Abstract
Missense substitutions of uncertain clinical significance in the BRCA1 gene are a vexing problem in genetic counseling for women who have a family history of breast cancer. In this study, we evaluated the functions of 29 missense substitutions of BRCA1 in two DNA repair pathways. Repair of double-strand breaks by homology-directed recombination (HDR) had been previously analyzed for 16 of these BRCA1 variants, and 13 more variants were analyzed in this study. All 29 variants were also analyzed for function in double-strand break repair by the single-strand annealing (SSA) pathway. We found that among the pathogenic mutations in BRCA1, all were defective for DNA repair by either pathway. The HDR assay was accurate because all pathogenic mutants were defective for HDR, and all nonpathogenic variants were fully functional for HDR. Repair by SSA accurately identified pathogenic mutants, but several nonpathogenic variants were scored as defective or partially defective. These results indicated that specific amino acid residues of the BRCA1 protein have different effects in the two related DNA repair pathways, and these results validate the HDR assay as highly correlative with BRCA1-associated breast cancer.
Keywords: BRCA1, homologous recombination, single-strand annealing, centrosome, VUS
Introduction
Among the more than 70,000 women who had their BRCA1 gene (MIM# 113705) sequenced by 2006, approximately 2.3% were found to carry a BRCA1 variant of uncertain significance (VUS). These variants were classified as uncertain because they occurred in families wherein segregation analysis had not been done or in whom the segregation analysis was not informative and thus the VUS had unknown effects on breast and ovarian cancer risk [Hall et al., 2009; Spearman et al., 2008; Sweet et al., 2009]. As whole genome and exome sequencing becomes a more prevalent practice, more VUSs in BRCA1 will be uncovered, and increasingly women will face the quandary of an uninformative genetic test. This leads to individuals with a VUS test result making decisions about cancer screening and prevention without concrete information on which to base their decisions. Women who have an indeterminate BRCA1 sequence result and a family history of breast cancer have a high level of distress, and there are no consistent clinical guidelines for advising them [Dorval et al., 2005; Petrucelli et al., 2002; van Dijk et al., 2006]. Of the 567 BRCA1 missense substitutions listed in the current Breast Cancer Information Core (BIC) database, 14 are described as pathogenic (Class 5) and 27 as nonpathogenic (Class 1). By including the data from Collaborators for the Investigation of Modifiers of BRCA1/2 (CIMBA), a total of 24 BRCA1 variants are pathogenic (CIMBA database). Less than 10% of the missense substitutions in the BIC database have known cancer predisposition. In the absence of definitive genetic information on BRCA1 missense substitutions, other methods are needed to determine whether missense substitutions are pathogenic. Multifactorial approaches have augmented genetic segregation analysis with additional information about the proband and, in some cases, the tumor [Easton et al., 2007; Goldgar et al., 2004; Lindor et al., 2012; Plon et al., 2008; Spearman et al., 2008; Sweet et al., 2009]. These approaches have been successful in reclassification of variants but are also incomplete because they cannot definitively determine whether any given variant affects the critical cancer suppressing function(s) of the protein [Millot et al., 2012]. In contrast, a biological functional assay has the potential to determine whether any given BRCA1 missense substitution is defective in a process, and if that function is predictive of cancer predisposition (i.e., 100% sensitive and 100% specific), then the functional assay can, in theory, be used in genetic counseling.
A variety of biological assays have been analyzed for BRCA1 function and have been correlated with cancer predisposition to various extents [Carvalho et al., 2007; Cotta-Ramusino et al., 2011; Kais et al., 2012; Lee et al., 2010; Millot et al., 2012; Morris et al., 2006; Ransburgh et al., 2010; Vallee et al., 2012]. Several of these assays test single domains of BRCA1, and several address the variant within the context of the full-length protein. In this study, we analyzed the effects of 29 missense substitutions in BRCA1 on the repair of double-strand DNA breaks (DSBs) by homologous recombination and by the single-strand annealing (SSA) pathways. Our results indicate that the effects of specific missense mutations in these pathways strongly correlate with breast cancer predisposition. Further, we find that specific amino acid substitutions affect the two double-strand break repair pathways differentially.
Materials and Methods
Plasmids and Cell Lines
All plasmids for the expression of human BRCA1 with missense substitutions were generated by site-directed mutagenesis from the wild-type sequence (GenBank: U14680.1). Several of these plasmids had been described in previous publications [Ransburgh et al., 2010; Wei et al., 2008]. The cell line for the homologous recombination assay, HeLa-DR, was based on the genomic integration of a specific vector that functions as a recombination substrate [Pierce et al., 2001]. The vector for homologous recombination had been the gift of M. Jasin (Memorial Sloan Kettering Cancer Institute, New York, NY). The HeLa-DR had been described before [Parvin et al., 2011; Ransburgh et al., 2010]. The repair of double-strand breaks by the SSA pathway was based on a vector kindly provided by J. Stark (City of Hope, Duarte, CA) [Bennardo et al., 2008; Stark et al., 2004] stably integrated into HeLa cells to make the HeLa–SSA cell line. The siRNA targeting the cellular BRCA1 3′ untranslated region (UTR) is the same as previously used [Ransburgh et al., 2010].
SSA Assay
HeLa–SSA cells were seeded in 15.6-mm-diameter wells in 24-well plates, and when cells were 50% confluent, cells were transfected with 5 pmol of the siRNA targeting the BRCA1 3′-UTR plus 0.3 μg of the BRCA1 expression plasmid in Lipofectamine 2000 reagent (Life Technologies, Grand Island, NY). At 24 hr after transfection, cells were transferred to 35-mm-diameter wells in six-well plates. At 48 hr, cells were transfected with 25 pmol of the BRCA1 3′-UTR-specific siRNA plus 0.75 μg BRCA1 expression plasmid plus 0.75 μg pCBASce (for the expression of I-SceI) in Lipofectamine 2000 reagent. After 3 days, cells are harvested by trypsinization, and the fraction of GFP-positive cells is determined using a FACScalibur flow cytometer (BD Biosciences, San Jose, CA) (Analytic Cytometry Shared Resource of The Ohio State University Comprehensive Cancer Center).
