Abstract
Purpose
This phase I clinical trial was conducted to determine the safety, efficacy, and molecular effects of sorafenib with temsirolimus in patients with advanced melanoma.
Patients and Methods
Patients with stage IV or unresectable or recurrent stage III melanoma and ECOG performance status of 0 to 1 were eligible. Sorafenib was given orally once or twice daily and temsirolimus was given intravenously weekly, both starting on day 1, with a 4-week cycle. Responses were assessed every 2 cycles per RECIST criteria. Consenting patients with accessible tumors underwent optional tumor biopsies prior to treatment and after the second infusion of temsirolimus. Tumor biopsies were analyzed for activating mutations in BRAF and NRAS, and for expression of P-ERK and P-S6 proteins.
Results
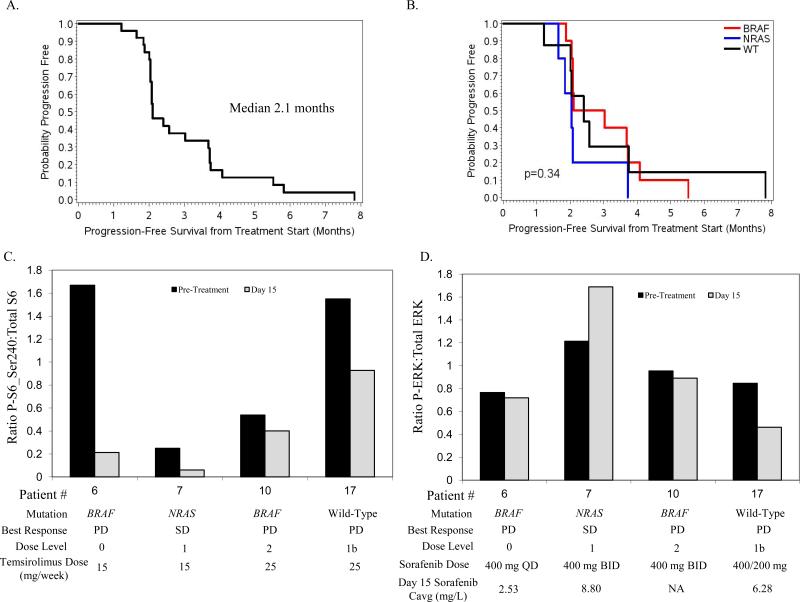
A total of 25 patients were accrued to the study. The MTD doses were sorafenib 400 mg qAM / 200 mg qPM daily and temsirolimus 25 mg IV weekly. Dose-limiting toxicities included thrombocytopenia, hand-foot syndrome (HFS), serum transaminase elevation and hypertriglyceridemia. There were no complete (CR) or partial (PR) responses with the combination; 10 patients achieved stabilization of disease as their best response. The median progression-free survival (PFS) was 2.1 months. Matching pre-treatment and day 15 tumor biopsies demonstrated marked inhibition of P-S6 with treatment in 3 of 4 evaluable patients, but minimal inhibition of P-ERK.
Conclusions
Combination therapy with sorafenib and temsirolimus resulted in significant toxicity at higher dose levels, failed to achieve any clinical responses in genetically unselected patient population, and did not inhibit P-ERK.
Keywords: phase I, sorafenib, temsirolimus, metastatic melanoma, BRAF
INTRODUCTION
The majority of melanomas have a mutation in the RAS-RAF-MEK-ERK signaling pathway, including activating mutations in the BRAF (~45%) and NRAS (~20%) genes (1). While activating mutations in BRAF are thought to predominantly activate downstream signaling via MEK and ERK, RAS GTPases activate additional signaling pathways (2). In particular, the PI3K-AKT-mTOR pathway plays a critical role in oncogenic signaling by RAS, and it is a promising combinatorial target for NRAS-mutant tumors.(3, 4) The PI3K-AKT-mTOR pathway is also activated in cancer by loss of function of the lipid phosphatases PTEN, which is detected in 10-30% of melanomas (5-7). PTEN loss is mutually exclusive with NRAS mutations, but often occurs in melanomas with a concurrent BRAF mutation. Functionally, loss of PTEN complements activating BRAF mutations to transform melanocytes in a murine model (8). Further, activation of PI3K-AKT-mTOR signaling has been implicated in both primary and secondary resistance to BRAF and MEK inhibitors (9-12). Thus, there is a strong rationale to test the clinical effects of combined inhibition of the RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways in melanoma.
Sorafenib (also called BAY 43-9006) is a small molecule inhibitor of several protein kinases, including wild-type and mutant BRAF, CRAF, c-KIT, and VEGFRs (13). Sorafenib is effective at inhibiting the growth and survival of melanoma cells lines and xenografts with BRAF or NRAS mutations in preclinical models (14). However, it achieves a clinical response in only 3% of patients (15, 16). One of these clinical studies evaluated the effects of sorafenib treatment on cyclin D1 and Ki67 expression in patient specimens (16). However, to date the effects of sorafenib treatment on ERK phosphorylation, which correlates with tumor regression with the selective BRAF inhibitor vemurafenib, has only been reported in one melanoma patient (17, 18).
