Abstract
Cell therapy for nervous tissue repair is limited by low transplant survival. We investigated the effects of a polyurethane-based reverse thermal gel, poly(ethylene glycol)-poly(serinol hexamethylene urethane) (ESHU) on bone marrow stromal cell (BMSC) transplant survival and repair using a rat model of spinal cord contusion. Transplantation of BMSCs in ESHU at three days post-contusion resulted in a 3.5-fold increase in BMSC survival at one week post-injury and a 66% increase in spared nervous tissue volume at four weeks post-injury. These improvements were accompanied by enhanced hindlimb motor and sensorimotor recovery. In vitro, we found that ESHU protected BMSCs from hydrogen peroxide-mediated death, resulting in a four-fold increase in BMSC survival with two-fold fewer BMSCs expressing the apoptosis marker, caspase 3 and the DNA oxidation marker, 8-Oxo-deoxyguanosine. We argue that ESHU protected BMSCs transplanted is a spinal cord contusion from death thereby augmenting their effects on neuroprotection leading to improved behavioral restoration. The data show that the repair effects of intraneural BMSC transplants depend on the degree of their survival and may have a widespread impact on cell-based regenerative medicine.
1. Introduction
Cell therapy is promising for repair of the damaged nervous system [1–3]. Bone marrow stromal cells (BMSCs) are candidate cells for such therapies because of their repair proficiency and relative accessibility [4–7]. BMSCs support repair of a myriad of other ailments including cardiomyopathy, muscle dystrophy, and wound healing [8–10]. Intraneural BMSC transplants are thought to elicit repair through paracrine effects [11–14]. However, these effects are likely limited due to low transplant survival in damaged nervous tissue [15–18].
Cell transplants may be lost due to various events including inflammation [19] and oxidative stress [20–24], which are initiated rapidly after injury. Thus, measures to protect cell transplants against these death-mediating mechanisms may increase transplant survival and potentially improve their reparative effects. One strategy to improve transplanted cell survival is by using the synthetic poly(ethylene glycol)-poly(serinol hexamethylene urethane) or ESHU, which is a reverse thermal gel with good biocompatibility and degradability [25,26]. ESHU is a copolymer with two hydrophilic poly(ethylene glycol) blocks flanking a hydrophobic poly(serinol hexamethylene urethane) block [25]. The presence of polyurethane [28,29] may provide ESHU with antioxidant capacity. ESHU dissolves in water and undergoes phase transition with increasing temperatures to form a physical gel at 37°C [25], which makes it especially practical for treatment of closed injuries [30].
ESHU was shown to have good biocompatibility with nervous tissue in the ocular system [26,27]. A beneficial feature of ESHU is that the repeating units of the polymer contain protected amine groups providing an easy path to functionalization using biomolecules or other signaling molecules that can offer enhanced bioactivity of the gel in vivo. One example is to functionalize ESHU through these amine groups with the pentapeptide, IKVAV, which in pilot experiments was shown to produce a neural interface similar to laminin.
We hypothesized that ESHU protects intraneural BMSC transplants from death leading to improved repair. This premise was tested in vivo using an adult rat model of spinal cord contusion [7,15,16] assessing BMSC transplant survival, inflammation, anatomical restoration, and functional recovery and in vitro using BMSC cultures determining the effects of ESHU on survival of BMSCs under oxidative stress.
2. Materials and Methods
2.1. Ethics and surgical approval
Before and after surgery, rats were housed following guidelines of the National Institutes of Health and the United States Department of Agriculture. The rats were kept within a double-barrier facility in standard rat cages with continuous supply of fresh air, water, and food. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
2.2. Transplant preparation
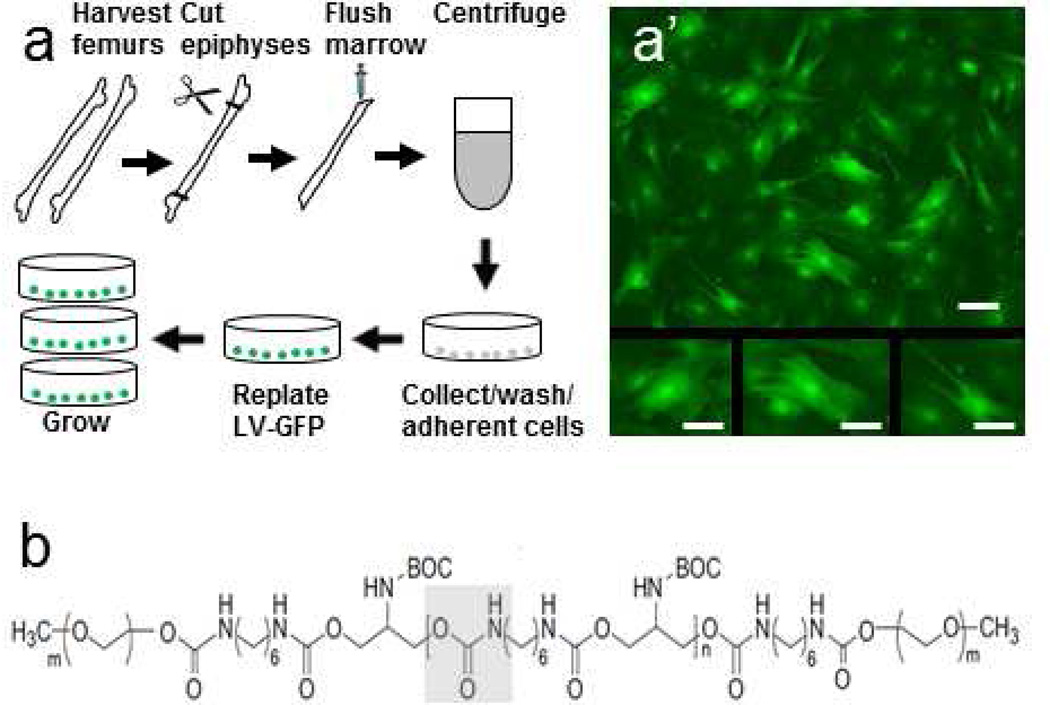
We harvested BMSCs from femurs of female adult Sprague Dawley rats according to a previously described protocol (Fig. 1a) [7,15,31]. Isolated cells were grown in Dulbecco’s Modified Eagle Medium (DMEM, Sigma-Aldrich, Allentown, PA, USA) with 10% fetal bovine serum (Mediatech, Manassas, VA, USA) and 1% penicillin/streptomycin (Invitrogen, Grand Island, NY, USA). To enable detection after transplantation, first passage cells were transduced to express green fluorescent protein (GFP) using lentiviral vectors (Fig. 1a’) [7,15,16]. Fourth passage cells positive for the BMSC markers, CD90 and CD105 and negative for blood cell markers, CD34, CD45 and HLA-DR [16,32,33] were used for the experiments.
Fig. 1. Schematic representations of transplant preparation and ESHU.
