Abstract
DYT1 dystonia is an inherited disease linked to mutation in the TOR1A gene encoding for the protein torsinA. Although the mechanism by which this genetic alteration leads to dystonia is unclear, multiple lines of clinical evidence suggest a link between dystonia and a reduced dopamine D2 receptor (D2R) availability. Based on this evidence, herein we carried out a comprehensive analysis of electrophysiological, behavioral and signaling correlates of D2R transmission in transgenic mice with the DYT1 dystonia mutation. Electrophysiological recordings from nigral dopaminergic neurons showed a normal responsiveness to D2-autoreceptor function. Conversely, postsynaptic D2R function in hMT mice was impaired, as suggested by the inability of a D2R agonist to re-establish normal corticostriatal synaptic plasticity and supported by the reduced sensitivity to haloperidol-induced catalepsy. Although an in situ hybridization analysis showed normal D1R and D2R mRNA expression levels in the striata of hMT mice, we found a significant decrease of D2R protein, coupled to a reduced ability of D2Rs to activate their cognate Go/i proteins.
Of relevance, we found that pharmacological blockade of adenosine A2A receptors (A2ARs) fully restored the impairment of synaptic plasticity observed in hMT mice.
Together, our findings demonstrate an important link between torsinA mutation and D2R dysfunction and suggest that A2AR antagonism is able to counteract the deficit in D2R-mediated transmission observed in mutant mice, opening new perspectives for the treatment of this movement disorder.
Keywords: Dystonia, D2 dopamine receptor, Adenosine
Introduction
Most cases of early-onset generalized torsion dystonia (DYT1) dystonia are caused by a GAG deletion in the TOR1A gene, coding for the protein torsinA (Ozelius et al., 1997). On a cellular level, the function of torsinA is still poorly understood, although it appears to perform chaperone-like functions and participate in membrane protein trafficking, vesicle fusion and secretory processing (for rev. see Breakefield et al., 2008; Tanabe et al., 2009). Neuropathological studies in DYT1 dystonia have been limited, and no explicit evidence for neurodegeneration has been reported, suggesting that the DYT1 mutation produces dystonia through neurochemical abnormalities within the basal ganglia, and more specifically within the striatum (Todd and Perlmutter 1998; Rostasy et al., 2003, Breakefield et al., 2008). In such a context, dopamine (DA) is the neurotransmitter most clearly linked to human dystonia (Augood et al., 2002, 2004; Perlmutter and Mink, 2004). Several imaging studies have identified DA D2 receptor (D2R) alterations in patients with primary dystonia (Perlmutter et al., 1997; Furukawa et al., 2000; Asanuma et al., 2005). Notably, recent PET studies demonstrate a significant reduction of D2R availability in the caudate/putamen of both manifesting and non-manifesting DYT1 mutation carriers (Carbon et al., 2009), suggesting that these alterations might represent a dystonic endophenotype of the disease.
Aberrant DA neurotransmission is evident also in mouse models of DYT1 dystonia. Mice overexpressing mutant torsinA display impaired electrophysiological responses to D2R activation (Pisani et al., 2006; Sciamanna et al., 2009), an increased DA turnover (Zhao et al., 2008) and an altered amphetamine-induced DA release (Balcioglu et al., 2007). These observations point directly to a decreased D2R function, although this has never been investigated in detail at cellular level.
Thus, to address if there is indeed a physiologically relevant defect in medium spiny neurons (MSNs) D2R function, we carried out an analysis of electrophysiological, behavioral and signaling responses in mice with the DYT1 mutation. Of note, extensive biochemical and physiological data demonstrate that adenosine A2A receptors (A2ARs) are co-localized on the same MSNs expressing D2Rs, where they counteract the activity of this latter class of receptors (Ferrè et al., 1997; Shen et al., 2008). This simple picture poses an obvious question. If D2R function is defective, can it be overcome by blocking A2ARs?
Our results demonstrate that overexpression of mutant torsinA produces severe alterations of D2R function at striatal postsynaptic sites without affecting dopaminergic neuron D2 autoreceptor function, further supporting the specific role played by striatal D2Rs in the pathophysiology of dystonia. More importantly, we show that pharmacological blockade of A2ARs restores the impairment of striatal synaptic plasticity observed in mutant mice, suggesting that the deficit in D2R function can be reverted by suppressing the negative control exerted by A2ARs on D2R function, and indicating A2ARs as a potential target for developing novel pharmacotherapies for dystonia.
Materials and methods
Animals
Experiments were carried out according to both EC and Italian guidelines (86/609/EEC; D.Lvo 116/1992, respectively) and were approved by the University of Rome “Tor Vergata” (n. 153/2001A). Transgenic mice (8-10 weeks old) were generated as previously described (Sharma et al., 2005), and displayed comparable increases of torsinA protein in the striatum versus their non transgenic littermates (Fig. S1). Animals were housed in a maximum of 5 per box in plexiglas cages (29 × 17.5 × 12.5 cm) and kept on constant temperature (22 ± 1 °C), 12 h light/dark cycle and food and water available ad libitum. Experiments were performed during the light phase and according to protocols approved by the veterinary department of the Italian Ministry of Health and in line with the ethical and safety rules and guidelines for the use of animals in biomedical research. All efforts were made to minimize the animals’ suffering.
Behavior
Novelty-induced exploratory test
Spontaneous motor activity was measured by novelty-induced exploratory test (Usiello et al., 2000) in NT, hWT and hMT naïve mice. Animals were individually placed into the experimental cages (35 × 25 × 30 cm), and videotaped for 60′ by using a computerized video tracking system (Videotrack, Viewpoint S.A., Champagne au Mont d'Or, France). Subsequently, an experimenter blinded to genotype has manually analyzed all movies. The total number of sector crossing (crossing the 6 squares in which the floor of the testing cage was subdivided on the monitor), as index of horizontal locomotor activity, was scored every 10 min for a total of 6 intervals.
Drugs and reagents
(±)-quinpirole dihydrochloride, haloperidol, CGS21680, dopamine (3-hydroxytyramine hydrochloride) and guanosine 5′-diphosphate sodium salt (GDP) were obtained from Sigma (St. Louis, MO), whereas KW6002 was kindly provided by Kiowa pharmaceutics Japan. All compounds were dissolved in saline solution, except for haloperidol that was prepared in 10% acetic acid in saline and the pH was brought to 6.0 with 1 M NaOH. All drugs were injected intraperitoneally (i.p.) in a volume of 10 ml/Kg. [35S] guanosine 5′-([gamma]-thio)triphosphate ([35S]GTP[gamma]S, 1250 Ci/mmol) was obtained from GE Healthcare, Amersham (Sweden).
Motor responses to drugs
Catalepsy
Mice were gently handled 5 min per day for a week before experiment. On testing day, 60 min before vehicle or haloperidol (1.5 or 2.5 mg/kg) injection, animals were placed one per cage to adapt to the new environment and then catalepsy time (s) was measured 120 min after injection. The procedures consist gently placing mice front limbs over a 4 cm high horizontal bar and then measuring the time each mouse remained in this position, with the limbs completely immobile for a maximum of 180 s.
Locomotor activity
Mice were gently handled 5 min per day for a week before experiment. On the testing day, animals were injected with vehicle or quinpirole (0.35 mg/kg) and immediately exposed to the experimental cage for 30 min. Another group of mice were administered with vehicle or CGS 21680 (0.25 or 0.5 mg/kg) and immediately exposed to the experimental cage for 30 min. Other naïve mice were treated with vehicle or KW6002 (1.5 or 3 mg/kg), after 1 h of cage habituation, and videotaped for 60 min.
Behavioral procedures were performed according to Usiello et al. (2000). Locomotor activity of animals (expressed as manually counted number of sector crossing) in the experimental cage (35 × 25 × 30 cm) was recorded by using a videocamera.
Statistical analyses
Locomotor activity in novelty-induced exploration test was analyzed using two-way (genotype × time) ANOVA with repeated measures. Motor responses to drugs were evaluated by twoway (genotype x treatment) ANOVA. Fischer's post-hoc comparisons were used when required. A significance level of p < 0.05 was accepted as statistically significant. All measures are expressed as mean ± standard error of the mean (SEM). All statistical analyses were performed with StatView software (version 5.0.1.0; SAS Institute, Cary, NC).