Western Blot Analysis of BRCA1 Protein
Immunoblots of the expressed BRCA1 protein were done as has been described previously [Ransburgh et al., 2010]. Cells that had been transfected as in the SSA assay were, in parallel, extracted in 0.1% NP-40, 0.05 M Hepes (pH 7.5), and 0.005 M EDTA. Protein contents of extracts were determined using the Bradford reagent; and 50 μg of protein was electrophoresed on 3–8% Nu-PAGE gels (Life Technologies, Grand Island, NY) M. Jasin (Memorial Sloan Kettering Cancer Institute, NewYork, NY), transferred to polyvinylidene fluoride membranes, and immunoblotted using antibody specific to BRCA1(400–1,100). Densitometry analysis was performed from immunoblots using Kodak 1D software (Eastman Kodak Company, Rochester, NY).
Statistical Analysis of Results
The raw results in the SSA assay for the missense substitutions were compared with the control samples (either GL2 siRNA or BRCA1 siRNA with BRCA1-wt add back) using the unpaired Student’s t-test and obtained the one-tail P values; for intermediate-phenotype mutants, besides the above-mentioned comparisons, we also conducted the unpaired Student’s t-test against the control sample that contains BRCA1 siRNA with empty vector adding back, and obtained the one-tail P values. P values of less than 0.01 were considered significant.
Results
Function of BRCA1 Variants in DNA Break Repair by Homologous Recombination
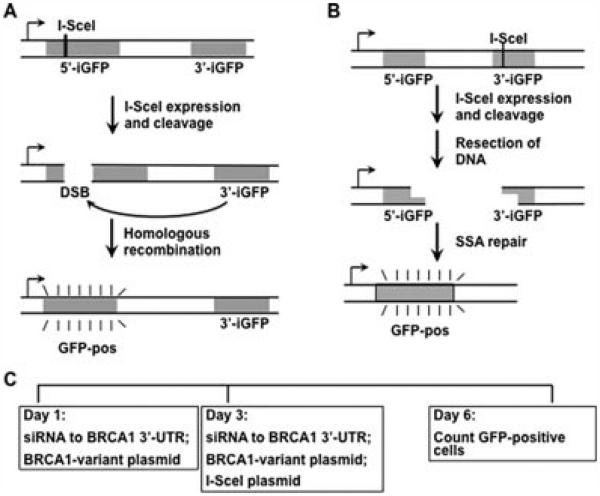
The BRCA1 protein has been shown to be a critical component of the response to DSBs. Cellular machinery can utilize homologous sequences to accurately repair the DSB, or the free ends can be joined by nonhomologous end joining. In addition to homologous recombination, the pathway of SSA exists by which the repair proteins resect DNA from the free ends of the DSB, and when short stretches of homologous sequence are exposed, then the DNA ends are rejoined [Stark et al., 2004]. The homologous recombination assay used in this study was based on a method developed by the Jasin laboratory in which a single integrated genomic locus contains two inactive GFP genes, and one of these contains the endonuclease cleavage site for the rare-cutting I-SceI enzyme [Pierce et al., 2001]. Upon expression of I-SceI in the cell, one inactive allele of GFP is cut, and if homologous recombination occurs using the second inactive GFP gene as the homology template, then the DNA repair results in gene conversion making the GFP gene become active (Fig. 1A). The SSA assay is based on an analogous strategy in which the GFP protein is not expressed because of intervening DNA sequence, with an I-SceI site at the 3′ end of the intervening DNA. Upon expression of the I-SceI endonuclease, the DSB is generated, and resection of the intervening sequence reveals homologous GFP sequences that are aligned and repaired [Stark et al., 2004] (Fig. 1B). These two processes depend on sequence homologies for the repair, but there are functional differences. The Rad51 and BRCA2 proteins are required for the homology-directed recombination (HDR) assay, but are not required for the SSA assay [Moynahan et al., 2001; Stark et al., 2004].
Figure 1.
The double-strand DNA break (DSB) repair assay system. A: The HDR recombination substrate [Pierce et al. 2001] is diagrammed. The upstream GFP allele is defective because of the inclusion of an I-SceI site in its sequence. The downstream GFP allele is defective because of another lesion and provides a donor sequence to repair via homologous recombination the I-SceI-generated DSB. B: The SSA recombination substrate [Stark et al., 2004] is diagrammed. After the I-SceI-generated DSB, intervening sequence is resected until homology in the GFP encoding sequences is revealed, permitting repair. C: The timeline of the typical experiment is indicated. Cells are transfected with a siRNA targeting the 3′-UTR of the BRCA1 mRNA plus a plasmid that expresses the BRCA1 variant and which is resistant to the siRNA. After 2 days, the siRNA and the BRCA1 variant expressing plasmid are transfected again along with a plasmid that expresses the I-SceI endonuclease. After 3 days, the cells are harvested and analyzed for GFP expression by flow cytometry.
For each assay, we have established HeLa-derived cell lines with the published recombination substrate [Pierce et al., 2001; Stark et al., 2004] integrated in the genome at a single site. The HeLa-DR cell line assays HDR, and the HeLa–SSA cell line is used to assay SSA. The HDR and the SSA assays have a similar experimental design with a depletion of BRCA1 by transfection of a siRNA that is specific for the 3′-UTR of the BRCA1 mRNA. We cotransfect a BRCA1 expression plasmid that is not affected by the siRNA and which expresses the variant BRCA1 protein. After 2 days, we repeat the transfection and also transfect a plasmid that encodes the expression of the I-SceI endonuclease to generate the DSB in the presence of the variant BRCA1. After 3 days, we assay the cells for GFP expression by flow cytometry. The timeline of the experiment is shown in Figure 1C.