(a) Isolation and transduction of bone marrow stromal cells. Plastic-adherent cells from femurs of adult Sprague-Dawley rats were collected, lentivirally transduced with green fluorescent protein, and passaged four times before used for transplantation. (a’) Green fluorescent protein-expressing bone marrow stromal cells in culture. Smaller panels show examples of cultured BMSC morphologies. (b) Structural formula of ESHU with the urethane bond in gray box. Bar = 50 µm in a’ and 25 µm in panels below.
2.3. ESHU
The preparation of ESHU (Fig. 1b) was previously described [25]. In brief, polyurethane blocks were synthesized by melting N-BOC-serinol (Sigma-Aldrich) under nitrogen and slowly adding hexamethylene diisocyanate (HDI; TCI America, Wellesley Hills, MA, USA) to initiate polymerization via urethane bonds. Both ends of polyurethane were capped with an isocyanate group using additional HDI and then dissolved in anhydrous dimethylformamide. Diethyl ether (Fisher Scientific, Pittsburgh, PA, USA) was used to precipitate out the polymer and remove unreacted hexamethylene diisocyanate. Polyethylene glycol (Alfa Aesar, Ward Hill, MA, USA) was coupled onto the polyurethane blocks under nitrogen, dissolved in dimethylformamide (EMD, Gibbstown, NJ, USA), and precipitated in and washed with diethyl ether. For purification, ESHU was dissolved in water and dialyzed (3500 MWCO) for 48h and finally freeze-dried. In our experiments, a 16% w/v ESHU solution in phosphate-buffered saline (PBS; pH 7.4) was prepared and sterile-filtered before use.
2.4. Surgical procedures
A model of adult rat spinal cord contusion [34,35] was used to test our hypothesis. Female adult Sprague Dawley rats (200 g, n=80; Charles Rivers Laboratory, Wilmington, MA, USA) were anaesthetized using intraperitoneal injection Ketamine (60 mg/ml; Butlerschein, Dublin, OH, USA) and Dexdomitor (0.5 mg/kg; Pfizer, New York, NY, USA) [7,15]. The tenth thoracic spinal cord segment was contused using a force of 200 kDyne (Infinite Horizon IH-0400 impactor; Precision Systems and Instrumentation, LLC, Versailles, KY, USA) [7,15,36]. The wound site was rinsed with sterile PBS with 0.1% gentamicin (VWR, Radnor, PA), the muscles were sutured in layers, and the skin was closed with Michel wound clips (Fine Science Tools, Foster City, CA, USA). All rats included in the studies had an impact within 5% of the intended force resulting in a 0.9–1.8 mm spinal cord compression and a Basso-Beattie- Bresnahan (BBB) [37,38] score ≤ 1 at day 1 and ≤ 5 at day 3 post-impact. Three days post-injury, rats were sedated and injected into the contusion [7,15,16] with 5 µl ESHU or PBS with 5×105 BMSCs, or ESHU or PBS only.
2.5. Post-surgery procedures
Antisedan (1.5 mg/kg; Pfizer) was injected subcutaneously to reverse the effects of Dexdomitor [7,15]. An intramuscular injection of gentamicin (6mg/kg; VWR), a subcutaneous injection of Rimadyl (5 mg/kg; Pfizer), and a subcutaneous injection of Ringer’s solution (10 ml on surgery day, 5 ml thereafter; Butlerschein) were administered daily for the first three days post-injury [7,15]. After the intraspinal injection at three days post-injury, the rats received gentamicin for four days and Ringer’s and Rimadyl for three days [7,15]. Bladders were manually emptied twice daily until reflex voiding occurred. Rats were monitored daily throughout the experiments. Rats were fixed at 15 min, one, four, or six weeks after injection. All rats survived without requiring pain or distress treatment.
2.6. Motor function assessment
Overground walking ability was assessed using the BBB test [37,38] weekly for six weeks post-injection (n = 10/group). Rats were tested for 4 min by two testers unaware of the treatments. Rats were familiarized with the open field and baseline values were determined before surgery. Scores were averaged per experimental group. Higher motor functions were assessed at six weeks post-injury using the BBB sub-score [39,40] as previously described (n = 10/group) [7]. Scores were averaged per experimental group. Sensorimotor function of the hind limbs was assessed before (baseline) and at six weeks post-injection using horizontal ladder walking (n = 10/group) [7,41,42]. Slips of the foot and part of lower leg and slips of the full leg were counted and expressed as a percentage of the total number of steps. Scores were averaged per experimental group.
2.7. Histological procedures
Rats were anaesthetized and transcardially perfused with 300 ml PBS followed by 400 ml 4 % paraformaldehyde (Sigma-Aldrich) in PBS. Spinal cords were dissected, post-fixed overnight in the same fixative, and transferred to 30% sucrose (Fisher Scientific) in PBS for 48 h. A 12 mm-long spinal cord segment centered at the injury epicenter was cut in 20 µm-thick horizontal cryostat sections (CM 1950; Leica Biosystems, Buffalo Grove, IL, USA). Every twelfth section was stained with cresyl violet (0.5 %; Sigma-Aldrich) for cytoarchitecture analysis and spared tissue volume assessment. Other section series were used for immunocytochemistry. Sections were analyzed using an Axio Observer Z1 fluorescent microscope (Zeiss, Thornwood, NY, USA) with StereoInvestigator® (MicroBrightField, Inc., Williston, VT, USA).
2.8. Immunocytochemistry
Tissue sections were incubated in 5% normal goat serum (Vector Labs, Burlingame, CA, USA) and 0.03% Triton X-100 (Sigma-Aldrich) in PBS for 1 h followed by the primary antibody for 2 h at room temperature and then overnight at 4 °C. Rabbit polyclonal antibodies against glial-fibrillary acidic protein (GFAP) were used to detect astrocytes (1:200; Dako North America, Inc., Carpinteria, CA). Mouse monoclonal antibodies against ED1 were used to detect macrophages (1:100; Millipore, Temecula, CA). BMSCs in vitro on 8-chamber culture slides (BD Falcon, Franklin Lakes, NJ; see also below) were fixed with 4 % paraformaldehyde in PBS for 10 min and stained with monoclonal antibodies against caspase 3 (rabbit, clone D3E9) to detect apoptotic cells (1:100; Millipore) and 8-Oxo-2’-deoxyguanosine (8-oxo-dG; mouse, clone 483.15) to detect cells with DNA damage (1:200; Millipore). After washing twice in PBS for 20 min, sections or cells were incubated with goat-anti-rabbit and goat anti-mouse Alexa Fluor 594 (1:200; Life Technologies, Grand Island, NY, USA) for 2 h at room temperature. DAPI (0.2 µl/ml; Sigma-Aldrich) was used to stain nuclei. Sections were covered with glass slips in fluorescent mounting medium (Dako North America, Inc.) and stored at 4 °C. Sections were analyzed using an Axio Observer Z1 fluorescent microscope (Zeiss, Thornwood, NY, USA) with StereoInvestigator® (MicroBrightField, Inc., Williston, VT, USA).