[35S]GTPγS binding assay
Striata from NT, hWT and hMT mice were sonicated in HEPES buffer (100 mM NaCl, 7 mM MgCl2, 1 mM EDTA, 20 mM HEPES, pH 7.6). Membranes were collected by centrifugation at 47,000 ×g for 20 min at 4 °C and the supernatant was discarded. The pellet was resuspended in HEPES buffer and centrifuged as described above. This procedure was repeated three times. The final pellet was resuspended in HEPES buffer, the protein concentration was determined using the BCA assay kit (Pierce Europe, Oud Beijerland, The Netherlands) and the membranes were used at a final protein concentration of 25 mg/ml.
Binding of [35S]GTPγS to striatal membranes was carried out in incubation buffer (HEPES buffer including 1 mM DTT) as described (Rinken et al., 1999). In brief, membranes were incubated with 0.1-0.2 nM [35S]GTPγS, 100 μM GDP and dopamine (DA) for 90 min at 30 °C. The reaction was terminated by rapid filtration through glass-fiber filters (GF/C, Whatman Int. Ltd., Maidstone, UK) and the filters were washed three times with 5 ml ice-cold buffer, containing 20 mM HEPES and 100 mM NaCl (pH 7.6). Non-specific binding was determined in presence of 100 μM of raclopride (Sigma-Aldrich Sweden AB, Stockholm, Sweden). Binding data were analyzed by means of the nonlinear least squares regression method, using GraphPad Prism version 2b (GraphPad, San Diego, CA, USA). All values are presented as means ± S.E.M. and expressed as percent [35S]GTPγS binding compared to control (100%) (no DA). Student's t-test was used to calculate statistical significances.
Western blotting
NT, hWT and hMT mice were sacrificed by decapitation without any previous pharmacological treatment or 30′ after vehicle or haloperidol (0.5 mg/kg) injection. The heads of the animals were immediately immersed in liquid nitrogen for 5 s. Brains were then removed and the striata were dissected out within 20 s on an ice-cold surface, sonicated in 1% SDS and boiled for 10 min. Aliquots (5 μl) of the homogenate were used for protein determination using a BCA (bicinchoninic acid) assay kit (Pierce Europe, Oud Beijerland, the Netherlands). Equal amounts of protein (30 μg) for each sample were loaded onto 10% polyacrylamide gels. Proteins were separated by SDS-PAGE and transferred overnight to membranes (PVDF) (Amersham Pharmacia Biotech, Uppsala, Sweden) (Towbin et al., 1979). Membranes from untreated mice were immunoblotted using antibodies against D2R (1:500, Chemicon), GRK2 (1:500), GRK3 (1:500), GRK6 (1:250), RGS9 (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-Arrestin2 (1:250, Everest Biotech, UK). Other membranes containing protein from haloperidol treated mice were immuno-blotted using antibodies against phosphoSer40-TH (Cell Signalling Technology). Detection was based on fluorescent secondary antibody binding, detected and quantitated using a Li-Cor Odyssey infrared fluorescent detection system (Li-Cor, Lincoln, NE). The levels of TH phosphoprotein were normalized for the amount of the corresponding total protein detected in the sample. Quantification of the bands intensity on autoradiographic films was achieved using a computer-assisted densitometer and the NIH Image software. In each membrane, results were normalized as percentage of the mean of controls.
NT, hWT and hMT mice were sacrificed to determine torsinA levels in the striatum. Protein extracts were obtained by homogenizing striatum in a buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.25% Na deoxycholate, 5 mM MgCl2, 0.1% SDS, 1 mM EDTA, and 1% protease inhibitor cocktail (Sigma, Milan, Italy). Crude lysates were sonicated and incubated on ice for 1 h. Samples were centrifuged at 13,000 rpm for 15 min at 4 °C and the supernatants were collected. 20 μg of striatal extract were denaturated at 98 °C for 5 min and loaded onto a (SDS) polyacrylamide gel (12%). Gels were blotted onto a PVDF membrane. Immunodetection was performed by rabbit polyclonal anti-torsinA, 1:500 overnight at 4 °C (Abcam, Cambridge, USA, cat. num. 34540) or mouse monoclonal anti-tubulin, 1:10,000 for 30 min (Sigma, cat. num. T4026) and horseradish peroxidize conjugated secondary antibodies (Bio-Rad lab., Milan, Italy), ECL-Plus reagent (GE Healthcare Europe GmbH, Milan, Italy) and the storm 840 acquisition system (GE Healthcare Europe GmbH). Quantification of the bands intensity on scanned filters was achieved by Quantity one analysis software (Bio-Rad, USA).
Electrophysiology
Slice preparation
Mice were sacrificed by cervical dislocation under ether anaesthesia. Coronal and parasagittal corticostriatal slices (250-300 μm thick) were cut with a vibratome in Krebs' solution (in mM: 126 NaCl, 2.5 KCl, 1.3 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 10 glucose, 18 NaHCO3), bubbled with 95% O2/ and 5% CO2. In parasagittal slices a knife cut was made between the striatum and the thalamus, to avoid contamination from thalamostriatal inputs (Ding et al., 2008; Martella et al., 2009). For recordings from substantia nigra, pars compacta, horizontal slices of the ventral midbrain (300 μm thick) were cut in ice-cold solution (Mercuri et al., 1995). After 30-60 min recovery, individual slices were transferred into a recording chamber (~0,5-1 ml volume), continuously superfused with oxygenated Krebs' medium, at 2.5-3 ml/min and maintained constantly at 32-33 °C.
Current-clamp recordings were performed blindly using sharp microelectrodes filled with 2 M KCl (40-60 MΩ). Signal acquisition and off-line analysis were performed with an Axoclamp 2B amplifier and pClamp9 software (Molecular Devices, USA). Nigral or striatal neurons were identified according to their electrophysiological characteristics (Lacey et al., 1987; Mercuri et al., 1995; Sciamanna et al., 2009).
Glutamatergic excitatory postsynaptic potentials (EPSPs) were evoked with a bipolar electrode placed either in the corpus callosum (coronal slices) or in the cortex (V-VI layer, parasagittal slices), in the presence of bicuculline (10 μM) to block GABAA receptors. For high-frequency stimulation (HFS, three trains: 3 sec duration, 100 Hz frequency, 20 sec intervals), stimulus intensity was raised to spike threshold level. The amplitude of EPSPs was averaged and plotted as % of the control amplitude for ~10 min pre-HFS. Magnesium (Mg2+) was removed from the perfusing solution to optimize LTP induction (LTP protocol) (Calabresi et al., 1992a). Synaptic depotentiation (SD) was induced by a low-frequency stimulation (LFS) protocol (2 Hz, 10 min), applied ~30 min after LTP induction.
Whole-cell recordings were performed from individual neurons visualized by means of IR-DIC videomicroscopy, as described (Sciamanna et al., 2009). Recordings were made with a Multiclamp 700b amplifier (Axon Instruments), using borosilicate glass pipettes (1.5 mm outer diameter, 0.86 inner diameter) pulled on a P-97 Puller (Sutter Instruments). Data were acquired with pClamp software and analyzed offline (Clampfit 10.2, Molecular Devices, USA; MiniAnalysis 6.0, Synaptosoft, USA and Prism 3.02, GraphPad software Inc., USA). Pipette resistance ranged from 2.5 to 5 MΩ. Membrane currents were continuously monitored and access resistance measured in voltage-clamp was in the range of 5–30 MΩ prior to electronic compensation (60–80% routinely used). For paired-pulse experiments and AMPA-and NMDA receptor-mediated currents, pipettes contained (mM): K+-gluconate (125), NaCl (10), CaCl2 (1.0), MgCl2 (2.0), 1,2-bis (2-aminophenoxy) ethane-N,N,N,N-tetra-acetic acid (BAPTA) (1), Hepes (10), GTP (0.3) Mg-ATP (2.0); pH adjusted to 7.3 with KOH. Cells were clamped at an holding potential (HP) of −80 mV.
Excitatory postsynaptic currents (EPSCs) were evoked in bicucul-line at 0.1 Hz. Paired-pulse facilitation was assessed by presenting two stimuli (interstimulus interval 50 ms), which evoked synaptic responses at ~50% of maximal amplitude, and measuring the ratio of the peak amplitude of the second EPSC (EPSC2), divided by the first (EPSC1).