Combined treatment with sorafenib and rapamycin, an mTOR inhibitor, decreases proliferation and invasion, and induces apoptosis, in BRAF-mutant human melanoma cell lines (19). Synergistic growth inhibition with this combination is observed in human melanoma cell lines with both mutant and wild-type BRAF (20). Clinical testing of mTOR inhibition in melanoma has previously been reported with temsirolimus (also called CCI-779), an mTOR inhibitor structurally related to rapamycin (21). Temsirolimus was well tolerated, but only 1 of 33 metastatic melanoma patients achieved a clinical response. While previous studies have examined the degree of inhibition of the phosphorylation (activation) of the mTOR effector S6 in circulating cells and head and neck carcinomas, no assessments have been reported in melanoma patients (22, 23).
We have conducted a phase I clinical trial of concurrent treatment with sorafenib and temsirolimus. The primary endpoint of the study was to evaluate the safety and toxicity of this regimen, and to determine the maximum tolerated dose for further testing. The secondary endpoint was to determine the clinical activity of this treatment in metastatic melanoma. Patients with accessible tumors underwent optional biopsies before and during treatment with sorafenib and temsirolimus for quantitative proteomic analysis by reverse phase protein arrays (RPPA) (24, 25). The presence of activating mutations in BRAF and NRAS, and effects on P-ERK and P-S6 protein expression, were compared to the clinical efficacy of the combination.
PATIENTS AND METHODS
Patient Selection
Patients with histologically confirmed stage IV or recurrent or unresectable stage III melanoma of non-uveal origin were enrolled. Major inclusion criteria included: ECOG performance status of ≤ 1; measurable disease defined by RECIST version 1.0; adequate organ functions; no active or symptomatic brain metastases and no steroid support for palliation; and uncontrolled blood pressure. There was no restriction in the number of prior therapies.
Treatment Plan
The protocol for this study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center, and all patients gave written informed consent before enrollment. The doses of oral sorafenib and intravenous temsirolimus in the first patient cohort were 400 mg twice a day and 15 mg weekly, respectively (dose level 1). Table 1 lists the dose escalation scheme. One cycle were defined as 4 weeks of treatment. The maximum number of treatment cycles planned was six; patients who continued to have disease regression were allowed to receive more than six cycles as long as they tolerated treatment well. Intrapatient dose escalation was not permitted.
TABLE 1.
Dose Escalation Scheme with Patient Mutation Status
| Dose level | Sorafenib (mg PO) | Temsirolimus (mg IV weekly) | Total Number Treated | BRAF # | NRAS # | WT # | NE # |
|---|---|---|---|---|---|---|---|
| 0 | 400 QD | 15 | 3 | 3 | |||
| 1* | 400 BID | 15 | 6 | 1 | 1 | 2 | 2 |
| 1a | 200 BID | 25 | 4 | 2 | 2 | ||
| 1b† | 400 in AM 200 in PM |
25 | 10 | 3 | 3 | 4 | |
| 2 | 400 BID | 25 | 2 | 1 | 1 | ||
| 3 | 400 BID | 50 | |||||
| 4 | 400 BID | 75 |
Dose levels 1a and 1b were added after the trial was initiated.
Starting dose level
Maximum tolerated dose: This dose level is recommended for a phase II study.
WT, wild-type BRAF and NRAS; NE, not evaluable.
Toxicity Evaluation
Toxicity was evaluated according to the US National Cancer Institute Common Terminology Criteria for Adverse Events v3.0.1 Patients were assessed for safety every 2 weeks during the first 2 cycles, and then once every cycle. Complete blood counts with differential counts and serum chemistry were obtained once a week during the first 2 cycles, and then every other week. Dose-limiting toxicity (DLT) was defined as grade 4 hematologic toxicity, ≥ grade 3 nonhematologic toxicity, any grade 2 nonhematologic or grade 3 hematologic toxicity requiring a dose reduction or treatment interruption for more than 7 days during the first cycle. Grade 3 to 4 nausea, vomiting, diarrhea were only considered DLTs if they occurred despite optimal medical management. Grade 3 electrolytes, uric acid, phosphorus abnormalities were not considered DLT if they were correctable within 1 week.
Response Evaluation
Responses were evaluated after every 2 cycles of the treatment using computed tomography and/or magnetic resonance imaging, and by direct ruler measurement for clearly visible cutaneous lesions. Clinical responses were evaluated using the international criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST) Committee (26).