2.9. Cell quantification
StereoInvestigator® (MicroBrightField, Inc.) was used to determine the numbers of GFP-positive BMSCs in the injury site [15,43] at seven days post-transplantation (n = 6/group) in every twelfth section and the numbers of caspase 3- and 8-oxo-dG-positive BMSCs in cultures (see below). All assessments were done by personnel blinded to the treatment groups. For GFP-positive BMSCs in the contusion, sections were 240 µm apart spanning the width of the spinal cord. In every section the area containing GFP-positive cells was outlined manually at 2.5 × magnification and covered with a 250 × 250 µm grid. At 60 × magnification with oil immersion, GFP-positive cells with a discernible DAPI-stained nucleus were marked using the optical fractionator with a 60 × 60 µm counting frame [7,15,43]. The numbers of immunostained cells in cultures were similarly determined. The numbers of GFP-positive cells were expressed as a percentage of the number of transplanted cells (± SEM). The numbers of caspase 3-and 8-oxo-dG-positive cells were expressed as a percentage of the number of seeded cells (± SEM). The numbers were averaged per experimental group.
Image J Software was used to determine the number of ED1-immunoreactive macrophages in the injury site at one and four weeks post-transplantation in every twelfth section by persons blinded to the treatment groups [16]. Numbers were averaged per experimental group.
2.10. Measurement of nervous tissue sparing
Cresyl violet-stained sections of rats that survived for four weeks post-injection were used to determine the volume of spared tissue in the damaged spinal cord segment using the Cavalieri estimator function of StereoInvestigator® (MicroBrightField, Inc.) [7,43]. Analysis was performed by personnel blinded to the experimental groups (n = 6/group). The Gundersen Coefficient of Error was < 0.05 for all measurements. Spared tissue volume was expressed as a percentage of the volume (± SEM) of an equally-sized comparable uninjured spinal cord segment and averaged per experimental group [7].
2.11. In vitro assessment of the protective effect of ESHU
To assess ESHU’s cell protective ability we kept BMSCs in vitro under oxidative stress, which is known to contribute to intraneural cell transplant loss [23,24]. A total of 4×105 cells were incubated in 100 µl ESHU or PBS with 200 µM hydrogen peroxide (H2O2; Sigma-Aldrich) for 24 h at 37°C. Then, 100 µl Trypan Blue (Sigma-Aldrich) was added and viable (Trypan Blue-negative) cells were quantified in a hematocytometer and expressed as a percentage of all counted cells. Results from nine samples from three independent experiments were averaged. In nine samples from three independent experiments, the average number of BMSCs expressing caspase 3, a marker for apoptosis, and 8-Oxo-2’-deoxyguanosine (8-oxo-dG), a marker for DNA damage, were determined (see above). Details on caspase 3 and 8-oxo-dG staining are described in 2.8. Immunocytochemistry.
2.12. Quantification of ESHU’s antioxidant ability
ESHU’s ability to scavenge H2O2 relative to PBS was measured using a H2O2 quantification kit (National Diagnostics, Atlanta, GA, USA) which colorimetrically measures Xylenol Orange-Ferric iron complex resulting from H2O2-mediated oxidation of ferrous iron. The linear standard curve of this assay is 15–100 ng/ml. We added 30 ng/ml H2O2 (Sigma-Aldrich) to ESHU or PBS which was kept in reagent buffer for 30 min following the manufacture’s guidelines. Absorbance was measured (Victor 2V 1420; Perkin-Elmer, Waltham, MA, USA) and the values from three independent experiments were averaged.
2.13. Statistical analysis
Two-tailed Student’s T-test was used to determine differences in cell numbers in vivo and in vitro and in H2O2 concentrations in vitro. One-way ANOVA with Tukey’s post-hoc test was used to assess differences in macrophages and nervous tissue sparing. Repeated measures ANOVA with Tukey’s post hoc test determined differences in functional performances. Differences between groups were considered significant when p < 0.05.
3. Results
3.1. BMSC transplant survival
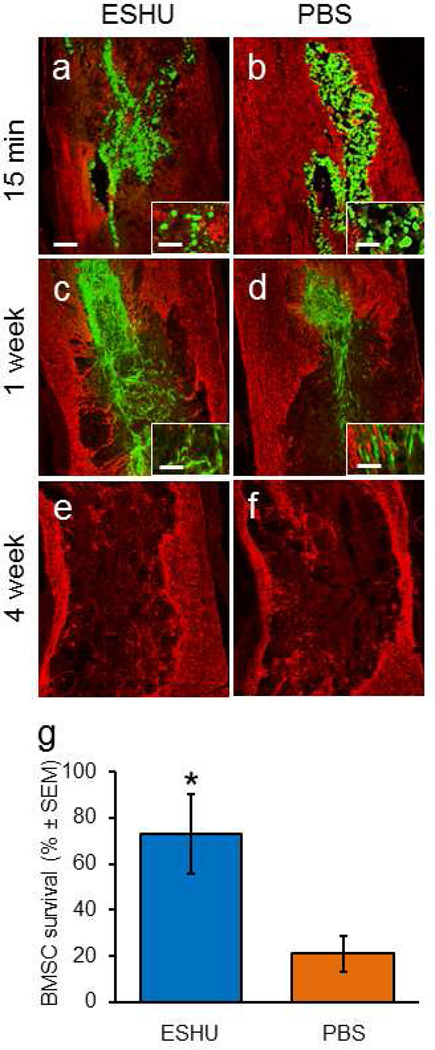
We investigated whether ESHU protects transplanted BMSCs from death in damaged nervous tissue using a spinal cord contusion model. At 15 min post-injection, rounded BMSCs were present in the injury when mixed in either ESHU (Fig. 2a) or PBS (Fig. 2b). One week post-injection, in both groups many spindle-shaped cells were also found (Figs. 2c, 2d). At 4 and 6 weeks, hardly any cells could be found in or near the contusion site after injection of BMSCs in ESHU (Fig. 2e) or in PBS (Fig. 2f). The temporal morphological profile of the grafted cells is in accordance with earlier observations [15]. Also, GFAP staining (Figs. 2a–f) was similar as previously described [7,15]. We found that 73 ± 17 % (SEM; n = 6) of transplanted cells had survived in ESHU while 21 ± 8 % (SEM; n = 6) survived in PBS (Fig. 2g), which represents a significant (p < 0.05) 3.5-fold increase in survival in ESHU compared with PBS. At four weeks post-injection, in both groups < 1 % of the cells has survived in the injury site. The data show that ESHU does not affect BMSC transplant morphology and protects against early death resulting in increased transplant presence at one week post-injection.
Fig. 2. ESHU improves the survival of bone marrow stromal cell transplants in a spinal cord contusion.