We have previously analyzed 16 variants of BRCA1 in a tissue-culture-cell-based assay for repair of DSBs via homologous recombination [Ransburgh et al., 2010]. Using the HeLa-DR cell line developed by us, up to 20% of the cells convert to GFP positive. This high level of recombination enables the quantitative analysis of variants in BRCA1 in this process. The endogenous BRCA1 mRNA is depleted by siRNA targeting the 3′-UTR, and a BRCA1 variant that is resistant to the siRNA is simultaneously expressed from a plasmid [Parvin et al., 2011; Ransburgh, et al., 2010]. A quantitative measure of the function of the BRCA1 variant is determined from the percentage of cells that convert to GFP positive.
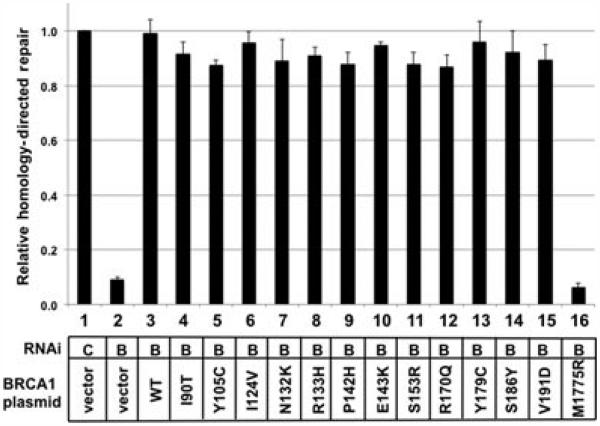
In this study, we first analyzed an additional 13 BRCA1 variants for function in HDR (Fig. 2). Twelve of the BRCA1 variants spanned amino acid residue 90 through 191 and were identified from the Breast Information Core (http://research.nhgri.nih.gov/bic/). These were expressed from the plasmid as full-length proteins. All 12 variants are listed in the BIC as having unknown phenotype with regard to predisposition to breast cancer. Several of these have been evaluated using a multifactorial classification system, and of these 12 variants, seven were nonpathogenic [Lindor et al., 2012]. These were chosen because the function of this domain was unknown and because many of these variants have been reported to be nonpathogenic. By testing the known nonpathogenic variants in this assay, we provide a specificity control for the functional test. The 13th variant tested is a known deleterious mutant in the carboxy terminus of the BRCA1 protein, M1775R. In these experiments, the percentage of cells that were GFP positive when transfected with the control siRNA and the control plasmid were set equal to 1%. All other results were normalized to this control, and all results were from three or more separate experiments. Depletion of BRCA1 and transfection with empty plasmid resulted in a value of 0.09 relative to the control (Fig. 2, lane 2). Thus, BRCA1 depletion has an over 10-fold effect reducing the level of homologous recombination. Depleting BRCA1 and transfecting a plasmid that expressed wild-type BRCA1 resulted in full restoration of homologous recombination (Fig. 2, lane 3). Among the variants with substitutions in residues from 90 to 191, all 12 functioned as wild type in the homologous recombination assay (Fig. 2, lanes 4–15) (P < 0.002 compared with depleted BRCA1). By contrast, the 13th tested missense substitution, a known pathogenic mutant BRCA1–M1775R, did not complement the depletion of BRCA1 in this assay (Fig. 2, lane 16) (Pv = 0.0014 compared with wild-type BRCA1).
Figure 2.
Analysis of 13 missense substitutions of BRCA1 in the homology-directed repair assay. HeLa-DR cells were transfected with a siRNA that depleted the endogenous BRCA1 and with a plasmid that expressed the indicated BRCA1 variant. After generating a double-strand DNA break by expressing the I-SceI endonuclease, functional homologous recombination results in the conversion of cells to positive for GFP. The fraction of GFP-positive cells was determined by flow cytometry, and results from each experiment were normalized according to the fraction of GFP-positive cells in the control transfection (lane 1). In each lane, the siRNA was targeted to a control (C; lane 1) or to the BRCA1 3′-UTR (B; lanes 2–16). The co–transfected plasmids were either empty vector (lanes 1 and 2), expressed wild-type BRCA1 (lane 3), or expressed the indicated BRCA1 variant (lanes 4–16). All experiments were done in triplicate. Error bars indicate the SEM.