To analyze the AMPA/NMDA ratio for EPSCs, electrodes were filled with a CsCl solution (in mM): 140 CsCl, 10 NaCl, 0.1 CaCl, 10 Hepes, 1 EGTA, 2 Mg-ATP and 0.5 Na-GTP, pH = 7.3. The AMPA-mediated EPSC component was studied at an HP of −60 mV, whereas the NMDA-mediated EPSC was analyzed by holding the cell at +40 mV. The different kinetics were used to discriminate these two synaptic components (Béïque et al., 2006).
The time of the peak current at -60 mV considered to be fully mediated by AMPA receptors was used to establish the time window for measuring the AMPA peak at +40 mV. The decay to baseline of the AMPA current at −60 mV was used to choose the time window for measurement of the NMDA current; a 5 ms measurement beginning 50 ms after stimulus artifact was used. This measurement represented the NMDA value (Beurrier et al., 2009).
AMPA- and NMDA-mediated membrane currents were induced by focal pressure ejection of 500 μM of Kainic acid and 500 μM NMDA, respectively, via a puffer pipette controlled by a Picospritzer (200 μsec; puff intensity at 3-5 psi). Experiments were performed in presence of Tetrodotoxin (TTX, 1 μM). One to six neurons per mouse were recorded. Each electrophysiological measure in the three groups of mice was obtained by pooling data from at least four different mice. One cell per slice was used for synaptic plasticity experiments.
Drugs were from Tocris Cookson (UK), except for DA and SCH 58621 from Sigma-Aldrich (Italy).
Statistical analysis
Electrophysiological results are means ± SEM. Student's t test and non-parametric Mann-Whitney test were used to compare means pre- and post-HFS/drug. Analysis of variance (ANOVA) test with a post-hoc Tukey test were performed among groups (p < 0.05; α = 0.01). For all analyses, p value <0.05 was considered statistically significant.
In situ hybridization
Radioactive in situ hybridization was performed using antisense 35S-radiolabelled riboprobes. D1R and D2R cDNA clones (Baik et al., 1998) were a kind gift from Prof. E. Borrelli. Pretreatment of slices and in situ hybridization were performed as already described (Errico et al., 2008). Following hybridization, sections were exposed to Kodak MR X-ray films for 6 days and then scanned to obtain a digital format. Quantification of the image intensity on autoradiographic films was achieved using a computer-assisted densitometer and the NIH Image software to detect density levels of D1R and D2R mRNA expression. Caudate-putamen (CPu) was divided into anterior (AP +0.98 mm from bregma, Paxinos and Franklin, 2001), medial (+0.26 mm from bregma) and posterior (−0.46 mm from bregma) levels. Nucleus Accumbens (Acb) (+1.34 mm from bregma) has also been investigated. Anatomical structures to be measured were outlined manually. Values representing the mean density of each area were obtained and adjusted to a background value, taken from corpus callosum of the same section, in order to control for slide-to-slide variability. Data were analyzed by two way (genotype × level) ANOVA with repeated measures (for CPu data) or one way (genotype) ANOVA (for Acb data) (StatView software, version 5.0.1.0; SAS Institute, Cary, NC), independently for each target.
Results
Unaltered DA D2R-mediated autoreceptor functions in hMT mice
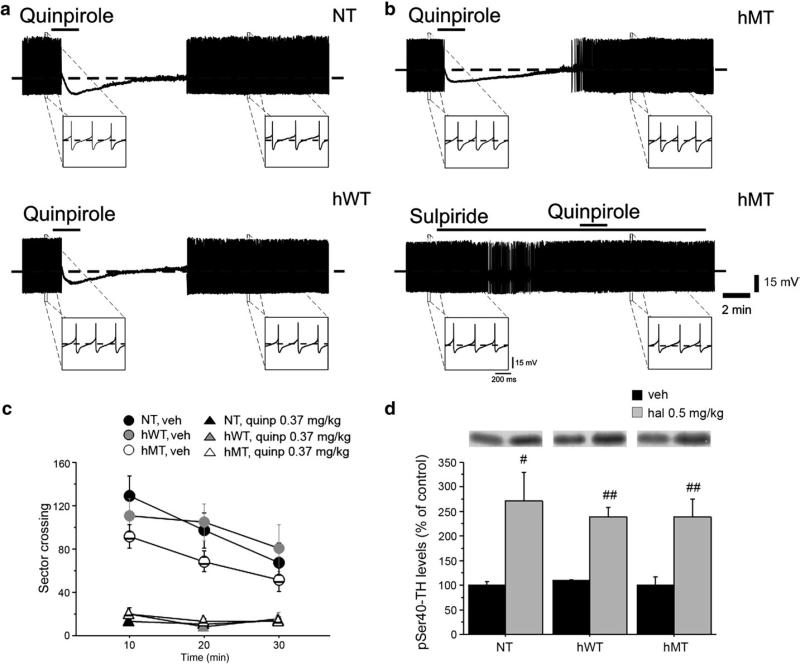
First, we explored the responsiveness of nigral neurons to D2 autoreceptor activation. Spontaneous, rhythmic firing activity of nigral neurons in hMT mice (n = 9) was comparable to that recorded from both hWT (n = 6) and NT (n = 9) mice (Fig. S2; NT: 3.9 ±1.5 Hz; hWT: 4.1 ± 1.6 Hz; hMT: 4.01 ± 1.7; p > 0.05). Similarly, DA application (100 μM, 45 sec) inhibited cell firing and hyperpolarized the cell membrane to a similar extent in slices from NT (16.4 ± 3.1 mV), hWT (14.3 ± 3 mV) or hMT mice (16.03 ± 4.2 mV) (p > 0.05) (Fig. S2). Such inhibitory effect is mediated specifically by somatodendritic D2 autoreceptors (Mercuri et al., 1997). Accordingly, the D2R agonist quinpirole (10 μM, 2 min) caused a membrane hyperpolarization and blockade of firing discharge (Figs. 1a,b; NT: 13.01 ± 2.7 mV; hWT: 15.1 ± 3 mV; hMT: 13.6 ± 3.3 mV), an effect that was prevented by the D2R antagonist sulpiride (3 μM). No significant difference was found among the three genotypes (p > 0.05).
Fig. 1.
Unaltered DA D2R-mediated autoreceptor function in hMT mice. Representative traces showing the effects of quinpirole (10 μM, 2 min) on nigral dopaminergic neurons. Bath-applied quinpirole hyperpolarizes the cell membrane and abolishes the spontaneous firing activity of neurons recorded from NT (a, upper trace), hWT (a, lower trace) and hMT slices (b, upper trace). Note that the D2R agonist elicits similar responses in the three groups of mice. Upon quinpirole washout, the membrane slowly returns to pre-drug level and firing activity is resumed. The quinpirole effect is prevented by pretreatment of the slice with the D2R antagonist sulpiride (3 μM) (b, lower trace), confirming the specificity of the response to quinpirole. (c) Quinpirole induced motor suppression in all genotypes. Locomotor activity, expressed as number of sector crossing (mean ± SEM), has been measured each 10 min over a total 30 min test. Mice were injected i.p. with vehicle (NT n = 11, hWT n = 9, hMT n = 18) or quinpirole 0.35 mg/kg (NT n = 20, hWT n = 11, hMT n = 14) immediately before the test. (d) Haloperidol increased phosphorylation of tyrosine hydroxylase (TH) at Ser40 in all groups tested. Mice were injected i.p. with vehicle (n = 6, each genotype) or haloperidol 0.5 mg/kg (NT n = 7, hWT n = 7, hMT n = 6) and killed 15 min later to perform western blotting analysis of striatal pSer40-TH. Upper panels show representative autoradiograms. Lower panels show summary of density values, normalized to DARPP32 and expressed as mean ± SEM. Genotypes and treatments are as indicated. veh: vehicle, quinp: quinpirole. # p < 0.05, ## p < 0.01, compared with vehicle group, within genotypes.
These results prompted us to investigate the effects of quinpirole on locomotor activity of hMT mice. This compound is commonly used in mice to suppress motor activity, an effect attributed to presynaptic D2R activation (Usiello et al., 2000). We found that quinpirole (0.35 mg/kg), inhibited spontaneous exploration to a similar extent in all genotypes (Fig. 1c). Indeed, two-way ANOVA revealed a significant treatment effect [F(2,105) =26.459, p < 0.0001] and a non significant treatment × genotype interaction [F(4,105) = 0.068, p = 0.9915].