Fifteen minutes after injection, transplanted cells (green) occupies most of the contusion regardless whether they were suspended in ESHU (a) or phosphate-buffered saline (PBS) (b). Staining for glial-fibrillary acidic protein (GFAP, red) was used to outline the contusion. Transplanted cells were mostly rounded in ESHU (insert panel a) and PBS (insert panel b). One week after injection, the transplant occupies only part of the contusion site but more so when suspended in ESHU (c) than PBS (d). The transplanted cells at this time point were mostly elongated with bipolar morphologies in ESHU (insert panel c) and PBS (insert panel d). Four weeks after injection, hardly any cells were detected in the contusion when injected with ESHU (e) or PBS (f). Similar results were observed after six weeks (not shown). (g) More transplanted cells survive in the contusion site the first week after injection when suspended in ESHU than PBS. Survival rate was measured against total number of injected cells. Error bars in bar graph display standard error of the mean (SEM). Asterisk = p < 0.05. Bar in a = 350 µm in a–d and 30 µm in inserts.
3.2. Effect of BMSC transplant survival on neuroprotection
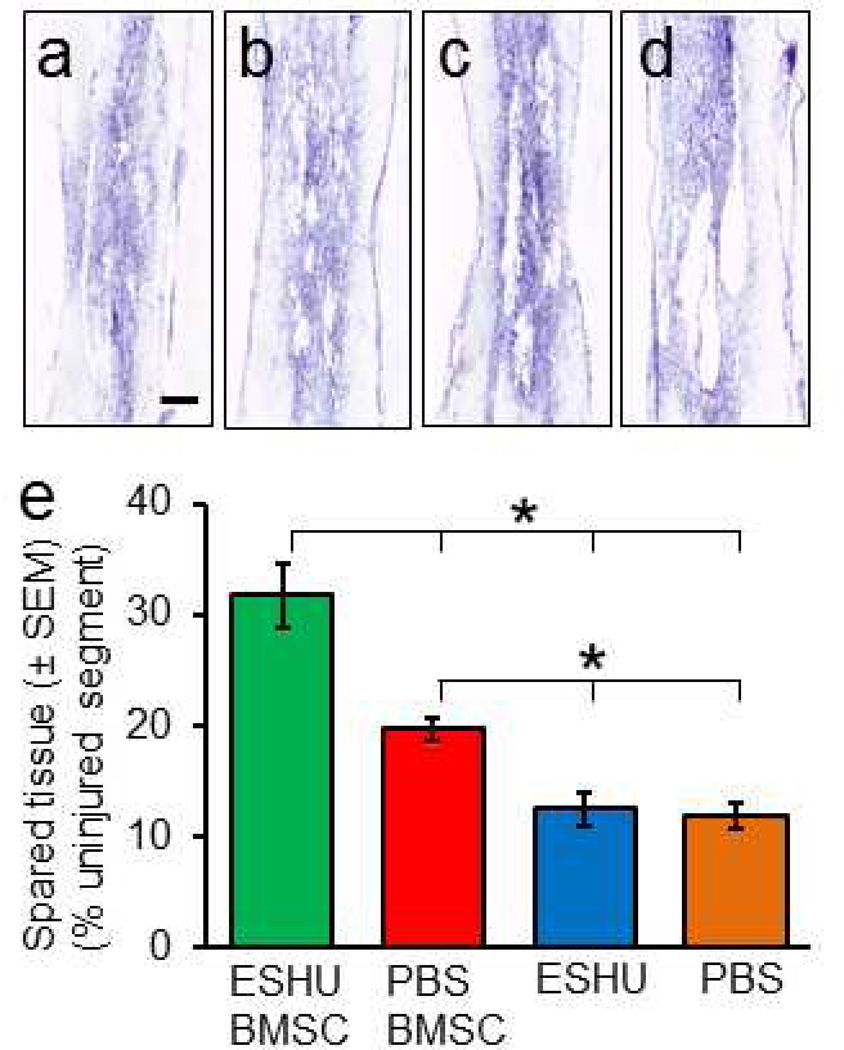
Because BMSC transplant survival is associated with neuroprotection [15] we assessed whether ESHU-promoted transplant survival rendered enhanced tissue sparing (Fig. 3a–d). The results demonstrated that the volume of spared tissue in rats with the transplant in ESHU is 66 % larger (p < 0.05; n = 6/group) than in rats with the transplant in PBS at four weeks post-transplantation (Fig. 3e). ESHU only had no effect on spared tissue volume in the damaged area (Fig. 3e). The data suggest that increased survival of intraneural BMSC transplants early after injection enhances neuroprotection of nervous tissue.
Fig. 3. ESHU augments neuroprotection by bone marrow stromal cell transplants in the contused spinal cord.
Damage and loss of nervous tissue was observed at four weeks after a bone marrow stromal cell (BMSC) transplant in ESHU (a) or PBS (b) or ESHU (c) or PBS (d) alone into the contused spinal cord. (e) Spared tissue volume was larger with the transplant in ESHU compared with all other groups. Error bars in bar graph display standard error of the mean (SEM). Asterisks = p < 0.05. Bar in a = 600 µm in a–d.
3.3. Effect of BMSC transplant survival on motor recovery
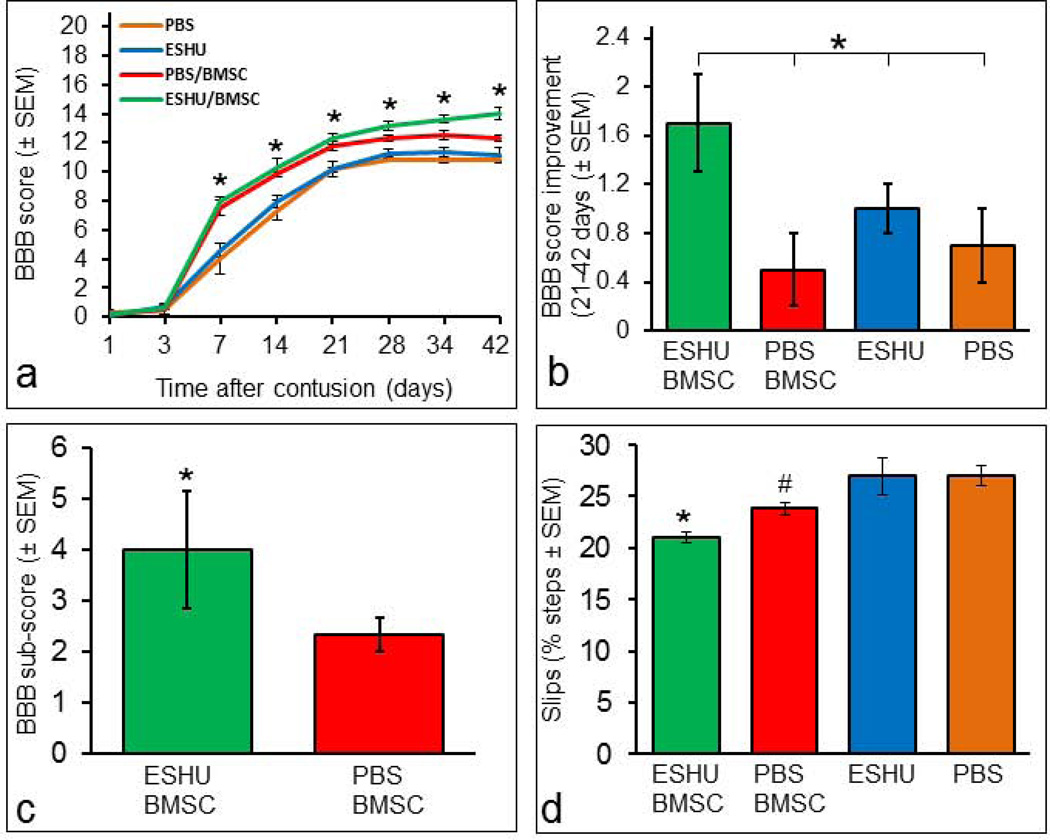
After spinal cord contusion, motor performance depends in part on the amount of nervous tissue at the injury site [7]. We examined whether augmented neuroprotection by BMSC transplants with ESHU-enhanced survival affected motor function recovery. We found that rats with the transplant in ESHU performed significantly (p<0.05; n = 10/group) better in overground walking than rats with BMSC in PBS at 4–6 weeks post-injury (Fig. 4a). Rats with BMSC in ESHU performed better than rats with ESHU or PBS alone at 1–6 weeks post-injury and