Function of 29 Variants of BRCA1 in the Repair of DNA Breaks by the SSA Pathway
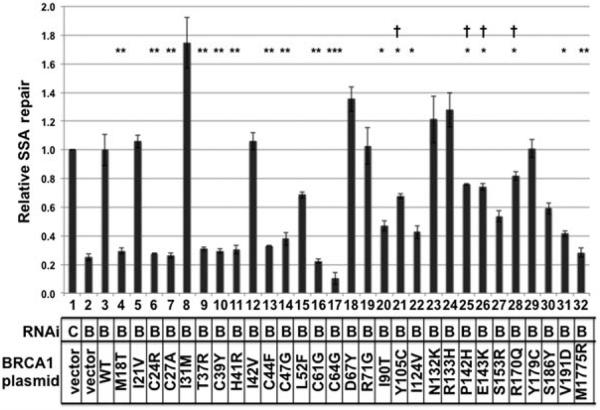
The 16 BRCA1 variants that had previously been tested for function in homologous recombination [Ransburgh et al., 2010] and the 13 BRCA1 variants tested in this study (Fig. 2) were analyzed for function in SSA repair. The recombination substrate [Bennardo et al., 2008; Stark et al., 2004] was based on similar design as the homologous recombination substrate with the exception that restoration of GFP activity occurs when the two inactive GFP genes are repaired by SSA. HeLa cells were stably transfected with the SA–GFP construct, and a clone was selected that had no detectable background GFP signal. Upon transfection of the I-SceI-expressing plasmid, about 5–7% of cells became GFP positive, indicative of a functioning SSA pathway. Depletion of BRCA1 resulted in a decrease to about 1–2% GFP-positive cells (Fig. 3, lane 2), indicating a role for BRCA1 in the SSA repair pathway that had been previously observed [Stark et al., 2004]. Depletion of BRCA1 resulted in about 25% of the activity found in nondepleted cells. As had been observed with the homologous recombination assay, transfection of a plasmid expressing wild-type BRCA1 fully complemented the defect in SSA due to depletion of the endogenous BRCA1 (Fig. 3, lane 3). By contrast, depletion of endogenous BRCA1 and expression of a known pathogenic mutant of BRCA1, M1775R, was as defective in SSA as had been transfection of the empty vector (Fig. 3, lane 32). Similar to previous observations with the zinc-coordinating mutants of BRCA1, these residues were all required for SSA repair of double-strand breaks (Fig. 3, lanes: 6, 7, 10, 11, 13, 14, 16, and 17). As was found for the homologous recombination assay, the M18T variant and the T37R variant were defective for SSA repair. The L52F variant had reduced levels of SSA repair, approximately 69% of wild-type activity (Fig. 3, lane 15), although this value was not significantly different from wild type. The D67Y variant, a known nonpathogenic variant [Easton et al., 2007], was fully active in SSA repair (lane 18).
Figure 3.
Analysis of 29 missense substitutions of BRCA1 in the SSA assay. HeLa–SSA cells were transfected as in Figure 2 with plasmids that express the 29 tested missense substitutions of BRCA1. Results of the fraction of cells that had converted to GFP positive within an experiment were normalized to the control experiment (lane 1). The Student’s t-test was applied to results, and if the SSA results were significantly different from the control lane, then it is indicated with asterisks. P values were: <0.01 (*), <0.001 (**), or <0.0001 (***). Results were also compared with fully defective (lane 2); and in some cases, the results were statistically different from the defective as well (indicated with a cross; P < 0.01). These latter variants were judged to be intermediate in phenotype. All experiments were done in triplicate. Error bars indicate the SEM.
Results diverged between the SSA and the homologous recombination assays for the variants in residues 90 through 191. The BRCA1–I90T variant functioned in the homologous recombination assay, but in the SSA assay, it was 47% as active as wild type (t-test for differing from wild-type P < 0.01; lane 20). The Y105C variant was statistically different from both wild-type BRCA1 and empty vector (indicated with both an asterisk and a cross; lane 21). This Y105C variant thus has intermediate phenotype, neither fully active nor fully defective. Similarly, the P142H, E143K, and the R170Q had intermediate phenotype (lanes 25, 26, and 28). The V191D variant was also defective for SSA repair (lane 31). Interestingly, the S153R and S186Y variants had diminished activity, but the change in activities was not statistically significant from the wild type. Thus, these variants were not scored as defective. Seven of the variants tested had assay-specific phenotypes, suggesting that how BRCA1 interacts with repair factors in the homologous recombination pathway is different from factors that BRCA1 binds in the SSA pathway.
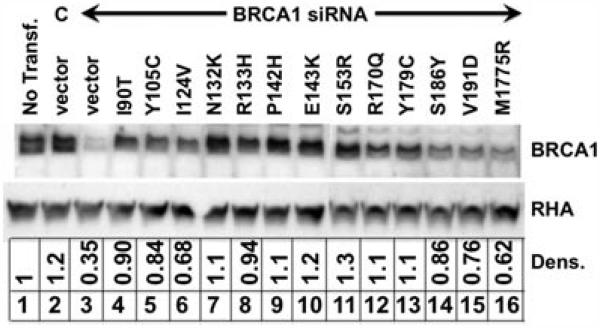
When replacing the endogenous BRCA1 protein with a variant BRCA1, there is the concern that the expression levels be consistent with the levels of the endogenous in nondepleted cells. A defective phenotype could be masked by high levels of overexpression of the variant protein, or the phenotype of a functional protein might not be observed because of poor protein expression. The 16 variants with missense substitutions between residues 18 and 71 had been tested in HeLa-derived cells and the expression of each variant was similar to the endogenous in each case [Ransburgh et al., 2010]. For expression, we tested the missense substitutions between residues 90 and 191 and also residue 1775, and found that they all express approximately similarly as the endogenous BRCA1 (Fig. 4). Normalized results from densitometry analysis are presented beneath each lane. Protein from nontransfected cells and from control siRNA and empty vector transfected cells had similar amounts of BRCA1 protein (Fig. 4, lanes 1 and 2). Depletion of BRCA1 and add back with empty plasmid depleted the level of BRCA1 protein, which densitometry analysis indicated to be about 0.35 relative to the nontransfected cells (lane 3). All variants were detected at higher levels than the lysate from BRCA1-depleted cells. Some variants were expressed at higher levels than others, but at most 1.3-fold higher than the BRCA1 levels in nontransfected cells (compare, for example, lane 7 with lane 2). Because none of the BRCA1 variants were expressed at levels much higher than the endogenous BRCA1, in no case could the phenotype of a defective variant be masked by overexpression. The S186Y, V191D, and M1775R variants all had somewhat lower expression, but these lower levels of expression did not correlate with function in either homologous recombination or SSA repair. As an example, the S186Y variant was a low expresser (0.86 relative to control), but it was functional in both assays. We note that the M1775R mutant was expressed at lower levels (0.62 relative to endogenous BRCA1) than any other BRCA1 variant (lane 16). We interpret this level of expression to be similar to other variants and not an explanation for the defect in each double-strand break repair pathway. However, we cannot exclude the interpretation that the M1775R was defective in the DNA repair assays because there was lower BRCA1 expression of this isoform in our cells.