D2 autoreceptors regulate also the state of phosphorylation of tyrosine hydroxylase (TH), the rate limiting enzyme in DA synthesis. Blockade of D2Rs with haloperidol (Jackson and Westlind-Danielsson, 1994) increases TH phosphorylation (Håkansson et al, 2004). This effect requires normal presynaptic D2R function, which provides a tonic inhibition of adenylate cyclase and cell firing (Lindgren et al., 2003). Western blotting analysis of pSer40-TH revealed a comparable haloperidol-induced enhancement of TH phosphorylation in all genotypes (Fig. 1d; two-way ANOVA [treatment effect: F(1,32) = 30.605, p < 0.0001; not significant genotype × treatment interaction: F(2,32) = 0.241, p = 0.7870]). In agreement with the electrophysiological and behavioral data, these results unambiguously indicate that D2 autoreceptor properties are preserved in hMT mice.
Impaired D2R-dependent postsynaptic functions in hMT mice
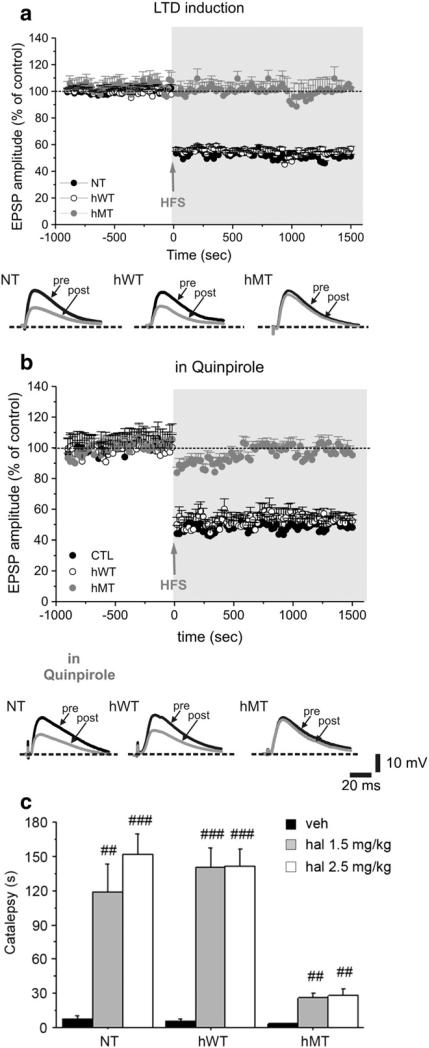
We then investigated postsynaptic D2R function by recording MSNs from striatal slices. MSNs were electrophysiologically identi-fied according to their peculiar intrinsic membrane properties, that were not significantly different among genotypes (Martella et al., 2009). High-frequency stimulation (HFS) of glutamatergic afferents led to the induction of robust LTD of glutamatergic EPSP in MSNs from NT (Fig. 2a; n = 41) and hWT mice (Fig. 2a; n = 48), but not in slices from hMT mice (Fig. 2a; n = 52). Because LTD induction requires D2R activation (Calabresi et al., 1997; Wang et al., 2006, Shen et al., 2008), we attempted to restore LTD by pretreating slices with quinpirole.
Fig. 2.
Impaired D2R-dependent postsynaptic function in hMT mice. Time-course of LTD induction in striatal medium spiny neurons from NT mice, hWT and hMT mice. (a) High-frequency stimulation (HFS, arrow) induces a robust LTD both in NT (filled circles) and hWT (open circles) mice, but not in hMT mice (grey circles). Representative traces of EPSPs recorded before (pre) and 20 min after (post) HFS in the three groups of mice. (b) Pretreatment of the slices with the D2R agonist quinpirole (10 μM, 20 min) does not modify the time-course of LTD in NT and hWT mice, nor it rescues LTD in hMT mice. Below, sample EPSPs are shown, measured before (pre) and 20 min after HFS (post). (c) Haloperidol induced a reduced cataleptic response in hMT animals. Mice were injected i.p. with vehicle (n = 8, each genotype), haloperidol 1.5 mg/kg (NT n = 8, hWT n = 8, hMT=7) or haloperidol 2.5 mg/kg (NT n = 8, hWT n = 8, hMT=7). Catalepsy time (s) was measured 120 min after injection. Data are expressed as mean ± SEM. Genotypes and treatments are as indicated. veh: vehicle, haloperidol: hal. ## p < 0.01, ### p < 0.0001, compared with vehicle group, within genotype; * p < 0.05, ** p < 0.01 compared with NT mice at the corresponding dose.
Quinpirole (10 μM, 30 min) did not affect intrinsic membrane (Sciamanna et al., 2009) and synaptic properties of MSNs (data not shown), in line with previous work on mouse MSNs (Goldberg et al., 2005; Kitada et al., 2007), and failed to restore LTD in hMT MSNs (Fig. 2b; NT: 49.9 ± 5.9, n = 22, t-test p < 0.001; hWT: 55.4 ± 4.2, n = 20, t-test p < 0.001; hMT: 91.2 ± 6.9, n = 17, Mann-Whitney p = 0.078).
To further explore postsynaptic D2R function in hMT animals, we determined the ability of haloperidol to induce catalepsy, which is manifested as an impaired ability to initiate movement. This behavioral response is typically produced by high doses of haloperidol (Jackson and Westlind-Danielsson, 1994) through blockade of postsynaptic D2Rs. Based on this rationale we used the Bar Test (Usiello et al., 2000) to determine the ability of haloperidol (1.5 and 2.5 mg/kg) to induce catalepsy in NT, hWT and hMT mice (Fig. 2c). Overall, haloperidol produced catalepsy in all genotypes, as indicated by two-way ANOVA [significant treatment effect: F(2,61) = 54.738, p < 0.0001]. However, the cataleptic response of hMT mice was lower than that observed in NT and hWT animals [significant genotype × treatment interaction: F(4,61) = 8.115, p < 0.0001].
Based on the critical role played by D2Rs in mouse motor behavior (Baik et al., 1995) we also analyzed the basal exploration of hMT mice (Fig. S3). The analysis of spontaneous motor activity revealed a slightly reduced locomotion in hMT mice, compared to the NT group, as revealed by one-way ANOVA [F(2,63) = 2.397, p = 0.0462].
In a final set of experiments, we analyzed D1R function and found no significant difference in both D1R-dependent motor behavior and GluR1 phosphorylation at Ser845 in the striatum of all genotypes (Fig. S4).
Altogether, electrophysiological and behavioral results indicate a reduced D2R postsynaptic activity, highlighting a dichotomy in pre- and postsynaptic D2R functionality in mice with mutant torsinA.
Postsynaptic D2Rs are not functionally coupled to Gi/Go proteins in hMT mice
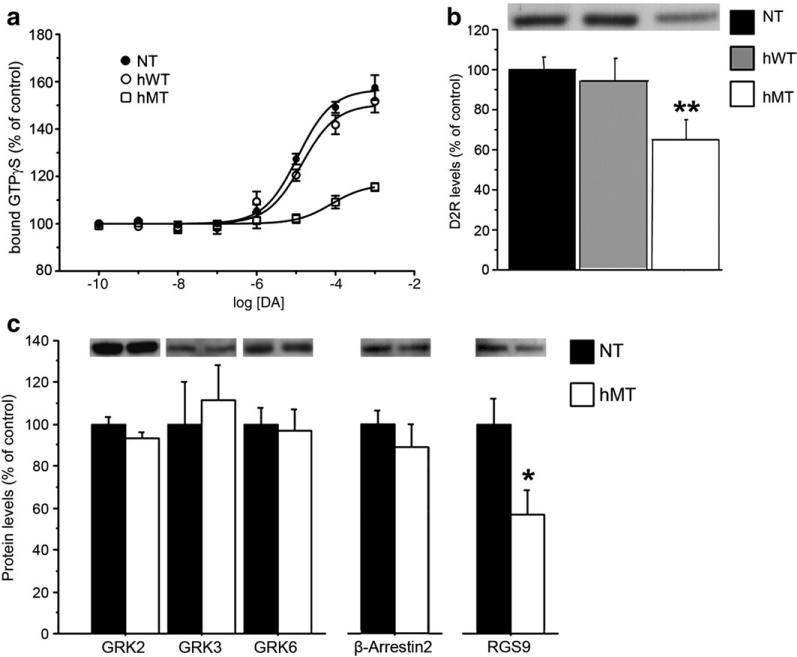
A large portion of the effects exerted by D2Rs are mediated via activation of Gi/Go proteins, which are coupled to inhibition of adenylyl cyclase and modulation of ion channels (Missale et al., 1998). Activation of G proteins is induced by binding to GTP, followed by dissociation of the α-GTP subunit from the β-γ complex. Therefore, in order to clarify the molecular basis of the reduced activity of postsynaptic D2R observed in hMT animals, we determined the ability of DA to promote GTP binding to striatal membranes prepared from the three different genotypes. In the striatum, [35S] guanosine 5′-([gamma]-thio)triphosphate ([35S]GTPγS) binding is activated by DA and largely reflects D2R activation (Geurts et al., 1999). In line with this notion, we found that incubation of striatal membranes with DA (10−10-10−3 M) produced a concentration-dependent increase in [35S]GTPγS binding (Fig. 3a), which was abolished by the D2R antagonist, raclopride (500 μM; data not shown). However, a significant difference in the maximal [35S]GTPγS binding was found between genotypes. In fact, in NT and hWT mice incubation of striatal membranes with 1 mM DA increased [35S]GTPγS binding by 56.7 ± 9.8% and 50.4 ± 8.6%, respectively, whereas in hMT mice the increase in [35S]GTPγS binding was significantly lower [16.6 ± 3.4%; p < 0.001 vs NT and hWT mice, one-way ANOVA and Bonferroni test]. These results demonstrate that the efficacy by which DA activates Gi/Go protein through D2Rs was significantly reduced (~75%) in hMT mice.