rats with BMSCs in PBS walked better overground than rats with ESHU or PBS alone only at 1–3 weeks post-injury (Fig. 4a). At 6 weeks, rats with BMSCs in ESHU showed consistent (>95%) weight-supported plantar steps with frontlimb-hindlimb coordination. The control transplanted rats were less consistent (50–95%) making such steps, whereas rats with ESHU or PBS only were less consistent and lacked frontlimb-hindlimb coordination. During the 4th–6th week after injection, overground walking was increased by 1.7 ± 0.4 points on the BBB scale in rats with the transplant in ESHU which was significantly (p < 0.05; n = 10/group) higher than the increase in the other groups (Fig. 4b). Higher motor functions of the hindlimbs were significantly improved (p < 0.05; n = 10/group) by 74% in rats with BMSC in ESHU compared with BMSCs in PBS at 6 weeks post-injury (Fig. 4c). Sensorimotor function was significantly increased (p < 0.05; n = 10/group) in rats with the transplant in ESHU compared with the other three groups at 6 weeks post-injury (Fig. 4d). Rats receiving the transplant in PBS had significantly improved sensorimotor function compared with rats with ESHU or PBS alone (Fig. 4d).
Fig. 4. ESHU leads to enhancement of motor function recovery by a bone marrow stromal cell transplant in the contused spinal cord.
(a) Overground walking ability was significantly improved in rats with a bone marrow stromal cell (BMSC) transplant in ESHU compared with BMSC in PBS at 4–6 weeks post-injury. Rats with BMSC in ESHU performed better than rats with ESHU or PBS alone at 1–6 weeks post-injury. Rats with BMSCs in PBS walked better overground than rats with ESHU or PBS alone only at 1–3 weeks post-injury. (b) Improvement in overground walking ability during the 4th–6th week post-injury was significantly improved in rats with a transplant in ESHU compared with all other groups. (c) Improved higher motor functions in rats with BMSCs in ESHU compared with PBS at 6 weeks post-injury. (d) Improved sensorimotor recovery in rats with the transplant in ESHU over all other groups and in rats with BMSCs in PBS over the control groups without BMSCs at 6 weeks post-injury. Error bars in bar graph display standard error of the mean (SEM). Asterisk = p < 0.05. Number sign = p < 0.05.
3.4. Inflammatory response
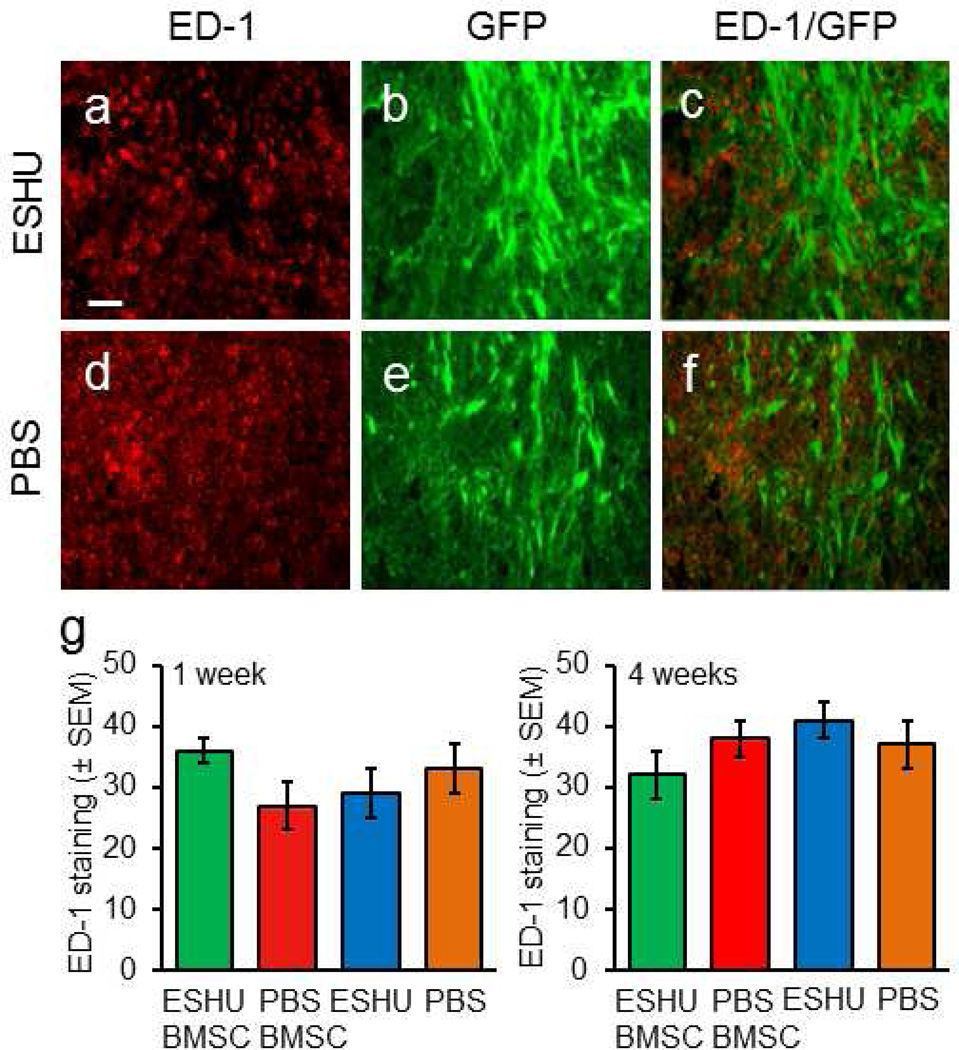
Macrophages invade damaged nervous tissue and contribute to cell death [19]. ESHU breakdown products could carry negative charges and so affect macrophage presence [44]. We tested the possible influence of macrophages on ESHU’s protective capacity by assessing their presence in the injury after injection of BMSCs in ESHU (Fig. 5a–c) or PBS (Fig. 5d–f), or ESHU or PBS only. The results demonstrated similar macrophage presence between all groups at one (Fig. 5g) and four (Fig. 5h) weeks post-injection, suggesting that ESHU is non-immunogenic. The data indicate that macrophages are not implicated in ESHU-mediated transplant survival.