Population Pharmacokinetic Analysis
Blood samples were collected on consenting patients at the following time points of cycle one: Day 1 - pretreatment, 1, 4, 6, 8, 10, and 24 hours after start of administration of sorafenib; Day 15 - pretreatment, 1, 4, 6, 8, 10, and 24 hours after treatment. Blood samples were collected in 5 mL sodium heparin tubes and obtained in Clinical and Translational Research Center at The University of Texas MD Anderson Cancer Center. Following collection, they were immediately centrifuged at 1500 rpm for 10 minutes at 4°C for separation. Plasma samples were aliquoted and stored in labeled-cryovials at approximately –70°C until analysis. Plasma sorafenib concentrations were determined by LC/MS/MS. A Waters Acquity UPLC system (Waters, Milford, MA) with a 4 °C autosampler was run in tandem with a Waters Quattro Premier mass spectrometer using MassLynx/Quanlynx software for data acquisition and integration. Sorafenib was extracted using a liquid-liquid method by adding 4 mL methyl tert-butyl ether (MTBE) to 100 μL of plasma and vortexed for 5 minutes. The resulting mixture was centrifuged at 2000 × g for 8 minutes at 4 °C and frozen at −80 °C for 30 minutes. The resulting supernatant was evaporated to dryness at 40 °C under gentle nitrogen gas stream and reconstituted with 100 μL of 50:50, methanol:water (v:v). Chromatographic separation was carried out following a 10 μL injection on a Phenomenex Kinetex C18 column (100 × 2.1 mm, 2.6 μm) over 5 minutes at 0.4 mL/min using 50:50, 0.2% formic acid:methanol (v:v) with a linear gradient to 98% methanol over the first 2 minutes. The monitored transition for sorafenib was 465 > 271 m/z with a peak retention time of 2.52 minutes in positive electrospray ionization mode (ESI+). The calibration curve ranged from 1-10,000 ng/mL in single donor human plasma. Pharmacokinetic parameter estimates were generated from individual patient concentration-time data using a non-compartmental model (WinNonLin v5.2, Pharsight Corp., Mountain View, CA).
Tumor Biomarker studies
After signing informed consent, each patient underwent a tumor biopsy (punch or core-needle biopsy), which was embedded in Optimum Cutting Temperature (OCT) solution, unless there was available frozen or OCT-embedded tumor samples obtained within 3 months prior to signing informed consent. An optional tumor biopsy was performed within 6 hours after the completion of temsirolimus infusion on day 15 of the first treatment cycle. H&E slides were generated from each tumor, which were reviewed by an experienced dermatopathologist. Tumors with regions of at least 70% viable tumor cells of sufficient size for DNA and protein extraction underwent H&E-guided macrodissection (7). Shears of the tumor-enriched isolates were prepared using a DNAse-free, RNAse free microtome at −20°C, then were stored at −80°C. DNA was extracted from the samples and underwent mass-spectroscopy based genotyping for mutations in BRAF (exon 15) and NRAS (exons 1and 2), as previously described (7, 27). Proteins were extracted from the samples as previously described (7). Protein lysates were normalized to the same concentration, denatured, and then submitted for RPPA analysis through the MD Anderson Cancer Center RPPA Core Facility2. Log2 expression data for each protein was corrected for measured differences in total protein loading as previously described (7). A detailed description of the RPPA procedure is provided in the Supplemental Materials.
Statistical Analysis
The MTD was defined as the highest dose at which the incidence of DLT was less than 33%. A conventional 3+3 dose-escalation design with predefined dose levels of sorafenib and temsirolimus was implemented. At the MTD, a total of 10 patients were enrolled to ensure that the safety profile was acceptable. Overall clinical response included both complete (CR) and partial responses (PR). Progression-free survival (PFS) and overall survival (OS) were measured from day 1 of treatment. One patient discontinued the study due to toxicity before disease progression and used different therapies; this patient was censored for PFS at the time of study discontinuation. Patients that did not experience death were censored at the time of their last clinic follow-up. The duration of clinical response was measured from the time a clinical response was first achieved until the time of disease progression or death, whichever occurred first.
The association between tumor mutation status and clinical response was determined using Fisher's exact test. Kaplan-Meier survival curves were used to estimate PFS and OS; log-rank testing was used to compare curves. Pharmacodynamic effects of treatment with sorafenib and temsirolimus on P-ERK and P-S6 were determined by calculating the ratio of phospho:total-protein expression in each sample. Pre-treatment and day 15 ratios were compared using paired Student's t-test.
RESULTS
Patient Characteristics
Twenty-five patients were enrolled and treated in the study (Table 2). The median age of the patients was 51 years. 40% of the patients had stage III melanoma, 12% had stage IV M1a, 28% stage IV M1b, and 20% stage IV M1c, respectively. 56% of the patients had received at least two prior systemic therapies for metastatic melanoma.
TABLE 2.