Fig. 3.
D2-like postsynaptic receptors are not functionally coupled to Gi/Go proteins in hMT mice. (a) DA-induced [35S]GTPγS binding was reduced in hMT mice. Striatal membranes of NT (n = 6), hWT (n = 6) and hMT (n = 6) mice were incubated with different concentrations of DA and 0.1 nM [35S]GTPγS for 90 min. Data are expressed as percent of [35S]GTPγS bound in the absence of DA and represent as mean ± SEM (n = 6). The dose–response curve from each animal was performed in duplicates. (b) Basal striatal D2R levels are reduced in hMT mice. Basal D2R expression levels in the striatum were determined by western blotting analysis in naïve NT (n = 25), hWT (n = 8) and hMT (n = 17) mice. (c) GRK2, GRK3, GRK6 and β-Arrestin2 content is unaltered in hMT mice (n = 8), compared to NT controls (n = 8). In contrast, RGS9 levels are significantly reduced in mutants (n = 16), compared to NT mice (n = 21). Upper panels in (b) and (c) show representative autoradiograms. The graphs show summary of values, normalized to DARPP32 and expressed as mean ± SEM. Genotypes are as indicated. * p < 0.05, ** p < 0.01, compared with NT mice.
We then analyzed whether the reduction in D2R-induced GTP binding could derive from decreased levels of receptors. In line with previous studies reporting reduced D2R binding sites in DYT patients (Perlmutter et al., 1997; Augood et al., 2002; Asanuma et al., 2005), western blotting analysis revealed a ~30% reduction of D2R levels in the striatum of hMT mice, compared to hWT and NT groups [One-way ANOVA: F(2,51) = 5.313, p = 0.0080] (Fig. 3b).
Activity of D2R is known to be regulated by G protein-coupled receptor kinases (GRKs). GRK-mediated phosphorylation of D2R induces the binding of arrestin which leads to receptor internalization and suppression of D2R-dependent signaling (DeWire et al., 2007). Signal termination at D2R is also enhanced by Regulator of G-protein Signaling 9 (RGS9) (Berman and Gilman, 1998). In order to gain further insight on the nature of the impairment of D2R-mediated transmission in mutant mice, we analysed the striatal levels of expression of GRK2, GRK3, GRK6, β-Arrestin2 and RGS9. Overall, western blotting analysis revealed comparable levels of GRK2, 3 and 6 between genotypes (Fig. 3c; Student's t test: p > 0.1 per each protein). Similarly, we did not find any statistical difference between control and mutant mice in the levels of β-Arrestin2 (p > 0.1). Conversely, the levels of RGS9 in mutant animals were significantly reduced (Fig. 3c; p < 0.05), suggesting that an adaptive down-regulation of RGS9 occurs in these mice, in attempt to compensate for the decreased availability of D2Rs.
Comparable striatal D2R mRNA levels between mutants and control mice
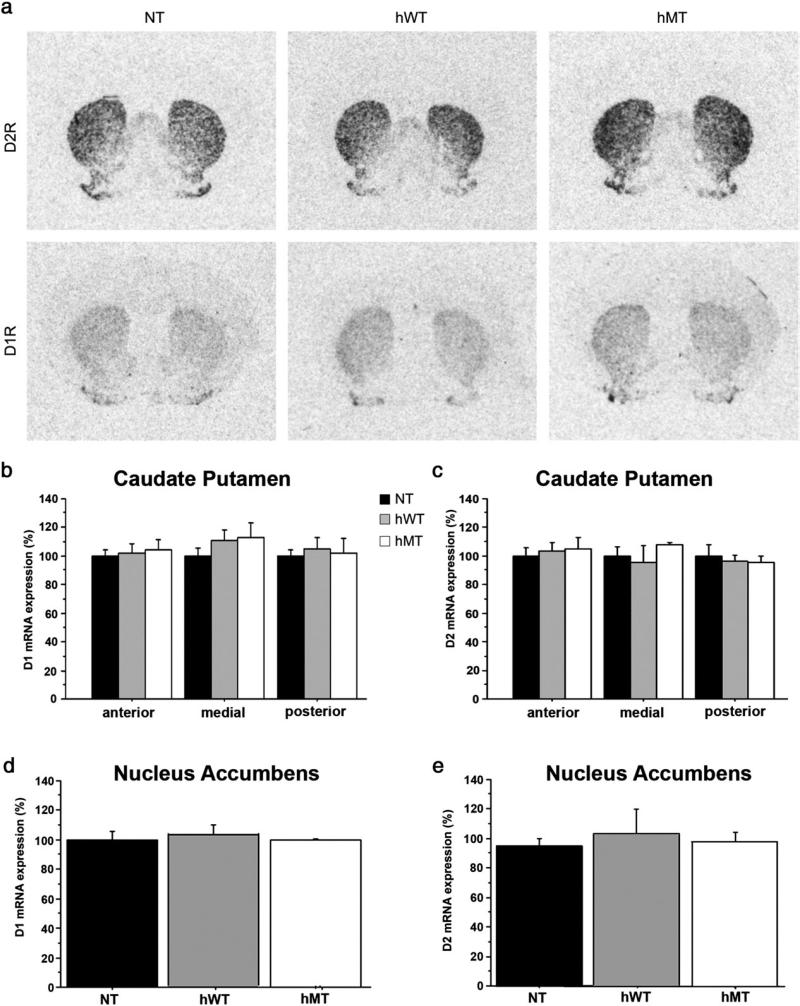
We next examined whether the reduction of striatal D2R protein observed in the mice overexpressing the mutant torsinA depended on transcriptional alterations. In situ hybridization analysis did not show any difference in D1R and D2R mRNA expression in the caudate putamen or in the nucleus accumbens of hMT, hWT and NT mice (Fig. 4a shows representative autoradiographic detections of D1Rs and D2Rs in the medial caudate putamen of each genotype group). Specifically, D2R mRNA expression in the three genotypes were comparable at all examined antero-posterior levels of caudate putamen [two-way ANOVA: non significant genotype effect: F(2,16) = 0.120, p = 0.8887, and non significant genotype × level interaction: F(4,16) = 1.102, p = 0.3896] (Fig. 4c) and in the nucleus accumbens [one-way ANOVA: F(2,8) = 0.099, p = 0.9064] (Fig. 4e). Similarly, the levels of D1R mRNA expression in hMT animals were comparable to those determined in hWT and NT mice, at all antero-posterior levels of the caudate putamen [two-way ANOVA: non significant genotype effect: F(2,16) = 0.423, p = 0.6688, and non significant genotype × level interaction: F(4,16) = 0.407, p = 0.8009] (Fig. 4b), and in the nucleus accumbens [one-way ANOVA: F(2,8) = 0.112, p = 0.8953] (Fig. 4d).
Fig. 4.