Fig. 5. ESHU does not affect the injury-induced macrophage response.
Macrophages (ED-1+, red) were found in the contusion with transplanted bone marrow stromal cell (green) in ESHU (a–c) or PBS (d–f). (g) ESHU as a transplant matrix or alone did not affect the presence of macrophages in the contusion at one and four weeks after transplantation. Error bars in bar graphs display standard error of the mean (SEM). Bar in a = 15 µm in a–f.
3.5. Oxidative stress-mediated cell death in vitro
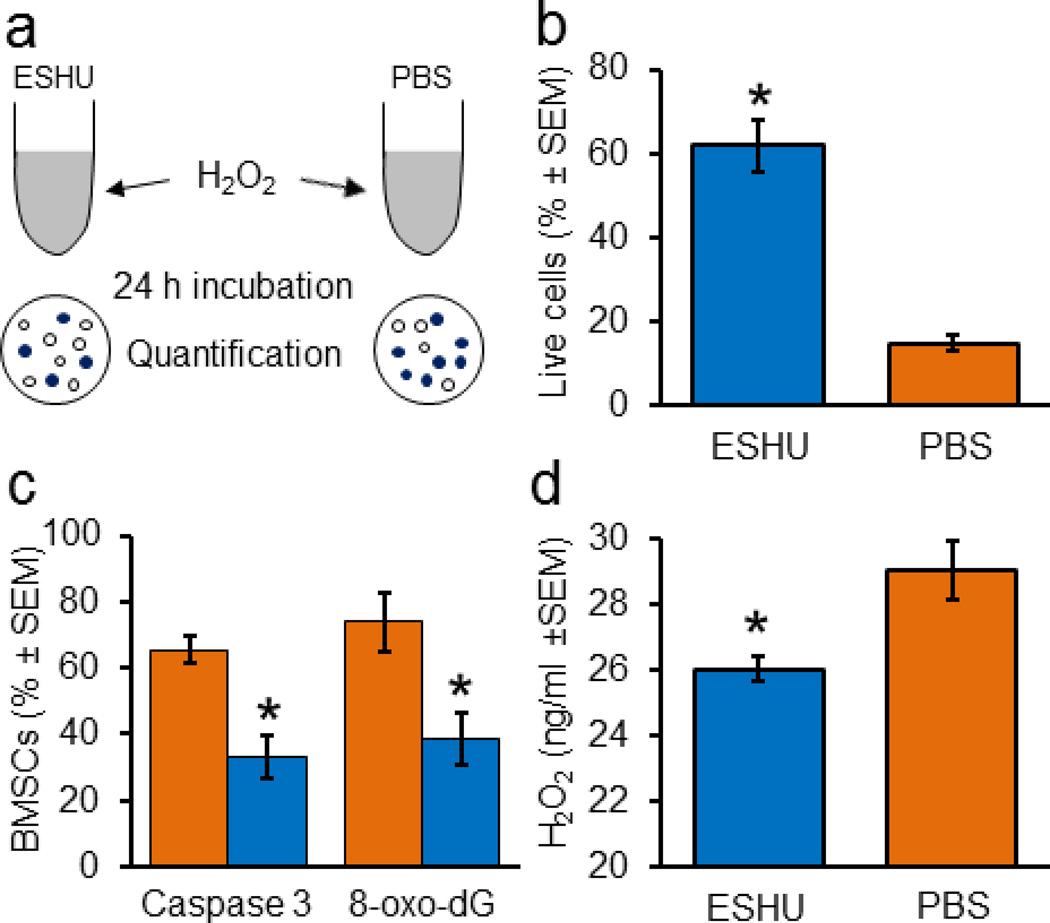
We tested whether ESHU protects BMSCs from H2O2-mediated death in vitro (Fig. 6a) and found that survival was increased four-fold in ESHU (62 ± 6%, SEM; n = 9) compared with PBS (15 ± 2%, p < 0.05, n = 9; Fig. 6b). ESHU resulted in an almost two-fold decrease in BMSCs positive for caspase 3 or 8-oxo-dG (p < 0.05, n = 9; Fig. 6c). To explore ESHU’s cell protective effect we assessed its proficiency in scavenging H2O2. We found that ESHU decreased the amount of H2O2 by 10 % (3 ng/ml) in 30 min compared with PBS (p < 0.05, n = 9; Fig. 6d), suggesting ESHU-mediated oxidation of H2O2. The data show that ESHU scavenges H2O2 and protects against oxidative stress-mediated cell death.
Fig. 6. ESHU protects bone marrow stromal cells in suspension and scavenges hydrogen peroxide in vitro.
(a) Schematic representation of in vitro assay of ESHU’s ability to protect bone marrow stromal cells from hydrogen peroxide (H2O2)-induced death. (b) Cell survival from H2O2-induced oxidative stress is better in ESHU than phosphate-buffered saline (PBS). (c) Fewer BMSCs positive for caspase 3 and 8-oxo-dG with ESHU (blue) than with PBS (orange). (d) ESHU scavenges H2O2 in PBS. Error bars in bar graphs display standard error of the mean (SEM). Asterisks = p < 0.05.
4. Discussion
We show that ESHU, a synthetic injectable reverse thermal gel, protects transplanted BMSCs from death thereby prolonging their presence in damaged nervous tissue and leading to enhanced tissue sparing accompanied by improved motor function recovery. Our study demonstrates that improved intraneural BMSC transplant survival enhances their effects on repair, which may have widespread impact on BMSC-based therapies for tissue repair.
The inclusion of ESHU enhanced BMSC presence in a contusion in the adult rat spinal cord. This effect was transient possibly due to degradation of ESHU [25]. When mixed in culture medium, BMSC presence was significantly lower in the contusion site [15–18,47]. Possibly ESHU retains BMSCs better in the contusion (i.e., the site of injection) compared with culture medium, resulting in the higher numbers. Previously we showed that about 2.4% of GFP-expressing BMSCs in culture medium leaked or migrated away from a contusion [15]. Therefore, the present results suggest that ESHU protects transplants in the contused spinal cord tissue during the first week postinjection, which is a critical time period for BMSC-mediated neuroprotection [15].