Patient Characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Total patients enrolled in the study | 25 (100) |
| Sex | |
| Male | 18 (72) |
| Female | 7 (28) |
| Age (years) | |
| Median [range] | 51 [22-76] |
| ECOG performance status | |
| 0 | 10 (40) |
| 1 | 15 (60) |
| Disease stage | |
| III (unresectable or recurrent) | 10 (40) |
| IV | |
| M1a | 3 (12) |
| M1b | 7 (28) |
| M1c | 5 (20) |
| Serum lactate dehydrogenase level | |
| Normal | 19 (76) |
| Higher than upper limit of normal | 6 (24) |
| Prior systemic treatment | |
| 0 | 4 (16) |
| 1 | 7 (28) |
| 2 | 8 (32) |
| 3 | 4 (16) |
| 4 | 2 (8) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group
Treatment
One of the first three patients treated at dose level 1 had a DLT (Grade 3 hand-foot syndrome and thrombocytopenia requiring dose reduction) (Table 3). However, no other DLTs were observed among three patients treated at dose level 0, nor among an additional three patients treated at dose level 1. At dose level 2, both of two patients had a DLT (Grade 3 serum transaminase elevation and hand-foot syndrome). To better define a maximum tolerated dose of the combination, two additional dose levels (level 1a and 1b) were evaluated. All four patients at dose level 1a tolerated the treatment without DLTs. The next six patients were treated at dose level 1b with only one patient experiencing reversible grade 3 hypertriglyceridemia. Four additional patients were treated at dose level 1b, and two of them experienced DLTs of ≥ grade 3 thrombocytopenia requiring dose reduction. Dose level 1b (sorafenib 400 mg in AM and 200 mg in PM daily and temsirolimus 25 mg weekly) was selected as the MTD.
TABLE 3.
Grade 3/4 Toxicities
| Dose Level | Sorafenib (mg) | Temsirolimus (mg weekly) | Toxicity |
|
|---|---|---|---|---|
| Grade 3 | Grade 4 | |||
| 0 (n=3) | 400 QD | 15 | Arthralgia (1) | None |
| Vomiting (1) | ||||
| 1 (n=6) | 400 BID | 15 | Hypophosphotemia (4) | None |
| Fatigue (3) | ||||
| Diarrhea (2) | ||||
| Thrombocytopenia (1) | ||||
| Dyspnea (1) | ||||
| Hand-Foot Syndrome (1) | ||||
| AST/ALT elevation (1) | ||||
| 1a (n=4) | 200 BID | 25 | Lymphopenia (1) | None |
| Anorexia (1) | ||||
| Hypophosphotemia (1) | ||||
| Fatigue (1) | ||||
| 1b (n=10) | 400 / 200 | 25 | Fatigue (3) | Thrombocytopenia (1) |
| Hypophosphotemia (2) | ||||
| Thrombocytopenia (1) | ||||
| Lymphopenia (1) | ||||
| Hypokalemia (1) | ||||
| Cough (1) | ||||
| Hypertriglyceridemia (1) | ||||
| AST/ALT elevation (1) | ||||
| 2 (n=2) | 400 BID | 25 | Fatigue (2) | None |
| Lymphopenia (1) | ||||
| Hand-Foot Syndrome (1) | ||||
| AST/ALT elevation (1) | ||||
| Hypophosphotemia (1) | ||||
Toxicity
The most common grade 3 or 4 non-hematologic adverse events were fatigue and hypophosphotemia (Table 4). The most common grade 3/4 hematologic toxicities were thrombocytopenia (n=3) and lymphopenia (n=3) (Table 4). Seventeen (68%) patients required dose reduction during the course of the treatment. All adverse events resolved upon treatment discontinuation.
TABLE 4.
Dose-limiting toxicities (DLTs) per dose level
| Dose Level | Sorafenib (mg) | Temsirolimus (mg weekly) | Number of DLTs | DLTs |
|---|---|---|---|---|
| 0 | 400 QD | 15 | 0 / 3 | None |
| 1 | 400 BID | 15 | 1* / 6 | G3 Hand-Foot Syndrome G3 Thrombocytopenia (requiring dosing reduction) |
| 1a | 200 BID | 25 | 0 / 4 | None |
| 1b | 400 / 200 | 25 | 3 / 10 | G3 Hypertriglyceridemia G4 Thrombocytopenia G3 Thrombocytopenia (requiring dosing reduction) |
| 2 | 400 BID | 25 | 2 / 2 | G3 serum transaminases elevation** G3 Hand-Foot Syndrome |
Dose level 1b was selected as maximum-tolerated dose
One patient had both DLTs at dose level 1
Both serum alanine aminotransferase and aspartate aminotransferase elevation
Pharmacokinetics
The plasma concentration-time profiles of sorafenib were characterized by a relatively slow absorption and elimination phase with primary and secondary peaks in a number of patients, as seen in a previous single agent phase 1 study (18). Sorafenib mean peak plasma concentrations (Cmax) and mean daily exposure, measured as the average concentration over 24 hours (Cavg), was 2-4 fold greater at steady-state on Day 15 when compared to Day 1 with low-to-moderate interpatient variability across all dosing levels (Table 5). Sorafenib mean Cavg at day 15 with 400 mg BID dosing was 8.45 mg/L, which was more than a dose proportional increase when compared to 400 mg QD (3.16 mg/L); mean Cmax increased proportionately (11.83 vs. 5.38 mg/L). Mean Cavg with sorafenib dosing of 400 mg AM/200 mg PM on Day 15 was 4.55 mg/L, which was similar to the result observed in the one patient treated with 200 mg BID with available Day 15 data (Cavg 5.12 mg/L). The sorafenib mean Cmax was similar for all dose levels on Day 1 but became more dose proportional by Day 15 (except for 200 mg BID). This trend and with the mean Cmax levels at 400 mg BID were similar to those observed in a review of four phase 1 trials with single agent sorafenib in patients with advanced cancer (28).