Comparable striatal D2R mRNA levels between mutants and control mice. (a) Representative images from the in situ hybridization study of D2R (upper row) and D1R (lower row) mRNA-positive neurons. Autoradiographs of coronal striatal sections of NT (left column), hWT (middle column) and hMT mice (right column). (b) Density values of D1R and (c) D2R mRNA in NT, hWT and hMT mice, measured in the anterior (AP +0.98 mm from bregma), medial (AP +0.26 mm from bregma) and posterior (AP −0.46 mm from bregma) caudate putamen. (d) Density values of D1R and (e) D2R mRNA in NT, hWT and hMT mice, measured in the nucleus accumbens (AP +1.34 mm from bregma). Data are expressed as mean ± SEM (n = 4, each genotype). Genotypes are as indicated.
Normal responsiveness of adenosine A2A receptors in hMT mice
Adenosine A2ARs and DA D2Rs are co-expressed in a large population of MSNs (Schiffmann et al., 1991; Fink et al., 1992). Based on the alteration found in D2R transmission at level of MSNs, we examined the state of functionality of A2ARs in hMT animals.
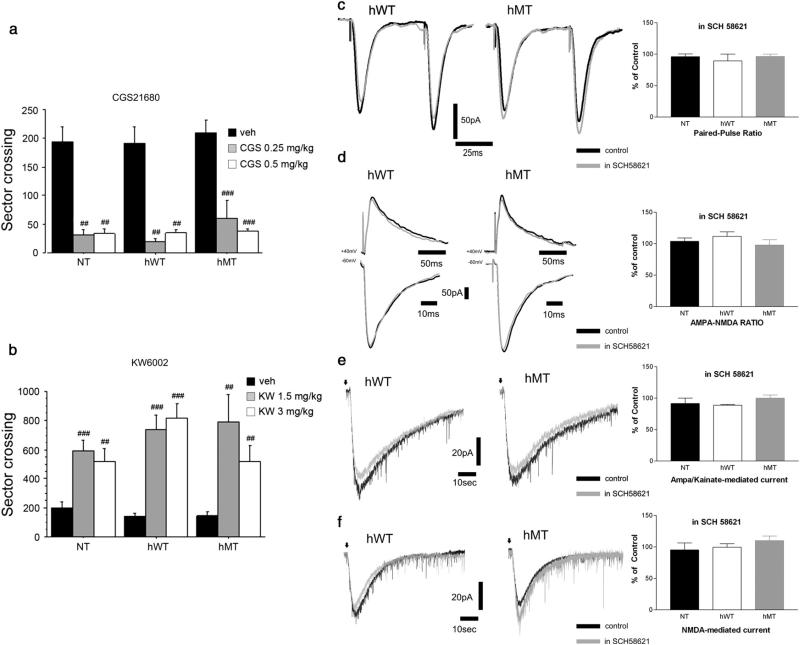
In a first series of experiments, we analyzed the A2AR agonist, CGS21680, and the antagonist, KW6002, for their ability to affect motor activity in NT, hWT and hMT mice. In agreement with previous work (Nikodijevic et al., 1990), administration of CGS 21680 (0.25 and 0.5 mg/kg) resulted in a strong depression of locomotion in all genotypes (Fig. 5a). Indeed, two-way ANOVA showed a significant treatment effect [F(2,108) =29.607, p < 0.0001] and non significant treatment × genotype interaction [F(4,108) = 0.062, p = 0.9928].
Fig. 5.
Unaltered striatal A2AR responses in hMT mice. (a) The A2AR agonist CGS21680 suppressed spontaneous locomotion in all genotypes. Animals were injected i.p. with vehicle (NT n = 27, hWT n = 29, hMT n = 13), CGS21680 0.25 mg/kg (n = 8, per genotype) or CGS21680 0.5 mg/kg (n = 8, per genotype). Locomotion is expressed as manually counted number of sector crossing (mean SEM) over a 30 min test. (b) The A2AR antagonist KW6002 stimulated motor activity in all tested groups. Mice were injected i.p. with vehicle (n = 13, per genotype), KW6002 1.5 mg/kg (NT n = 8, hWT n = 7, hMT n = 8) or KW6002 3 mg/kg (n = 8, per genotype). Locomotion is expressed as manually counted number of sector crossing (mean ± SEM) over 60′ test, after 60′ habituation. Genotypes and treatments are as indicated vehicle: veh, CGS21680: CGS, KW6002: KW. ## p < 0.01, ### p < 0.0001 compared with vehicle group, within genotype. (c) Sample EPSCs showing that paired-pulse ratio (PPR, 50 ms interstimulus interval) was unaffected by bath-application of SCH 58261 (grey traces) both in hWT and hMT mice. Right. Summary plot of the changes in PPR in the three genotypes. (d). Superimposed traces showing the lack of effect of SCH 58261 on the two synaptic components, NMDA and AMPA, recorded at +40 mV and −60 mV, respectively, in hWT and hMT mice. Right. Summary plot of the effect of SCH 58261 on AMPA/NMDA ratio. e. Representative traces show that SCH 58261 does not modify the inward current induced by focal application of both AMPA (e) or NMDA (f) in both hWT and hMT mice in control conditions and in the presence of SCH 58261 (grey traces).
In contrast, the A2AR antagonist KW6002 (1.5 and 3 mg/kg), which stimulates locomotion in mice (Kase et al., 2003), elicited a vigorous hyperlocomotion regardless of genotype (Fig. 5b) [Two-way ANOVA indicated a significant treatment effect: F(2,77) =43.141, p < 0.0001, and a non-significant treatment × genotype interaction: F(4,77) = 2.036, p = 0.976]. Altogether these studies show unaltered behavioral responses in hMT mutants to both activation or blockade of striatal A2ARs.
These in vivo results led us to analyze the electrophysiological responses of MSNs to the A2AR antagonists SCH 58621. Pretreatment of striatal slices with 50 nM SCH 58621 did not affect basal intrinsic or synaptic properties of MSNs recorded from the different groups of mice. Indeed, SCH 58621 (50 nM, 15 min) did not affect paired-pulse ratio (Fig. 5c; NT: 96.2 ± 4% of pre-drug value; hWT: 89.3 ± 10.8%; hMT: 96.4 ± 3.7%; n = 6 per genotype, p > 0.05). Similarly, AMPA/NMDA ratio of the recorded EPSCs (n = 6 per genotype) were not modified by SCH 58621 (Fig. 5d; NT: 103.9 ± 5%; hWT: 111.7 ± 7.2%; hMT: 97.9 ± 8.5%; n = 6 per genotype; p > 0.05). Moreover, SCH 58621 did not affect the amplitude of the currents generated by focal application of either AMPA (Fig. 5e; NT: 91.7 ± 8.4%; hWT: 88.9 ± 1.3%; hMT: 100 ± 5%; n = 4 per genotype, p > 0.05) or NMDA (Fig. 5f; NT: 95.4 ± 11%; hWT: 99.5 ± 5.7%; hMT: 110 ± 7%; n = 4 per genotype, p > 0.05), suggesting that A2AR antagonism did not, per sè, affect ionotropic glutamatergic transmission at striatal synapses.
A2AR antagonism rescues striatal synaptic plasticity in hMT mice
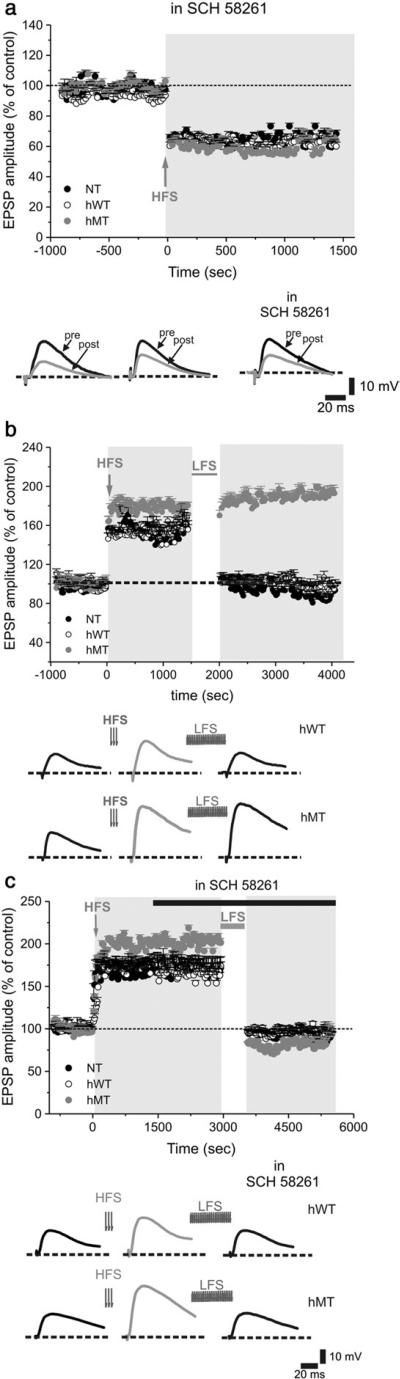
Interestingly, although SCH 58621 did not affect basal synaptic properties in the three genotypes (Figs. 5c–f), it fully restored the LTD impairment in hMT mice (Fig. 6a; NT: 65.04 % ± 4.8, n = 21; hWT: 61.7 ± 2.9, n = 19; hMT: 65.9 ± 1.9, n = 22, Mann-Whitney p < 0.0001).
