by polyfunctionality in vitro, is similar or in some cases smaller in alloreactive populations compared to CMV-responsive populations, despite the starkly different numbers of responding cells. Most of the responding allospecific population is composed of incompletely developed responders, or alternatively, cells that when stimulated with something other than their cognate antigen respond in a less robust fashion. This finding is easily reconciled when considering the intentional maturation of a protective response to a viral pathogen compared with the cross-reactive nature of alloreactivity (at least in non-allosensitized patients). Thus, as most of the direct alloreactive T cell repertoire is perhaps best considered non-intentional, and thus of lesser potency, estimates of the size of the alloimmune repertoire should consider the potency of the cells involved rather than simply their numbers.
Many aspects of viral-specific and allospecific cells appear similar, in particular that CD8+ effector cells responding in vitro display a CD197−CD45RA− TEM phenotype. However, one marked difference appears in the CD4 compartment, where CD28-negative cells make up a substantially higher proportion of the allospecific repertoire than is seen in viral specific cells These CD28− memory T cells are clearly resistant to B7 inhibitor belatacept when compared with CD28+ cells, regardless of their apparent capacity to produce cytokines. That there is an apparent effect of belatacept on these cells in bulk culture may relate to by-stander effects of belatacept reducing the stimulation of CD28+ cells. Such an effect could be anticipated in vivo if CD28+ cells competed for resources or antigen presentation such that their inhibition but survival would mute the effects of CD28− cells. Regardless, one area to be considered in evaluating a patient’s risk for belatacept-resistant rejection may be the degree to which their heterologous alloreactive T cell help is derived from matured versus naïve CD4+ cells. This is consistent with recent observations that we have made identifying patients who have failed belatacept therapy as having a significantly larger percentage of CD4+ T cells characterized as exhausted or senescent, and notably CD28 negative(17). It would appear that CMV-specific maturation produces a cell population that is highly functional and resists inhibition with belatacept regardless of CD28 expression. However, we cannot discount the possibility that cells responding to other viruses, including EBV, may have different maturation and CD28 expression traits that could influence the generalizability of these findings.(18, 19)
The concern for heterologous immunity as a barrier to costimulation blockade-based therapies has been well established in animals models(20,21). Indeed, viral specific T cells have been identified with alloantigen cross reactivity(22). This has led to reasonable speculation that prior viral exposure would significantly alter the size of the allospecific repertoire. In our specific assessment of the magnitude of an alloreactive response based solely on prior CMV exposure, we find no such relationship. This might be a risk factor when considering broad viral exposure or allo-sensitization, rather than exposure to a particular virus, given that the virus-specific response is relatively narrow. Thus, we do not interpret these findings as supporting or refuting the importance of heterologous immunity on clinical belatacept resistance. However, we submit that studies investigating heterologous immunity through the use of identical cross reactive antigens may present a highly focused response that does not reflect the stochastic nature of cross reactivity between similar but non-identical antigens and overly estimate the influence of viral exposure on belatacept resistance.
The differential response of belatacept based on maturation state is consistent with the known differences in mechanism of belatacept and CNIs. CNIs target downstream of the TCR, a signal required for both naïve and memory T cell activation, while belatacept directly targets the CD28-B7 pathway, a pathway required largely for naïve responses but decreasingly required for activation in T cells that have prior antigen experience. Thus, belatacept’s capacity for inhibiting T cell activation should be expected to be limited to cells in early stages of effector development whereas CNIs logically have inhibitory capacity irrespective of the stage of T cell maturation. Herein, we have shown that this differential mechanism has consequence in altering T cell responsiveness. Our data indicate that belatacept’s immunosuppressive effect will manifest based on the breadth, quality, capacity and CD28 expression of polyfunctional cytokine production of ones T cell repertoire. Highly matured immune responses, be them allo- or pathogen specific, will be less well inhibited. In the case of allospecific clones, this will manifest as increased rejection, but in the case of pathogen specific clones, this will present as a sparing of protective immunity. Conversely, when the repertoire is largely undeveloped, or when cross-reactivity evokes an incomplete response, responses will be effectively controlled. For the allospecific repertoire, this will result in satisfactory control of rejection, but in the case of viral naiveté, this will result in loss of protective immune competence. Our findings are consistent with the clinical effects of belatacept, and may be relevant in assessing the appropriateness of belatacept therapy for an individual patient, assigning belatacept to individuals with low risk of belatacept resistant rejection and low viral risk, and relegating patients at a higher risk of rejection to a more toxic but more consistent CNI alternative.