Figure 4.
Analysis of the expression levels of 13 variants in HeLa–SSA cells. The expression levels of 16 previously tested BRCA1 substitution variants were tested in the HeLa-derived cell line [Ransburgh et al., 2010]. Thirteen variants, with substitutions at residues from 90 to 1775, were expressed according to the same protocol as in Figure 2. Protein extracts were analyzed by gel electrophoresis and immunoblotting. Samples were: no transfection (lane 1), control siRNA plus empty plasmid vector (lane 2), BRCA1-specific siRNA (lanes 3–16) with empty plasmid vector (lane 3), and plasmid expressing the indicated BRCA1 variant (lanes 4–16). The same membrane was probed using antibody specific for RNA helicase A (RHA) as a loading control (bottom). Densitometry analysis was performed to compare the expression of BRCA1 from the plasmid to the endogenous BRCA1 in nontransfected cells.
Comparison of Variants in Different Assays
The 29 BRCA1 variants analyzed in this study were compared for function in the two DNA repair assays and with available knowledge about cancer association (Table 1). Many of the variants were consistent across the two assays, but there were several variants demonstrating separation of function. All of the zinc-coordinating residues were required for function in both repair assays. The M18T variant was clearly defective, but it is currently classified as unknown clinical significance. There is one genetic model that classified this M18T variant as likely pathogenic (see below).
Table 1.
Summary of BRCA1 Variants
| Variant | HDR | SSA | Clinical impact [literature] |
|---|---|---|---|
| M18T | − a | − | Unknown [Easton et al., 2007; Langston et al., 1996]; Class 4; LOVD] |
| I21V | +a | + | Unknown [nonpathogenic; [Ruffner et al., 2001] |
| C24R | − a | − | Unknown [pathogenic; [Morris et al., 2006] |
| C27A | − a | − | Unknown |
| I31M | +a | + | Unknown [nonpathogenic; [Ruffner et al., 2001] |
| T37R | − a | − | Unknown [pathogenic; [Abkevich et al., 2004] |
| C39Y | − a | − | Pathogenic |
| H41R | − a | − | Unknown [pathogenic; [Morris et al., 2006] |
| I42V | +a | + | Unknown [neutral; [Ruffner et al., 2001] |
| C44F | − a | − | Pathogenic [BIC] |
| C47G | − a | − | Pathogenic [BIC] |
| L52F | +a | + | Unknown |
| C61G | − a | − | Pathogenic [Class 5; LOVD] |
| C64G | - a | - | Pathogenic |
| D67Y | + a | + | Nonpathogenic [Easton et al., 2007]; Class 1; LOVD] |
| R71G | +a | + | Pathogenic [Class 5 BIC; splicing] |
| I90T | + | − | Unknown [neutral; [Morris et al., 2006] |
| Y105C | + | Intermed | Unknown [nonpathogenic; [Easton et al., 2007; Judkins et al., 2005]; Class 1; LOVD] |
| I124V | + | − | Unknown [nonpathogenic; [Easton et al., 2007]; Class 1; LOVD] |
| N132K | + | + | Unknown [pathogenic; [Caligo et al., 2009]; nonpathogenic, [Easton, et al., 2007]; Class 1, LOVD]. |
| R133H | + | + | Unknown |
| P142H | + | Intermed | Unknown [nonpathogenic; [Chenevix-Trench et al., 2006]; Class 1; LOVD] |
| E143K | + | Intermed | Unknown [Nonpathogenic; [Easton et al., 2007]; Class 1, LOVD] |
| S153R | + | + | Unknown |
| R170Q | + | Intermed | Unknown [nonpathogenic; [Abkevich et al., 2004] |
| Y179C | + | + | Unknown [nonpathogenic; [Osorio et al., 2007; Spurdle et al., 2008]; Class 1; LOVD] |
| S186Y | + | + | Unknown [Uncertain, S186Y, [Tavtigian, et al., 2006]; Class 1, LOVD). |
| V191D | + | − | Unknown [nonpathogenic; [Judkins et al., 2005]; Class 1; LOVD] |
| M1775R | − | − | Pathogenic [pathogenic; [Carvalho et al., 2007]; Class 5; LOVD] |
“Intermed” indicates that the results for the repair assay were significantly different from those with the wild-type BRCA1 and significantly different from those with the depleted BRCA1 and vector add-back.
Results from [Ransburgh et al. (2010)].
LOVD, Leiden Open Variation Database: International Agency for Research on Cancer Database Classification.
Benchmarking Function to Clinical Relevance
Recently, a new classification system for variants has been proposed [Lindor et al., 2012; Plon et al., 2008]. According to this system, Class 1 denotes variants that are not pathogenic. Class 2 is likely nonpathogenic. Class 3 is uncertain. Class 4 is likely pathogenic (probability of being pathogenic is 0.95–0.99), and Class 5 is definitely pathogenic (probability >0.99). According to variants described in the [Lindor et al. (2012)] system, three of the variants in this study (M18T, C61G, and M1775R) were of Class 4, either likely pathogenic or definitely pathogenic. An additional five mutants are treated clinically as Class 5, pathogenic, (C39Y, C44F, C47G, C64G, and R71G). One of these pathogenic mutants, R71G, does not affect the BRCA1 protein, but rather the splicing of the mRNA [Vega et al., 2001]. Since the assays tested express BRCA1 from spliced cDNA, the R71G defective phenotype would not be detected. We thus exclude this mutant from the comparison of benchmarking these assays against known mutants. There were then seven pathogenic mutants (Class 4 or 5) analyzed and eight nonpathogenic variants (Class 1; Table 1). When comparing the results of our functional analyses with these previously classified variants, the HDR assay scored all seven pathogenic or likely pathogenic mutants as defective, and all eight nonpathogenic variants as functional. The results of the assay were, in the case of every variant, either fully active or fully defective (see examples in Fig. 2). To date, no variant tested in the HDR assay produced an intermediate score.