The ESHU-mediated increase in BMSC survival in the contused spinal cord resulted in anatomical (tissue sparing) and functional (motor/sensorimotor) improvements. Spared tissue volume was not affected by ESHU alone, indicating that the neuroprotection was elicited by the increased survival of the transplant. Previously, we showed that the neuroprotective effects of BMSC transplants are greatest during the first week post-injury [15]. The current finding demonstrates that the efficacy of an intraspinal BMSC transplant to elicit neuroprotection depends on its degree of survival and that increased survival leads to increased spared tissue volumes. Neuroprotection by intraneural BMSC transplants is thought to result from paracrine effects [11–14]. Our finding that increased transplant survival results in increased tissue sparing may imply that the magnitude of neuroprotection elicited by the transplants depends on the concentration and/or availability of secreted growth factors mediating paracrine actions.
Overground walking and higher motor and sensorimotor functions of the paralyzed hindlimbs were further improved in rats that received BMSCs in ESHU. The improvements in overground walking were particularly evident during the second half of the 6-week period. It was demonstrated that BMSC transplant-mediated improvements in motor function recovery after spinal cord [4–7,18] and brain [50–52] injury are correlated with the amount of spared nervous tissue [7,53]. Thus, in our study, neuroprotection elicited by BMSC transplants with ESHU-increased survival likely contributes to the observed improved motor function recovery. At present the anatomical correlates underlying improved motor recovery are not completely known but may involve increased numbers of descending axons conducting the actual motor activity [7] and/or increased myelination providing better signal conduction [53,54], which both could result from neuroprotection.
In search of potential mechanisms underlying ESHU-mediated BMSC protection, we assessed macrophage presence in the contusion. Macrophages are naturally present in damaged nervous tissue and contribute to the death of neural and transplanted cells [16,17,19]. Oxidation of ESHU could lead to carboxylates whose negative charges might inhibit adhesion of macrophages [44] thereby limiting their contribution to cell death. We found that the number of macrophages in the contusion was similar with or without the presence of ESHU. BMSCs are hypoimmunogenic, lacking MHC class II and co-stimulatory molecules for effector T cell induction [48], and suppress T cell proliferation [49]; thus the adaptive immune response is unlikely to be largely involved in allogeneic BMSC death. Our data suggest that the protective effects of ESHU result from direct effects on the transplant rather than indirect effects involving macrophages.
Another possible mechanism underlying ESHU-promoted BMSC survival is antioxidation. Reactive oxygen species (ROS) accumulate rapidly in damaged nervous tissue and induce oxidative stress leading to cell death [20–24]. We used H2O2 to determine whether ESHU has the ability to scavenge ROS and thus mediate antioxidant effects. H2O2 is amply present in damaged nervous tissue [45]. We found that a 16 % ESHU solution removed 3 ng H2O2 in a 30 min time period. Assuming continuous activity at 3 ng/30 min, ESHU removed ~ 20% of added H2O2 in our in vitro assay of ESHU’s ability to protect BMSCs, which elicited a 47 % increase in their survival relative to PBS. The ability to scavenge ROS may be exerted through its urethane groups [28,29]. Possibly ESHU’s antioxidant effects may be increased with higher concentrations [46]. Our observations point at antioxidation as a potential mechanism of ESHU-promoted BMSC transplant survival. ROS are known to contribute to transplanted cell death [23,24]. Future studies need to define molecular factors in ESHU’s protective actions and whether the protection by ESHU in vivo is concentration-dependent.
5. Conclusions
We demonstrate that the reparative effects of a BMSC transplant are enhanced by promoting their survival. This finding critically impacts current and future BMSC-based therapies for the central nervous system. ESHU’s ability to gel at body temperature allows for injection (i.e., minimally invasive) into closed injuries. Besides reducing oxidative stress and serving as a matrix for cells, ESHU can also be used to deliver drugs and/or functionalized with bioactive molecules to affect targeted biological events. These benefits render ESHU an important candidate in future therapies for the traumatized or degenerated nervous system. Furthermore, because oxidative stress is part of many diseases where BMSCs can be effective, such as cardiac myopathy and peripheral arterial disease, ESHU may have wide therapeutic relevance.
Acknowledgements
Financial support was provided by LUMC (grant # 30229/5000) to GJR, NIH 5T32EB003392-07 (BR), NSF #DMR-1206589 (YW), and the Department of Physical Medicine and Rehabilitation at the University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112(3):523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 2.Ruff CA, Fehlings MG. Neural stem cells in regenerative medicine: bridging the gap. Panminerva Med. 2010;52(2):125–147. [PubMed] [Google Scholar]
- 3.Forraz N, Wright K, Jurga M, McGuckin C. Experimental therapies for repair of the central nervous system: stem cells and tissue engineering. J Tissue Eng Regen Med. 2013;7(7):523–536. doi: 10.1002/term.552. [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99(4):2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, et al. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20(2):278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood A, Lu D, Wang L, Chopp MJ. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. Neurotrauma. 2002;19(12):1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- 7.Ritfeld GJ, Nandoe Tewarie RD, Vajn K, Rahiem ST, Hurtado A, Roos RAC, et al. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. 2012;21(7):1561–1575. doi: 10.3727/096368912X640484. [DOI] [PubMed] [Google Scholar]
- 8.Tschöpe C, Miteva K, Schultheiss HP, Linthout SV. Mesenchymal stromal cells: a promising cell source for the treatment of inflammatory cardiomyopathy. Curr Pharm Des. 2011;17(30):3295–3307. doi: 10.2174/138161211797904136. [DOI] [PubMed] [Google Scholar]
- 9.Ichim TE, Alexandrescu DT, Solano F, Lara F, Campion Rde N, Paris E, et al. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010;260(2):75–82. doi: 10.1016/j.cellimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Zou JP, Huang S, Peng Y, Liu HW, Cheng B, Fu XB, et al. Mesenchymal stem cells/multipotent mesenchymal stromal cells (MSCs): potential role in healing cutaneous chronic wounds. Int J Low Extrem Wounds. 2012;11(4):244–253. doi: 10.1177/1534734612463935. [DOI] [PubMed] [Google Scholar]
- 11.Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in regenerative medicine? Bone Marrow Transplant. 201;47(2):164–171. doi: 10.1038/bmt.2011.81. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 204;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 13.Caplan Al. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawryluk GW, Mothe A, Wang J, Wang S, Tator C, Fehlings MG. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21(12):2222–2228. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandoe Tewarie RD, Hurtado A, Ritfeld GJ, Rahiem ST, Wendell DF, Barroso MM, et al. Bone marrow stromal cells elicit tissue sparing after acute but not delayed transplantation into the contused adult rat thoracic spinal cord. J Neurotrauma. 2009;26(12):2313–2322. doi: 10.1089/neu.2009.0987. [DOI] [PubMed] [Google Scholar]