Fig. 6.
A2AR antagonism rescues striatal synaptic plasticity impairments in hMT mice. Preincubation of the slices with the A2AR antagonist SCH 58261 (50 nM, 20 min) restores LTD in hMT mice, without modifying the time-course in NT and hWT mice (a). Below, sample EPSPs recorded before (pre) and after (post) HFS. (b) In the absence of external magnesium, HFS (arrow) induces a robust LTP in the three genotypes. Once obtained a stable LTP, a low-frequency stimulation protocol (LFS) is able to revert synaptic potentiation to resting level, both in NT and hWT mice, but not in hMT slices. Below, representative traces showing EPSPs before and after HFS, as well as after LFS in hWT and hMT mice. (c) Pretreatment of the tissue with SCH 58261 rescues synaptic depotentiation. Sample EPSPs recorded before (left), after HFS (middle trace), and after the LFS protocol (right). In hMT mice, SCH 58261 restores a physiological synaptic depotentiation.
In MSNs from hMT mice, HFS induced a LTP which was higher in magnitude when compared to NT or hWT mice (Fig. 6b; 201.6 ± 8.2%; n = 69, Mann-Whitney p < 0.001). Once striatal LTP is stabilized, a low-frequency stimulation (LFS) protocol can reverse synaptic strength to resting levels, a phenomenon termed synaptic depotentiation (SD) (Rioult-Pedotti et al., 2007). In line with recent evidence (Martella et al., 2009), LFS induced a SD only in NT and hWT mice (Fig. 6b, NT: 96.0 ± 3.3%, n = 40, t-test p < 0.0001; hWT: 93.8 ± 3.4%, n = 36, t-test p < 0.0001), whereas LFS failed to depotentiate corticostriatal synapses in hMT mice (Fig. 6b, 196.2 ± 8.5%, n = 40, t-test p = 0.39). Together, the loss of LTD (see Fig. 2) and SD on one hand, and the increase in LTP on the other, demonstrate that a “disinhibition” represents a hallmark of this model of DYT1 dystonia.
In accord with the hypothesis of a pivotal role of A2AR activation in LTP induction (Shen et al., 2008), we tested whether A2AR antagonism could also influence SD in hMT mice. Notably, while SCH 58621 did not affect SD in NT or hWT mice, it fully restored SD in hMT mice (Fig. 6c; 80.2 ± 2.6 %, n = 40, t-test p < 0.001).
Discussion
Multiple lines of experimental and clinical evidence link dopaminergic dysfunction to the occurrence of distinct forms of primary dystonia (Wichmann, 2008; Carbon et al., 2009; Tanabe et al., 2009).
Here, we provide evidence for a substantial decrease in postsynaptic D2R functionality in mice overexpressing mutant torsinA. Furthermore, based on the evidence that D2R activity is under a negative control exerted by A2ARs co-localized on the same population of MSNs, we show that blockade of A2ARs restores physiological D2R activity.
A prominent alteration of DA D2Rs was found in the striatum of hMT mice, consisting of a ~30% significant reduction in receptor expression. In an attempt to clarify the cause for decreased striatal D2R expression, we performed a quantitative in situ hybridization analysis. Overall, the changes in D2R protein content contrast with the similar levels of D2R mRNA measured in the striata of hMT mice in comparison to NT, or hWT mice, suggesting that mutation of torsinA leads to reduced striatal D2R content, irrespective of DA D2R transcriptional activity. Intriguingly, these data are in good agreement with a recent imaging study, that showed a decrease in striatal D2R binding sites not only in manifesting but also in non-manifesting carriers of the DYT1 mutation (Carbon et al., 2009), supporting the notion that alterations in DA D2R-mediated transmission in dystonia gene carriers may represent an endophenotype of the disease.
Although the molecular mechanisms at the basis of the reduction of striatal D2Rs in hMT mice remain to be clarified, we found that this decrease is accompanied by a dramatic decline in the ability of D2Rs to activate their cognate Go/i proteins, which have a crucial role in the regulation of cAMP/PKA signal transduction pathway (Borgkvist and Fisone, 2007). On the other hand, the reduction of D2R expression observed in mutant mice is not accompanied by changes in GRKs or β-Arrestin2, which are involved in D2R desensitization. However, mutant mice display lower levels of striatal RGS9. This protein acts as GTPase-accelerating protein, thereby promoting G protein inactivation. A reasonable interpretation is that reduced RGS9 expression represents an adaptive response elicited in mutant mice to compensate for reduced D2R functioning. Therefore, the reduction in GTPγS binding observed in hMT mice appear to be a direct consequence of reduced D2R availability at membrane level.
Our results suggest that the impairment of DA D2R-mediated transmission observed in the striata of hMT mice affects primarily postsynaptic D2R-related functions. In fact, although overexpression of torsinA occurred also in dopaminergic midbrain neurons, we did not observe any alteration in the ability of presynaptic D2R to control the state of excitability of nigral dopaminergic neurons and regulate the state of phosphorylation of TH. Taken together, these observations support the existence of specific and yet unidentified factors required for mutant torsinA to manifest its aberrant influences on D2R expression. In this regard, future in vitro studies are mandatory to elucidate this important issue, and in turn, to establish at which molecular level mutated torsinA interferes with D2R expression at membrane sites.
Besides displaying preserved D2R-dependent presynaptic functions, hMT transgenic mice do not show any functional alteration associated to striatal dopaminergic D1R transmission. In fact D1R stimulation with the selective full agonist SKF81297, induced similar activation of PKA pathway and behavioral hyperactivity in all genotypes tested (Fig. S4).
Conversely, in agreement with altered D2R postsynaptic functions, we found that hMT mice display a significant decrease in the ability to respond to haloperidol, a drug which depresses motor activity and induces catalepsy by blocking D2Rs located on MSNs (Usiello et al., 2000). Because the action of haloperidol depends on tonic activation of D2Rs, this finding suggests that overexpression of the mutated form of torsinA overall results in a diminished ability of DA to finely tune striatal D2Rs.
Along with abnormal behavioral responses to haloperidol, dys-functional D2R on MSNs also appears to contribute to LTD loss in the striatum of hMT mice. Two major findings support this assumption: i) quinpirole could not rescue the LTD deficit, while ii) recovery of LTD was obtained through A2AR blockade.
The requirement of intact D2R signaling for the induction of corticostriatal LTD has been recognized since its first description (Calabresi et al., 1992b), and confirmed in many subsequent studies (Lovinger et al., 1993, Kreitzer and Malenka, 2007; Shen et al., 2008). Less clear, however, remains the location of D2Rs required for LTD induction in striatal cellular elements, since both cholinergic interneurons (Wilson 2006) and MSNs (Shen et al., 2008) have been proposed to bear D2Rs required for LTD expression. In previous works, we have found that cholinergic interneurons express abnormal excitatory responses to D2R stimulation (Pisani et al., 2006), and that exaggerated acetylcholine release is a final step for LTD disruption in these mice (Martella et al., 2009). The results of the present work, however, also emphasize the role of D2Rs on MSNs in the control of corticostriatal LTD, providing support to the hypothesis that cholinergic tone is also regulated by a D2R/A2AR functional interaction in distant cellular elements. This idea would be consistent with the observation that, in our experimental conditions, the large majority of MSNs undergo LTD (Martella et al., 2009; present work).