Compared with these benchmarks, the variants tested in the SSA assay did not score as well as the HDR assay. All pathogenic mutants were defective for SSA repair of double-strand breaks, but one nonpathogenic variant (I124V) was defective for SSA, and three Class 1 variants had intermediate phenotype in the SSA assay. Although the HDR assay was both highly sensitive and highly specific, the SSA assay was highly sensitive at association with cancer risk, but it was less specific. Of interest, it had been noted that four BRCA1 variants, Y105C, P142H, E143K, and Y179C, had decreased accumulation at the sites of DNA breaks [Wei et al., 2008]. Although these variants were normal for HDR, three of these had intermediate levels of function in the SSA assay (Table 1). Perhaps there is a link between the accumulation of BRCA1 at DNA repair sites and a factor important for the SSA assay.
Discussion
Functional Assays as Predictive Tools for Assessing BRCA1 Variants
In this study, we analyzed a total of 29 BRCA1 variants in the repair of DSBs by homologous recombination and by SSA. For the sake of this discussion, we exclude the BRCA1–R71G variant that affects splicing [Vega et al., 2001] because all of our assays used prespliced cDNAs and would not be sensitive to a splicing defect. By comparing our results with those BRCA1 missense substitutions that have a known effect on breast cancer predisposition, we found that the HDR assay was 100% accurate: seven previously classified pathogenic mutants were defective for HDR, and eight previously classified nonpathogenic variants in BRCA1 were functional for HDR. Further, the results for the HDR assay were either fully active or fully defective. We interpret these results to indicate that the HDR assay is validated as a predictive tool for assessing BRCA1 variants.
By contrast to the HDR assay, the function of BRCA1 variants in the repair of DSBs by SSA had a number of differences that diminished the accuracy of this assay. On the basis of the benchmarks of variants that were classified using a genetic model [Lindor et al., 2012], the SSA assay was sensitive because it did not misclassify any pathogenic variants, but it was not specific because it misclassified several nonpathogenic variants as either intermediate or defective. Interpretation of the SSA assay was also complicated by intermediate results; by comparison, the HDR assay had no intermediate results among the 29 variants tested. It is possible, but remains to be determined, whether missense variants that are proficient for HDR, but deficient or intermediate for SSA, confer a lower or moderate risk of cancer. We thus conclude that the HDR assay is a more reliable predictive tool for assessing the function of BRCA1 variants than is the SSA assay.
We had previously performed a similar analysis of the control of centrosome duplication by BRCA1 variant proteins [Kais et al., 2012]. Fewer variants of the BRCA1 protein were analyzed in this earlier study using the centrosome assay, but it has several differences from the HDR assay. With regard to those variants with inferred breast cancer association, the centrosome duplication assay had an intermediate result for the BRCA1–D67Y variant, which is nonpathogenic. The T37R variant was defective for both DNA repair assays, but was functional in control of the centrosome duplication. Five amino acid residues away, the I42V variant had the opposite pattern as T37R, with defective centrosome control but fully functional in both DNA repair assays. Experimentally, the centrosome regulation assay is much more difficult to perform than the HDR assay, making it not as desirable an assay for analysis of BRCA1 variants. In addition, the centrosome assay appears, based on the limited set of variants, to be less accurate than the HDR assay. On the contrary, like the SSA, none of the tested seven variants that were previously classified as Class 4 or 5, were functional in the centrosomal assay, suggesting that this assay is sensitive.
It will be necessary to analyze many more variants in the HDR assay and then compare those results with the clinical experiences before this assay can be used for clinical interpretation. On the basis of the accuracy of the currently tested variants, we believe that the HDR assay will help with difficult to classify variants of unknown significance.
Functional Assays for Probing BRCA1 Residues Important in Biological Function
Separate from the clinical utility of the different functional tests, important biological information about the BRCA1 protein is available from the various functional assays. Very little structure–function information exists for BRCA1, and there have been limited systematic analyses of the effects of BRCA1 amino acid variants in a given function. A variety of variants have been analyzed in the context of short BRCA1 amino-terminal truncations for ubiquitin ligase activity [Morris et al., 2006]. In a similar vein, a variety of variants in the context of a short BRCA1 carboxy-terminal fragment have been analyzed for the activation of transcription [Carvalho et al., 2007; Lee et al., 2010]. Several variants in the context of the full-length protein have been analyzed in terms of resistance to ionizing radiation [Ruffner et al., 2001] and reversal of a lethal phenotype in murine embryonic stem cells [Chang et al., 2009].
We find that there are some discordancies between functional assays of the variants. The most striking are at T37R and I42V. BRCA1–T37R was defective for both DNA repair processes, but was fully functional for control of centrosomes. Conversely, BRCA1–I42V was defective for control of centrosome duplication [Kais et al., 2012] but functional in the DNA repair processes. From this result, we hypothesize that the protein–BRCA1 contacts in the DNA repair pathways depend on the T37 residue, whereas a different protein–BRCA1 contact is important for regulating the centrosome duplication. Such a notion is possible because the centrosome regulation occurs in the cytoplasm and the DNA repair occurs in the nucleus, and it is reasonable to suppose that different proteins are involved.