Table 5.
Sorafenib pharmacokinetics
| Sorafenib Concentration (mg/L) | ||||
|---|---|---|---|---|
| Patient | Sorafenib Dose (mg, frequency) | Temsirolimus Dose (mg, weekly) | Cavg/Cmax, Day 1 | Cavg/Cmax, Day 15 |
| 1 | 400, BID | 15 | 1.43/2.09 | 7.10/8.91 |
| 2 | 400, BID | 15 | 2.07/2.53 | 7.70/12.46 |
| 3 | 400, BID | 15 | 1.23/1.94 | 9.79/13.53 |
| 7 | 400, BID | 15 | 2.70/3.70 | 8.80/12.46 |
| 8 | 400, BID | 15 | 2.23/3.61 | 8.84/11.80 |
| Mean | 1.93/2.77 | 8.45/11.83 | ||
| SD | 0.60/0.83 | 1.06/1.75 | ||
| CV% | 31.1/30.0 | 12.5/14.8 | ||
| 4 | 400, QD | 15 | 1.33/1.82 | 3.45/4.87 |
| 5 | 400, QD | 15 | 1.40/2.94 | 3.49/7.01 |
| 6 | 400, QD | 15 | 1.11/1.78 | 2.53/4.27 |
| Mean | 1.28/2.18 | 3.16/5.38 | ||
| SD | 0.15/0.66 | 0.54/1.44 | ||
| CV% | 11.8/30.2 | 17.2/26.8 | ||
| 12 | 200, BID | 25 | 0.73/1.04 | 5.12/6.75 |
| 13 | 200, BID | 25 | 0.67/2.47 | ND |
| 14 | 200, BID | 25 | 0.83/2.96 | ND |
| 15 | 200, BID | 25 | 0.47/1.42 | ND |
| Mean | 0.68/1.97 | ND | ||
| SD | 0.15/0.89 | ND | ||
| CV% | 22.5/45.3 | ND | ||
| 16 | 400 AM, 200 PM | 25 | 0.90/1.40 | 4.19/6.80 |
| 17 | 400 AM, 200 PM | 25 | 1.30/2.19 | 3.17/5.39 |
| 20 | 400 AM, 200 PM | 25 | 1.00/1.66 | 6.28/9.08 |
| Mean | 1.07/1.75 | 4.55/7.09 | ||
| SD | 0.21/0.40 | 1.59/1.86 | ||
| CV% | 19.5/23.0 | 34.9/26.3 | ||
Cavg, average daily sorafenib plasma concentration; Cmax, peak sorafenib plasma concentration; BID=twice daily; QD=once daily; AM=morning dose; PM=evening dose; SD, standard deviation; CV%, coefficient of variation; ND, no data available.
Clinical Efficacy and Molecular Correlates
None of the patients had a clinical response. Ten patients had disease stabilization (SD) for at least 8 weeks; only three patients had disease stabilization for more than 16 weeks. The median number of cycles received per patient was 2 (range <1-8). Overall the median PFS was 2.1 months (Figure 1), and the median OS was 1.3 years (Supplemental Figure 1). Among the 10 patients treated at the MTD, two achieved stable disease, the median PFS was 2.0 months, and the median OS was 1.2 years.
Fig 1. Clinical and pharmacodynamic effects of treatment with sorafenib and temsirolimus.
A, Progression-free survival from the start of treatment with sorafenib and temsirolimus for all patients enrolled in the trial. B, Progression-free survival from the start of treatment with sorafenib and temsirolimus for all patients with known mutation status for BRAF and NRAS. Red, activating BRAF mutation; Blue, activating NRAS mutation; Black, wild-type BRAF and NRAS genes. P-value determined by log-rank testing. C, Changes in S6 Ser240 phosphorylation with treatment. The expression of phosphorylated (P) and total (T) S6 protein was determined by RPPA. The ratio of P:T S6 protein in the pre-treatment (Black bars) and day 15 (Gray) tumor biopsies in the patients with matching samples are shown. The patient mutation status, best clinical response, dose level, and temsirolimus dose are shown below each patient label. SD, stable disease; PD, progressive disease. D, Changes in ERK phosphorylation with treatment. In addition to mutation status, best clinical response, dose level, and sorafenib dose, the sorafenib Day 15 average serum concentration (Cavg) for each patient is indicated. Day 15 Cavg was not available for one patient (NA).