- 16.Ritfeld GJ, Nandoe Tewarie RD, Rahiem ST, Hurtado A, Roos RA, Grotenhuis A, et al. Reducing macrophages to improve bone marrow stromal cell survival in the contused spinal cord. Neuroreport. 2010;21(3):221–226. doi: 10.1097/WNR.0b013e32833677cd. [DOI] [PubMed] [Google Scholar]
- 17.Swanger SA, Neuhuber B, Himes BT, Bakshi A, Fischer I. Analysis of allogeneic and syngeneic bone marrow stromal cell graft survival in the spinal cord. Cell Transplant. 2005;14(10):775–786. doi: 10.3727/000000005783982594. [DOI] [PubMed] [Google Scholar]
- 18.Parr AM, Kulbatski I, Wang XH, Keating A, Tator CH. Fate of transplanted adult neural stem/progenitor cells and bone marrow-derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol. 2008;70(6):600–607. doi: 10.1016/j.surneu.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Kigerl KA, Ankeny DP, Garg SK, Wei P, Guan Z, Lai W, et al. System x(c)(−) regulates microglia and macrophage glutamate excitotoxicity in vivo. Exp Neurol. 2012;233(1):333–341. doi: 10.1016/j.expneurol.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18(6):667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong Q, Lin CL. Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life SCI. 2010;67(11):1817–1829. doi: 10.1007/s00018-010-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 2008;107(3):712–721. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siriphorn A, Chompoopong S, Floyd CL. 17beta-estradiol protects Schwann cells against H2O2-induced cytotoxicity and increases transplanted Schwann cell survival in a cervical hemicontusion spinal cord injury model. J Neurochem. 2010;115(4):864–872. doi: 10.1111/j.1471-4159.2010.06770.x. [DOI] [PubMed] [Google Scholar]
- 24.Syntichaki P, Tavernarakis N. The biochemistry of neuronal necrosis: rogue biology? Nat. Rev. Neurosci. 2003;4:672–684. doi: 10.1038/nrn1174. [DOI] [PubMed] [Google Scholar]
- 25.Park D, Wu W, Wang Y. A functionalizable reverse thermal gel based on a polyurethane/PEG block copolymer. Biomaterials. 2011;32(3):777–786. doi: 10.1016/j.biomaterials.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park D, Shah V, Rauck BM, Friberg TR, Wang Y. An anti-angiogenic reverse thermal gel as a drug-delivery system for age-related wet macular degeneration. Macromol Biosci. 2013;13(4):464–469. doi: 10.1002/mabi.201200384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friberg TR, Park D, Shah V, Rauck B, Medina C, Wang Y. Biodegradable and injectable in situ gelling solution controls the release of bevacizumab (Avastin) in vivo. ARVO Annual Meeting. 2012 [Google Scholar]
- 28.Christenson EM, Anderson JM, Hiltner A. Oxidative mechanisms of poly(carbonate urethane) and poly(ether urethane) biodegradation: in vivo and in vitro correlations. J Biomed Mater Res A. 2004;70(2):245–255. doi: 10.1002/jbm.a.30067. [DOI] [PubMed] [Google Scholar]
- 29.McBane JE, Santerre JP, Labow RS. The interaction between hydrolytic and oxidative pathways in macrophage-mediated polyurethane degradation. J Biomed Mater Res A. 2007;82(4):984–994. doi: 10.1002/jbm.a.31263. [DOI] [PubMed] [Google Scholar]
- 30.Oudega M. Schwann cell and olfactory ensheathing cell implantation for repair of the contused spinal cord. Acta Physiol (Oxf) 2007;189(2):181–189. doi: 10.1111/j.1748-1716.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 31.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95(7):3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181(1):67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 33.Seshi B, Kumar S, Sellers D. Human bone marrow stromal cell: coexpression of markers specific for multiple mesenchymal cell lineages. Blood Cells Mol Dis. 2000;26(3):234–246. doi: 10.1006/bcmd.2000.0301. [DOI] [PubMed] [Google Scholar]
- 34.Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med. 1999;22(2):119–124. doi: 10.1080/10790268.1999.11719557. [DOI] [PubMed] [Google Scholar]
- 35.Bunge R, Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 36.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 37.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 38.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 39.Lankhorst AJ, Verzijl MR, Hamers FPT. Experimental spinal cord contusion injury: comparison of different outcome parameters. Neurosci Res Comm. 1999;24:135–148. [Google Scholar]
- 40.Joosten EA, Veldhuis WB, Hamers FPT. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J Neurosci Res. 2004;77(1):127–142. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 41.Bolton DA, Tse AD, Ballermann M, Misiaszek JE, Fouad K. Task specific adaptations in rat locomotion: runway versus horizontal ladder. Behav Brain Res. 2006;168(2):272–279. doi: 10.1016/j.bbr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Kunkel-Bagden E, Dai HN, Bregman BS. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp Neurol. 1992;116:40–51. doi: 10.1016/0014-4886(92)90174-o. [DOI] [PubMed] [Google Scholar]
- 43.Boyce RW, Dorph-Petersen KA, Lyck L, Gundersen HJ. Design-based stereology: introduction to basic concepts and practical approaches for estimation of cell number. Toxicol Pathol. 2010;38(7):1011–1025. doi: 10.1177/0192623310385140. [DOI] [PubMed] [Google Scholar]
- 44.Brodbeck WG, Nakayama Y, Matsuda T, Colton E, Ziats NP, Anderson JM. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18(6):311–319. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 45.Moon YJ, Lee JY, Oh MS, Pak YK, Park KS, Oh TH, et al. Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J Neurosci Res. 2012;90(1):243–256. doi: 10.1002/jnr.22734. [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Shan Y, Liu D. Stability, disposition, and penetration of catalytic antioxidants Mn-porphyrin and Mn-salen and of methylprednisolone in spinal cord injury. Cent Nerv Syst Agents Med Chem. 2012;12(2):122–130. doi: 10.2174/187152412800792742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill CE, Hurtado A, Blits B, Bahr BA, Wood PM, Bartlett Bunge M, et al. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur J Neurosci. 2007;26(6):1433–1445. doi: 10.1111/j.1460-9568.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- 48.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 49.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 50.Kawabori M, Kuroda S, Ito M, Shichinohe H, Houkin K, Kuge Y, et al. Timing and cell dose determine therapeutic effects of bone marrow stromal cell transplantation in rat model of cerebral infarct. Neuropathology. 2013;33(2):140–148. doi: 10.1111/j.1440-1789.2012.01335.x. [DOI] [PubMed] [Google Scholar]
- 51.Yasuhara T, Matsukawa N, Hara K, Maki M, Ali MM, Yu SJ, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. 2009;18(10):1501–1514. doi: 10.1089/scd.2009.0011. [DOI] [PubMed] [Google Scholar]
- 52.Bonilla C, Zurita M, Otero L, Aguayo C, Rico MA, Vaquero J. The severity of brain damage determines bone marrow stromal cell therapy efficacy in a traumatic brain injury model. J Trauma Acute Care Surg. 2012;72(5):1203–1212. doi: 10.1097/TA.0b013e318248bdcf. [DOI] [PubMed] [Google Scholar]
- 53.Zhang YJ, Zhang W, Lin CG, Ding Y, Huang SF, Wu JL, et al. Neurotrophin-3 gene modified mesenchymal stem cells promote remyelination and functional recovery in the demyelinated spinal cord of rats. J Neurol Sci. 2012;313(1–2):64–74. doi: 10.1016/j.jns.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 54.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26(13):3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]