Acknowledgments
The authors would like to gratefully acknowledge the members of the Emory Transplant Center Biorepository and the clinical research nurse staff of the Emory Transplant Center for their invaluable technical assistance.
Funding
This work was funded in part by grants from the US Food and Drug Administration (1R01 FD003539-01; ADK), the National Institutes of Health (1R01 AI097423; ADK), the Georgia Research Alliance, and the Roche Organ Transplant Research Foundation grant (346678023; HX).
Abbreviations
- CMV
cytomegalovirus
- CFSE
carboxyfluorescein succinimidyl ester
- CNIs
calcineurin inhibitors
- IFN-γ
interferon gamma
- TNF-α
tumor necrosis factor alpha
- PBMCs
peripheral blood mononuclear cells
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- PBS
phosphate-buffered saline
- FBS
fetal bovine serum
- ICCS
intracellular cytokine staining.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ekberg H, Bernasconi C, Tedesco-Silva H, Vitko S, Hugo C, et al. Calcineurin inhibitor minimization in the symphony study: observational results 3 years after transplantation. American Journal of Transplantation. 2009;9:1876–1885. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske B, Skeans M, Leighton T, Ghimire V, Leppke S, Israni A. OPTN/SRTR 2011 annual data report: international data. American Journal of Transplantation. 2011;11:199–225. doi: 10.1111/ajt.12026. [DOI] [PubMed] [Google Scholar]
- 3.Fisher R. Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transpl Infect Dis. 2009;11:195–202. doi: 10.1111/j.1399-3062.2009.00372.x. [DOI] [PubMed] [Google Scholar]
- 4.Issa N, Fishman J. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772–786. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 5.Jevnikar A, Mannon R. Late kidney allograft loss: what we know about it, and what we can do about it. Clin J Am Soc Nephrol. 2008;3:S56–S57. doi: 10.2215/CJN.03040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom R, Doyle A. Kidney disease after heart and lung transplantation. American Journal of Transplantation. 2006;6:671–679. doi: 10.1111/j.1600-6143.2006.01248.x. [DOI] [PubMed] [Google Scholar]
- 7.Naesens M, Kuypers D, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 8.Vincenti F, Larsen C, Durrbach A, Wekerie T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran P, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B Belatacept Study Group. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 9.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) American Journal of Transplantation. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Larsen C, Alberu J, Bresnahan B, Garcia V, Kothari J, et al. Three-year outcomes from BENEFIT, a randomized active-controlled, parallel-group study in adult kidney transplantation. American Journal of Transplantation. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 11.Badell I, Russell M, Thompson P, Turner A, Weaver T, Robertson J, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. The Journal of Clinical Investigation. 2010;120:4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CR, Kirk AD. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. American Journal of Transplantation. 2011;11:22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 14.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T cell in immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 15.Kannanganat S, Ibegbu C, Chennareddi L, Bobinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Rusell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature Medicine. 2009;15:746–759. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinosa J, Cheeseman J, Mehta A, Stempora L, Townsend R, Kirk AD. CD4+ T Cell Differentiation and Belatacept Resistant Rejection. Am J Transplant. 2013;13(S5):44. [Google Scholar]
- 18.Amyes E, Hatton C, Montamat-Sicotte D, Gudgeon N, Rickinson AB, McMichael AJ, Callan MFC. Characterization of the CD4+ T cell response to Epstein-Barr Virus during primary and persistent infection. J. Exp. Med. 2003;198:903–911. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iancu EM, Corthesy P, Baumgaertner P, Devevre E, Voelter V, Romero P, et al. Clonotype selection and composition of human CD8 T cells specific for persistent herpes viruses varies with differentiation but is stable over time. Journal of Immunology. 2009;183:319–331. doi: 10.4049/jimmunol.0803647. [DOI] [PubMed] [Google Scholar]
- 20.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003 Jun;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh R, Selin L. No one is naïve: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 22.Amir AL, D'Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, van der Hoorn MA, Kester MG, Doxiadis II, Falkenburg JH, Claas FH, Heemskerk MH. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]