BRAF and NRAS tumor mutation status was determined for the patients. Ten (40%) patients had activating BRAF mutations, five (20%) had NRAS mutations, eight (32%) had wild-type BRAF and NRAS genes; mutation status could not be determined in two patients (8%). There was no significant difference in median PFS (p=0.34, Figure 1B) or OS (p=0.98, Supplemental Figure 1) by mutation status. Tumor mutation status was also not associated with the chance of achieving SD as a best response (p=0.75, Supplemental Table 1). A review of patient records identified nine patients with clinical testing for c-KIT gene mutations. One patient, who had wild-type BRAF and NRAS genes, was found to have a c-KIT L576P mutation. This patient had stable disease as the best response, and remained on treatment for 8 cycles before progressing, which was the longest progression-free survival of any patient in the trial. None of the other patients with c-KIT testing results had a mutation in the gene.
Ten patients with pre-treatment tumor biopsies also underwent an optional tumor biopsy on day 15 of cycle 1. Four patients underwent paired core needle biopsies of lymph node metastases, and six underwent paired punch biopies of cutaneous metastases. Review of the core needle biopsies revealed none of the samples contained isolates of >70% viable tumor of sufficient size for the planned proteomic analyses, while four of the six pairs of punch biopsies were sufficient for reverse phase protein array (RPPA) analysis. Two of the tumors were BRAF-mutant (1 V600E, 1 V600K), one was NRAS-mutant (Q61K), and one was wild-type for both genes. The NRAS mutant had stable disease as best response and received 4 cycles of treatment; the other three patients had progressive disease and received only 2 cycles. Comparison of the levels of the ratio of phospho:total S6 protein demonstrated that all four paired samples had at least a 25% decrease, and two had at least 75% decrease, in P-S6 following treatment with sorafenib and temsirolimus, consistent with inhibition of mTOR by temsirolimus (Figure 1C). In contrast, only one of the four samples showed a decrease of phospho:total ERK levels of greater than 25% (wild-type, progressive disease) (Figure 1D).
DISCUSSION
Clinical experiences in multiple cancers have demonstrated the promise of targeted therapies. However, these experiences have also identified the frequent, and often rapid, emergence of resistance as an important challenge (29). Based on the pattern of molecular alterations observed in melanoma and the results of preclinical studies, we performed a phase I clinical trial to determine the efficacy and toxicity of combined treatment with sorafenib, an inhibitor of BRAF and other kinases, and temsirolimus, an mTOR inhibitor, in patients with advanced melanoma. Our study identified significant toxicity with the combination at higher doses, and failed to achieve clinical responses in any of the patients. Biomarker analyses failed to identify any relationship between activating mutations in the RAS-RAF-MEK-ERK signaling pathway with progression-free survival. Further, pharmacodynamic analysis demonstrated that the combination achieved minimal inhibition of ERK activation.
Sorafenib was the first BRAF inhibitor to undergo clinical testing in melanoma following the discovery of frequent activating BRAF mutations in this disease. Although it had demonstrated promising results in preclinical models, only one of 37 metastatic melanoma patients achieved a clinical response with sorafenib monotherapy (15). Recently, the mutant-selective, second-generation BRAF inhibitors vemurafenib (also known as PLX4032 or RG7204) and GSK2118436 have demonstrated dramatic efficacy in metastatic melanoma patients with BRAF mutations (29, 30). In the phase III trial comparing vemurafenib to dacarbazine, treatment with vemurafenib resulted in significant improvement in the clinical response rate, PFS, and OS (31). Analysis of patients treated with vemurafenib in a phase I study demonstrated that clinical responses correlated with achievement of >60% inhibition of P-ERK expression in the tumor cells (17). Preclinical studies demonstrated that sorafenib inhibits P-ERK in human melanoma cell lines with activating BRAF mutations in vitro (14). In contrast to both the vemurafenib trial and the preclinical studies, in this trial of combined treatment with sorafenib and temsirolimus quantitative analysis of P-ERK levels in four patients with evaluable matching pre-treatment and day 15 biopsies demonstrated <10% inhibition in three of the patients, and 46% inhibition in one. Pharmacokinetic analysis found that the maximum and average levels of sorafenib on Day 15 detected in this trial (Table 5) are similar to those previously reported in sorafenib single-agent phase I trials (32). Thus, it is unlikely that the failure of sorafenib to inhibit P-ERK is due to a pharmacokinetic interaction with temsirolimus. While the in vivo pharmacodynamic failure of sorafenib to inhibit the RAS-RAF-MEK-ERK pathway has been hypothesized to contribute to its lack of efficacy, the results of this trial provide direct evidence to support this conclusion. These findings are consistent with an analysis of matched specimens from melanoma patients treated with sorafenib that did not identify significant changes in Ki67 or cyclin D1 expression, although these are not direct pharmacodynamic readouts of RAF kinases (16). Another study reported a positive correlation between pre-treatment expression levels of VEGFR2 and clinical responsiveness to treatment with sorafenib, paclitaxel, and carboplatin (33). VEGFR2 is a known target of sorafenib, and likely it responsible for its clinical activity in renal cell carcinoma. In all three studies the presence of activating BRAF mutations failed to correlate with clinical benefit from sorafenib. Taken together, the data supports that sorafenib should not be used to inhibit the RAS-RAF-MEK-ERK pathway in melanoma patients, particularly those with activating BRAF mutations.