Pharmacological blockade of A2AR-mediated transmission has been shown to mimic striatal postsynaptic D2R activation in MSNs (Ferrè et al., 1997). Accordingly, A2ARs and D2Rs oppose each other in the induction of bidirectional synaptic plasticity, with D2R promoting LTD, and A2ARs favouring LTP induction (Shen et al., 2008). Indeed, we found that SCH 58261, a specific A2AR antagonist, rescued LTD in hMT mice, suggesting that the deficit in D2R function can be reverted by eliminating the negative tone exerted by A2ARs on D2R function. SCH 58261 was also able to restore SD in hMT mice, although through a mechanism still unidentified. Nonetheless, the present work, together with our recent observations (Martella et al., 2009) suggests that in this mouse model of DYT1 dystonia, plasticity at glutamatergic synapses had lost its bidirectionality, because the balance between the opponent processes controlling LTD/LTP and SD is disrupted. To this respect, the balance D2R/A2ARs appears to play a relevant, though complex role in the regulation of this phenomenon.
Of note, blockade of A2ARs is currently regarded as a promising therapeutic strategy for Parkinson's disease (Morelli et al., 2007), which is characterized by loss of DA-mediated transmission. However, the use of A2ARs antagonists has never been postulated for the treatment of dystonia. Based on our evidence that A2AR receptors blockade rescues physiological striatal synaptic plasticity in mice with the DYT1 dystonia mutation, we provide a plausible rationale for indicating A2ARs as a potential target for the development of novel therapies for dystonia.
Supplementary Material
Acknowledgments
We wish to thank Drs. N. Sharma and P. Popoli for their support. This work was supported by grants from Bachmann-Strauss Dystonia & Parkinson's Foundation and Dystonia Medical Research Foundation to AP; Ministero Salute (Prog. Finalizzato and Art. 56) to GB, AP, and NBM, Istituto Superiore Sanità (Malattie Rare) to AP; Agenzia Spaziale Italiana (DCMC grant) to GB, and USPHS grant P50NS37409 to DGS. AU represents Mariano Scippacercola Foundation.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.nbd.2010.03.003.
References
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB, Eidelberg D. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, Klein C, Breakefield XO, Standaert DG. Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology. 2002;59:445–448. doi: 10.1212/wnl.59.3.445. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung J, Breakefield XO, Standaert DG. Dopamine transmission in DYT1 dystonia. Adv. Neurol. 2004;94:53–60. [PubMed] [Google Scholar]
- Baik JH, Ricetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Baik MG, Lee MJ, Choi YJ. Gene expression during involution of mammary gland. Int. J. Mol. Med. 1998;2(1):39–44. [PubMed] [Google Scholar]
- Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. J. Neurochem. 2007;102(3):783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Bonvento G, Kerkerian-Le Goff L, Gubellini P. Role of glutamate transporters in corticostriatal synaptic transmission. Neuroscience. 2009;158:1608–1615. doi: 10.1016/j.neuroscience.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gate. J. Biol. Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci. Biobehav. Rev. 2007;31:79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur. J. Neurosci. 1992a;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J. Neurosci. 1992b;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J. Neurosci. 1997;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Niethammer M, Peng S, Raymond D, Dhawan V, Chaly T, Ma Y, Bressman S, Eidelberg D. Abnormal striatal and thalamic dopamine neurotransmission: Genotype-related features of dystonia. Neurology. 2009;72:2097–2103. doi: 10.1212/WNL.0b013e3181aa538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J. Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico F, Rossi S, Napolitano F, Catuogno V, Topo E, Fisone G, D'Aniello A, Centonze D, Usiello A. D-aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J. Neurosci. 2008;28(41):10404–10414. doi: 10.1523/JNEUROSCI.1618-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrè S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. Mol. Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Hornykiewicz O, Fahn S, Kish SJ. Striatal dopamine in early-onset primary torsion dystonia with the DYT1 mutation. Neurology. 2000;54(5):1193–1195. doi: 10.1212/wnl.54.5.1193. [DOI] [PubMed] [Google Scholar]
- Geurts M, Hermans E, Maloteaux JM. Assessment of striatal D1 and D2 dopamine receptor-G protein coupling by agonist-induced [35S]GTP gamma S binding. Life Sci. 1999;65:1633–1645. doi: 10.1016/s0024-3205(99)00412-9. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Håkansson K, Pozzi L, Usiello A, Haycock J, Borrelli E, Fisone G. Regulation of striatal tyrosine hydroxylase phosphorylation by acute and chronic haloperidol. Eur. J. Neurosci. 2004;20:1108–1112. doi: 10.1111/j.1460-9568.2004.03547.x. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther. 1994;64:291–370. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Kase H, Aoyama S, Ichimura M, Ikeda K, Ishii A, Kanda T, Koga K, Koike N, Kurokawa M, Kuwana Y, Mori A, Nakamura J, Nonaka H, Ochi M, Saki M, Shimada J, Shindou T, Shiozaki S, Suzuki F, Takeda M, Yanagawa K, Richardson PJ, Jenner P, Bedard P, Borrelli E, Hauser RA, Chase TN, KW-6002 US-001 Study Group Progress in pursuit of therapeutic A2A antagonists: the adenosine A2A receptor selective antagonist KW6002: research and development toward a novel nondopaminergic therapy for Parkinson's disease. Neurology. 2003;61:S97–S100. doi: 10.1212/01.wnl.0000095219.22086.31. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc. Natl. Acad. Sci. USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J. Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc. Natl. Acad. Sci. USA. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J. Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- Martella G, Tassone A, Sciamanna G, Platania P, Cuomo D, Viscomi MT, Bonsi P, Cacci E, Biagioni S, Usiello A, Bernardi G, Sharma N, Standaert DG, Pisani A. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain. 2009;132(Pt 9):2336–2349. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur. J. Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Ricetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog. Neurobiol. 2007;83(5):293–309. doi: 10.1016/j.pneurobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Nikodijevic O, Daly JW, Jacobson KA. Characterization of the locomotor depression produced by an A2-selective adenosine agonist. FEBS Lett. 1990;261:67–70. doi: 10.1016/0014-5793(90)80638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Jacoby D, Penney J, Risch NJ, Fahn S, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat. Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; San Diego: 2001. [Google Scholar]
- Perlmutter JS, Mink JW. Dysfunction of dopaminergic pathways in dystonia. Adv. Neurol. 2004;94:163–170. [PubMed] [Google Scholar]
- Perlmutter JS, Tempel LW, Black KJ, Parkinson D, Todd RD. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystonia. Neurology. 1997;49(5):1432–1438. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- Pisani A, Martella G, Tscherter A, Bonsi P, Sharma N, Bernardi G, Standaert DG. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol. Dis. 2006;24:318–325. doi: 10.1016/j.nbd.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Rinken A, Ferré S, Terasmaa A, Owman C, Fuxe K. Serotonergic agonists behave as partial agonists at the dopamine D2 receptor. NeuroReport. 1999;10:493–495. doi: 10.1097/00001756-199902250-00009. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Donoghue JP, Dunaevsky A. Plasticity of the synaptic modification range. J. Neurophysiol. 2007;98:3688–3695. doi: 10.1152/jn.00164.2007. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Augood SJ, Hewett JW, Leung JC, Sasaki H, Ozelius LJ, Ramesh V, Standaert DG, Breakefield XO, Hedreen JC. TorsinA protein and neuropathology in earl onset generalized dystonia with GAG deletion. Neurobiol. Dis. 2003;12:11–24. doi: 10.1016/s0969-9961(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Libert F, Vassart G, Vanderhaeghen JJ. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci. Lett. 1991;130:177–181. doi: 10.1016/0304-3940(91)90391-6. [DOI] [PubMed] [Google Scholar]
- Sciamanna G, Bonsi P, Tassone A, Cuomo D, Tscherter A, Viscomi MT, Martella G, Sharma N, Bernardi G, Standaert DG, Pisani A. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiol. Dis. 2009;34:133–145. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Baxter MG, Petravicz J, Bragg DC, Schienda A, Standaert DG, Breakefield XO. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J. Neurosci. 2005;25:5351–5355. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nat. Rev. Neurol. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD, Perlmutter JS. Mutational and biochemical analysis of dopamine in dystonia: evidence for decreased dopamine D2 receptor inhibition. Mol. Neurobiol. 1998;16:135–147. doi: 10.1007/BF02740641. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wichmann T. Dopaminergic dysfunction in DYT1 dystonia. Exp. Neurol. 2008;212:242–246. doi: 10.1016/j.expneurol.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Striatal D2 receptors and LTD: yes, but not where you thought they were. Neuron. 2006;50:347–348. doi: 10.1016/j.neuron.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Zhao Y, DeCuypere M, LeDoux MS. Abnormal motor function and dopamine neurotransmission in DYT1 DeltaGAG transgenic mice. Exp. Neurol. 2008;210:719–730. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.