There were variants that distinguished between BRCA1 residues important for DSB repair by homologous recombination versus SSA. The observed differences between the SSA and HDR assays involved residues from 90 to 191. Several of these (Y105C, P142H, and E143K) that had intermediate phenotype in the SSA assay had been shown to have slow accumulation at sites of DSBs. Especially, P142H mutation abolished the association with Ku80 protein, which plays an important role in the repair of DSBs [Wei et al., 2008]. Perhaps the SSA pathway contributes to the accumulation of BRCA1 at sites of DNA damage in cells.
In summary, in this study, we evaluated missense variants of BRCA1 in two different DNA repair pathways. Data support that the function of BRCA1 in the HDR assay is consistent with the known cancer association of these variants, and we suggest that this test is predictive for breast cancer. By contrast, the repair of DNA by the related SSA pathway is not perfectly consistent with what is known about cancer predisposition, and the SSA assay yields intermediate results. On the basis of these current results, we suggest that the HDR assay is validated for predicting whether substitution at any residue of BRCA1 is disease associated.
Acknowledgments
National Institutes of Health
R01 CA141090
State University Comprehensive Cancer Center
We thank Dr. J. Stark of the Beckman Research Institute at the City of Hope for the plasmid construct that was used for making the cell line for the SSA assay, and we thank Tapahsama Banerjee for technical assistance.
Footnotes
Disclosure statement: The authors declare no conflict of interest.
References
- Abkevich V, Zharkikh A, Deffenbaugh AM, Frank D, Chen Y, Shattuck D, Skolnick MH, Gutin A, Tavtigian SV. Analysis of missense variation in human BRCA1 in the context of interspecific sequence variation. J Med Genet. 2004;41:492–507. doi: 10.1136/jmg.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennardo N, Cheng A, Huang N, Stark JM. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 2008;4:e1000110. doi: 10.1371/journal.pgen.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligo MA, Bonatti F, Guidugli L, Aretini P, Galli A. A yeast recombination assay to characterize human BRCA1 missense variants of unknown pathological significance. Hum Mutat. 2009;30:123–133. doi: 10.1002/humu.20817. [DOI] [PubMed] [Google Scholar]
- Carvalho MA, Marsillac SM, Karchin R, Manoukian S, Grist S, Swaby RF, Urmenyi TP, Rondinelli E, Silva R, Gayol L, Baumbach L, Sutphen R, et al. Determination of cancer risk associated with germ line BRCA1 missense variants by functional analysis. Cancer Res. 2007;67:1494–1501. doi: 10.1158/0008-5472.CAN-06-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Biswas K, Martin BK, Stauffer S, Sharan SK. Expression of human BRCA1 variants inmouse ES cells allows functional analysis of BRCA1mutations. J Clin Invest. 2009;119:3160–3171. doi: 10.1172/JCI39836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevix-Trench G, Healey S, Lakhani S, Waring P, Cummings M, Brinkworth R, Deffenbaugh AM, Burbidge LA, Pruss D, Judkins T, Scholl T, Bekessy A, et al. Genetic and histopathologic evaluation of BRCA1 and BRCA2 DNA sequence variants of unknown clinical significance. Cancer Res. 2006;66:2019–2027. doi: 10.1158/0008-5472.CAN-05-3546. [DOI] [PubMed] [Google Scholar]
- Cotta-Ramusino C, McDonald ER, 3rd, Hurov K, Sowa ME, Harper JW, Elledge SJ. A DNA damage response screen identifies RHINO, a 9-1-1 and TopBP1 interacting protein required for ATR signaling. Science. 2011;332:1313–1317. doi: 10.1126/science.1203430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval M, Gauthier G, Maunsell E, Dugas MJ, Rouleau I, Chiquette J, Plante M, Laframboise R, Gaudet M, Bridge PJ, Simard J. No evidence of false reassurance among women with an inconclusive BRCA1/2 genetic test result. Cancer Epidemiol Biomarkers Prev. 2005;14:2862–2867. doi: 10.1158/1055-9965.EPI-05-0512. [DOI] [PubMed] [Google Scholar]
- Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, Allen-Brady K, Tavtigian SV, Monteiro AN, Iversen ES, Couch FJ, Goldgar D. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet. 2007;81:873–883. doi: 10.1086/521032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgar DE, Easton DF, Deffenbaugh AM, Monteiro AN, Tavtigian SV, Couch FJ. Integrated evaluation of DNA sequence variants of unknown clinical significance: application to BRCA1 and BRCA2. Am J Hum Genet. 2004;75:535–544. doi: 10.1086/424388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, Wenstrup RJ, Ward BE, Scholl TA, Noll WW. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judkins T, Hendrickson BC, Deffenbaugh AM, Eliason K, Leclair B, Norton MJ, Ward BE, Pruss D, Scholl T. Application of embryonic lethal or other obvious phenotypes to characterize the clinical significance of genetic variants found in trans with known deleterious mutations. Cancer Res. 2005;65:10096–10103. doi: 10.1158/0008-5472.CAN-05-1241. [DOI] [PubMed] [Google Scholar]
- Kais Z, Chiba N, Ishioka C, Parvin JD. Functional differences among BRCA1 missense mutations in the control of centrosome duplication. Oncogene. 2012;31:799–804. doi: 10.1038/onc.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston AA, Malone KE, Thompson JD, Daling JR, Ostrander EA. BRCA1 mutations in a population-based sample of young women with breast cancer. N Engl J Med. 1996;334:137–142. doi: 10.1056/NEJM199601183340301. [DOI] [PubMed] [Google Scholar]