In contrast to sorafenib, the measurement of P-S6 levels in the biopsies supports that temsirolimus was able to inhibit mTOR signaling at doses that were tolerated in this trial. This result is consistent with clinical studies with temsirolimus in other cancers (22, 23). While mTOR inhibitors have been well tolerated as single agents, they have failed to demonstrate significant clinical activity in several solid tumors, including melanoma (21). Preclinical studies demonstrated that inhibition of the mTORC1 complex, which occurs with rapalogs like temsirolimus, disrupts a negative feedback loop, resulting in hyperactivation of AKT (34). Consistent with this, biopsies from patients treated with everolimus, another mTOR inhibitor, showed a decrease in P-S6, but an increase in P-AKT Ser473 (35). In our study, there was no increase in P-AKT Ser473 levels with combined treatment with temsirolimus and sorafenib (Supplemental Figure 2). It is possible that concurrent treatment with sorafenib affected this feedback loop, as there appear to be multiple interactions between the RAS-RAF-MEK-ERK and PI3K-AKT-mTOR signaling pathways (10, 36, 37). However, a similar lack of increase in P-AKT Ser473 following single-agent temsirolimus was also recently observed in matching biopsies in head and neck cancer patients (23).
While there was no significant association between BRAF or NRAS mutations and disease stabilization or progression free survival, the patient in the trial with the longest treatment duration (8 months) was found to have a c-KIT L576P mutation. Two prior reports have described clinical responses and symptomatic benefit from sorafenib in metastatic melanoma patients with c-KIT V560D (38) and c-KIT D820Y (39) mutations, respectively. Interestingly, a biopsy performed after progression on sorafenib in this patient identified the continued presence of the c-KIT L576P mutation, and failed to identify any secondary mutations (40). As secondary resistance to KIT inhibitors in melanoma patients with c-KIT mutations is frequent, and as yet cannot be attributed to the presence of additional mutations in the c-KIT gene, it is possible that combinatorial approaches involving inhibitors targeting other pathways may be clinically beneficial in these patients.
In conclusion, we report here that treatment with sorafenib and temsirolimus is toxic and provides minimal clinical benefit in patients with advanced melanoma. This regimen was developed to test the anti-tumor effects of combined inhibition of the RAS-RAF-MEK-ERK and the PI3K-AKT-mTOR signaling pathways. Based on our pharmacodynamic analysis, we do not believe that this regimen achieved enough inhibition of the RAS-RAF-MEK-ERK signaling pathway to test the clinical benefit of such a therapeutic approach. A number of additional potent inhibitors against each of these pathways have become available for clinical testing since the design of this trial, and likely will be tested in combinatorial approaches in the near future. Our results, and the studies that led to the development of clinically effective BRAF inhibitors (17), support the critical need for pharmacodynamic evaluations in order to appropriately interpret the results of such trials.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Targeted therapies against the RAS-RAF-MEK-ERK signaling pathway have recently demonstrated dramatic activity in patients with melanoma, but their clinical benefit has been limited by the rapid development of resistance. A number of lines of evidence support that combined inhibition of the RAS-RAF-MEK-ERK and the PI3K-AKT pathways may be an effective strategy to overcome resistance. This report describes the results of the first clinical trial in melanoma assessing both the anti-tumor effects and molecular correlates of combined therapy against both of these pathways, with sorafenib (RAF inhibitor) and temsirolimus (mTOR inhibitor). The combination had significant toxicity and failed to achieve clinical responses in any patients, including patients with activating BRAF mutations. Pharmacodynamic analysis supports that the regimen was able to inhibit signaling by mTOR, but it did not inhibit ERK activation. The results reinforce the need for pharmacodynamic analysis in the evaluation of novel targeted therapies and combinations.
Acknowledgments
Supported in part by NCI grants UO1 CA062461 and N01 CM17003, the NIH through the MD Anderson Cancer Center Support Grant CA016672, the P50 SPORE Career Development Grant (K.B.K.), the ASCO Young Investigator Award (M.A.D.), and the MD Anderson Cancer Center Physician-Scientist Program (M.A.D., A.J.F.L.).
Footnotes
REFERENCES
- 1.Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–88. doi: 10.1002/humu.20481. [DOI] [PubMed] [Google Scholar]
- 2.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 3.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 4.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic Interaction Between NRAS and BRAF Mutations and PTEN//MMAC1 Inactivation in Melanoma. J Investig Dermatol. 2004;122:337–41. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao H, Zhang X, Fowlkes K, Haluska FG. Relative Reciprocity of NRAS and PTEN/MMAC1 Alterations in Cutaneous Melanoma Cell Lines. Cancer Res. 2000;60:1800–4. [PubMed] [Google Scholar]
- 7.Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, et al. Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma. Clin Cancer Res. 2009;15:7538–46. doi: 10.1158/1078-0432.CCR-09-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen A, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res. 2010;70:8736–47. doi: 10.1158/0008-5472.CAN-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired Resistance to BRAF Inhibitors Mediated by a RAF Kinase Switch in Melanoma Can Be Overcome by Cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SJ, Kim JS, Kim SW, Brantley E, Yun SJ, He J, et al. Macitentan (ACT-064992), a tissue-targeting endothelin receptor antagonist, enhances therapeutic efficacy of paclitaxel by modulating survival pathways in orthotopic models of metastatic human ovarian cancer. Neoplasia. 2011;13:167–79. doi: 10.1593/neo.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc) 2005;41:773–84. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- 14.Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–8. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 15.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott PA, Hamilton A, Min C, Safarzadeh-Amiri S, Goldberg L, Yoon J, et al. A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One. 2010;5:e15588. doi: 10.1371/journal.pone.0015588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark JW, Eder JP, Ryan D, Lathia C, Lenz H-J. Safety and Pharmacokinetics of the Dual Action Raf Kinase and Vascular Endothelial Growth Factor Receptor Inhibitor, BAY 43-9006, in Patients with Advanced, Refractory Solid Tumors. Clinical Cancer Research. 2005;11:5472–80. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 19.Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, Maczey E, et al. Combined Inhibition of MAPK and mTOR Signaling Inhibits Growth, Induces Cell Death, and Abrogates Invasive Growth of Melanoma Cells. J Invest Dermatol. 2008;128:2013–23. doi: 10.1038/jid.2008.44. [DOI] [PubMed] [Google Scholar]
- 20.DiVito KA, Charette LA, Rimm DL, Camp RL. Long-term preservation of antigenicity on tissue microarrays. Lab Invest. 2004;84:1071–8. doi: 10.1038/labinvest.3700131. [DOI] [PubMed] [Google Scholar]
- 21.Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–8. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 22.Peralba JM, deGraffenried L, Friedrichs W, Fulcher L, Grünwald V, Weiss G, et al. Pharmacodynamic Evaluation of CCI-779, an Inhibitor of mTOR, in Cancer Patients. Clinical Cancer Research. 2003;9:2887–92. [PubMed] [Google Scholar]
- 23.Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev. 2004;13:667–72. [PubMed] [Google Scholar]
- 24.Hennessy BT, Lu Y, Gonzalez-Angulo AM, Carey MS, Myhre S, Ju Z, et al. A Technical Assessment of the Utility of Reverse Phase Protein Arrays for the Study of the Functional Proteome in Non-microdissected Human Breast Cancers. Clin Proteomics. 2011;6:129–51. doi: 10.1007/s12014-010-9055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–21. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–8. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strumberg D, Voliotis D, Moeller JG, Hilger RA, Richly H, Kredtke S, et al. Results of phase I pharmacokinetic and pharmacodynamic studies of the Raf kinase inhibitor BAY 43-9006 in patients with solid tumors. Int J Clin Pharmacol Ther. 2002;40:580–1. doi: 10.5414/cpp40580. [DOI] [PubMed] [Google Scholar]
- 29.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kefford RF, Arkenau H, Brown MP, Milward M, Infante JR, Long GV, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28:8503. [Google Scholar]
- 31.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, Pharmacokinetics, and Preliminary Antitumor Activity of Sorafenib: A Review of Four Phase I Trials in Patients with Advanced Refractory Solid Tumors. The Oncologist. 2007;12:426–37. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 33.Jilaveanu L, Zito C, Lee SJ, Nathanson KL, Camp RL, Rimm DL, et al. Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res. 2009;15:1076–85. doi: 10.1158/1078-0432.CCR-08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and Schedule-Dependent Inhibition of the Mammalian Target of Rapamycin Pathway With Everolimus: A Phase I Tumor Pharmacodynamic Study in Patients With Advanced Solid Tumors. J Clin Oncol. 2008;26:1603–10. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Muller M, Smith D, Dutta B, Komurov K, Iadevaia S, et al. Kinome siRNA-phosphoproteomic screen identifies networks regulating AKT signaling. Oncogene. 2011 doi: 10.1038/onc.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–72. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintas-Cardama A, Lazar AJ, Woodman SE, Kim K, Ross M, Hwu P. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nature clinical practice. 2008;5:737–40. doi: 10.1038/ncponc1251. [DOI] [PubMed] [Google Scholar]
- 39.Handolias D, Hamilton AL, Salemi R, Tan A, Moodie K, Kerr L, et al. Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Br J Cancer. 2010;102:1219–23. doi: 10.1038/sj.bjc.6605635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009;8:2079–85. doi: 10.1158/1535-7163.MCT-09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.