- Lee MS, Green R, Marsillac SM, Coquelle N, Williams RS, Yeung T, Foo D, Hau DD, Hui B, Monteiro AN, Glover JN. Comprehensive analysis of missense variations in the BRCT domain of BRCA1 by structural and functional assays. Cancer Res. 2010;70:4880–4890. doi: 10.1158/0008-5472.CAN-09-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor NM, Guidugli L, Wang X, Vallee MP, Monteiro AN, Tavtigian S, Goldgar DE, Couch FJ. A review of a multifactorial probability-based model for classification of BRCA1 and BRCA2 variants of uncertain significance (VUS) Hum Mutat. 2012;33:8–21. doi: 10.1002/humu.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millot GA, Carvalho MA, Caputo SM, Vreeswijk MP, Brown MA, Webb M, Rouleau E, Neuhausen SL, Hansen TV, Galli A, Brandão RD, Blok MJ, et al. A guide for functional analysis of BRCA1 variants of uncertain significance. Hum Mutat. 2012;33:1526–1537. doi: 10.1002/humu.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JR, Pangon L, Boutell C, Katagiri T, Keep NH, Solomon E. Genetic analysis of BRCA1 ubiquitin ligase activity and its relationship to breast cancer susceptibility. Hum Mol Genet. 2006;15:599–606. doi: 10.1093/hmg/ddi476. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Osorio A, Milne RL, Honrado E, Barroso A, Diez O, Salazar R, de la Hoya M, Vega A, Benitez J. Classification of missense variants of unknown significance in BRCA1 based on clinical and tumor information. Hum Mutat. 2007;28:477–485. doi: 10.1002/humu.20470. [DOI] [PubMed] [Google Scholar]
- Parvin J, Chiba N, Ransburgh D. Identifying the effects of BRCA1 mutations on homologous recombination using cells that express endogenouswild-typeBRCA1. J Vis Exp. 2011;48:2468. doi: 10.3791/2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucelli N, Lazebnik N, Huelsman KM, Lazebnik RS. Clinical interpretation and recommendations for patients with a variant of uncertain significance in BRCA1 or BRCA2: a survey of genetic counseling practice. Genet Test. 2002;6:107–113. doi: 10.1089/10906570260199357. [DOI] [PubMed] [Google Scholar]
- Pierce AJ, Hu P, Han M, Ellis N, Jasin M. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev. 2001;15:3237–3242. doi: 10.1101/gad.946401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle AB, Tavtigian SV. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransburgh DJ, Chiba N, Ishioka C, Toland AE, Parvin JD. Identification of breast tumor mutations in BRCA1 that abolish its function in homologous DNA recombination. Cancer Res. 2010;70:988–995. doi: 10.1158/0008-5472.CAN-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffner H, Joazeiro CA, Hemmati D, Hunter T, Verma IM. Cancer-predisposing mutations within the RING domain of BRCA1: loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc Natl Acad Sci U S A. 2001;98:5134–9. doi: 10.1073/pnas.081068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman AD, Sweet K, Zhou XP, McLennan J, Couch FJ, Toland AE. Clinically applicable models to characterize BRCA1 and BRCA2 variants of uncertain significance. J Clin Oncol. 2008;26:5393–5400. doi: 10.1200/JCO.2008.17.8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurdle AB, Lakhani SR, Healey S, Parry S, Da Silva LM, Brinkworth R, Hopper JL, Brown MA, Babikyan D, Chenevix-Trench G, Tavtigian SV, Goldgar DE, kConFab Investigators Clinical classification of BRCA1 and BRCA2 DNA sequence variants: the value of cytokeratin profiles and evolutionary analysis—a report from the kConFab Investigators. J Clin Oncol. 2008;26:1657–1663. doi: 10.1200/JCO.2007.13.2779. [DOI] [PubMed] [Google Scholar]
- Stark JM, Pierce AJ, Oh J, Pastink A, Jasin M. Genetic steps of mammalian homologous repair with distinctmutagenic consequences. Mol Cell Biol. 2004;24:9305–9316. doi: 10.1128/MCB.24.21.9305-9316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet K, Senter L, Pilarski R, Wei L, Toland AE. Characterization of BRCA1 ring finger variants of uncertain significance. Breast Cancer Res Treat. 2009;119:737–743. doi: 10.1007/s10549-009-0438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavtigian SV, Deffenbaugh AM, Yin L, Judkins T, Scholl T, Samollow PB, de Silva D, Zharkikh A, Thomas A. Comprehensive statistical study of 452 BRCA1missense substitutions with classification of eight recurrent substitutions as neutral. J Med Genet. 2006;43:295–305. doi: 10.1136/jmg.2005.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee MP, Francy TC, Judkins MK, Babikyan D, Lesueur F, Gammon A, Goldgar DE, Couch FJ, Tavtigian SV. Classification of missense substitutions in the BRCA genes: a database dedicated to Ex-UVs. Hum Mutat. 2012;33:22–28. doi: 10.1002/humu.21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk S, Timmermans DR, Meijers-Heijboer H, Tibben A, van Asperen CJ, Otten W. Clinical characteristics affect the impact of an uninformative DNA test result: the course of worry and distress experienced by women who apply for genetic testing for breast cancer. J Clin Oncol. 2006;24:3672–3677. doi: 10.1200/JCO.2005.03.7259. [DOI] [PubMed] [Google Scholar]
- Vega A, Campos B, Bressac-De-Paillerets B, Bond PM, Janin N, Douglas FS, Domenech M, Baena M, Pericay C, Alonso C, Carracedo A, Baiget M, Diez O. The R71G BRCA1 is a founder Spanish mutation and leads to aberrant splicing of the transcript. Hum Mutat. 2001;17:520–521. doi: 10.1002/humu.1136. [DOI] [PubMed] [Google Scholar]
- Wei L, Lan L, Hong Z, Yasui A, Ishioka C, Chiba N. Rapid recruitment of BRCA1 to DNA double-strand breaks is dependent on its association with Ku80. Mol Cell Biol. 2008;28:7380–7393. doi: 10.1128/MCB.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]