Abstract
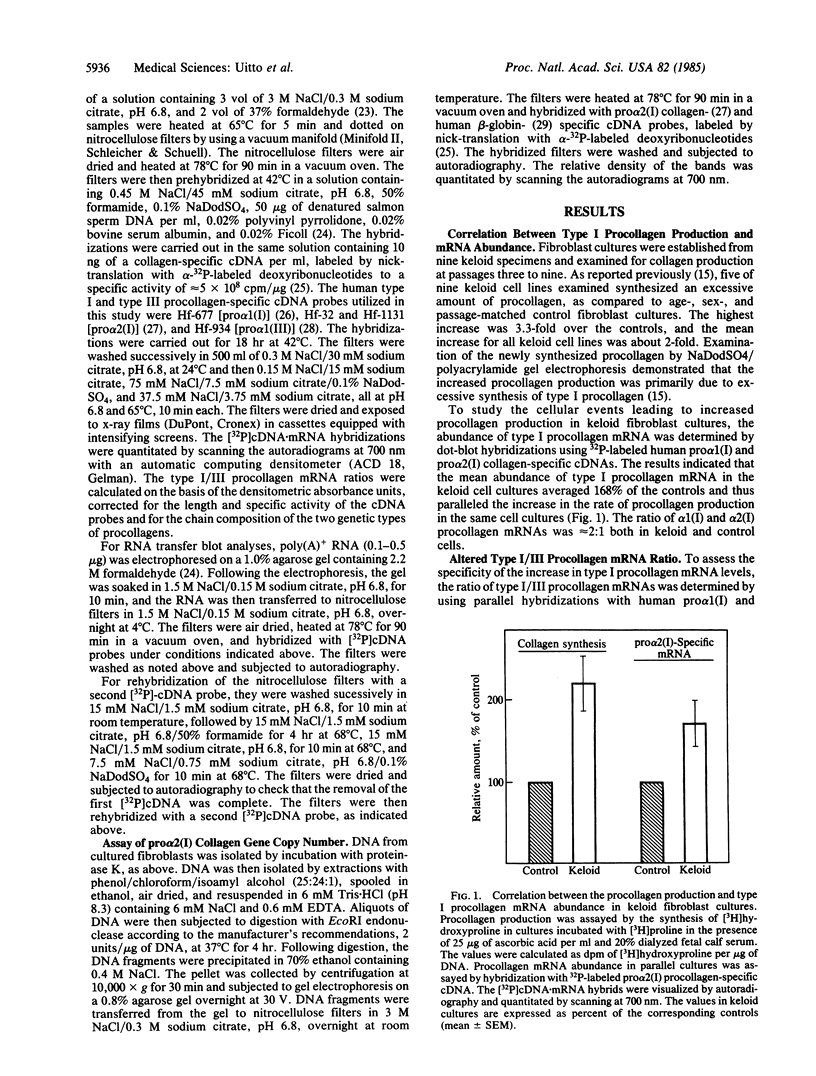
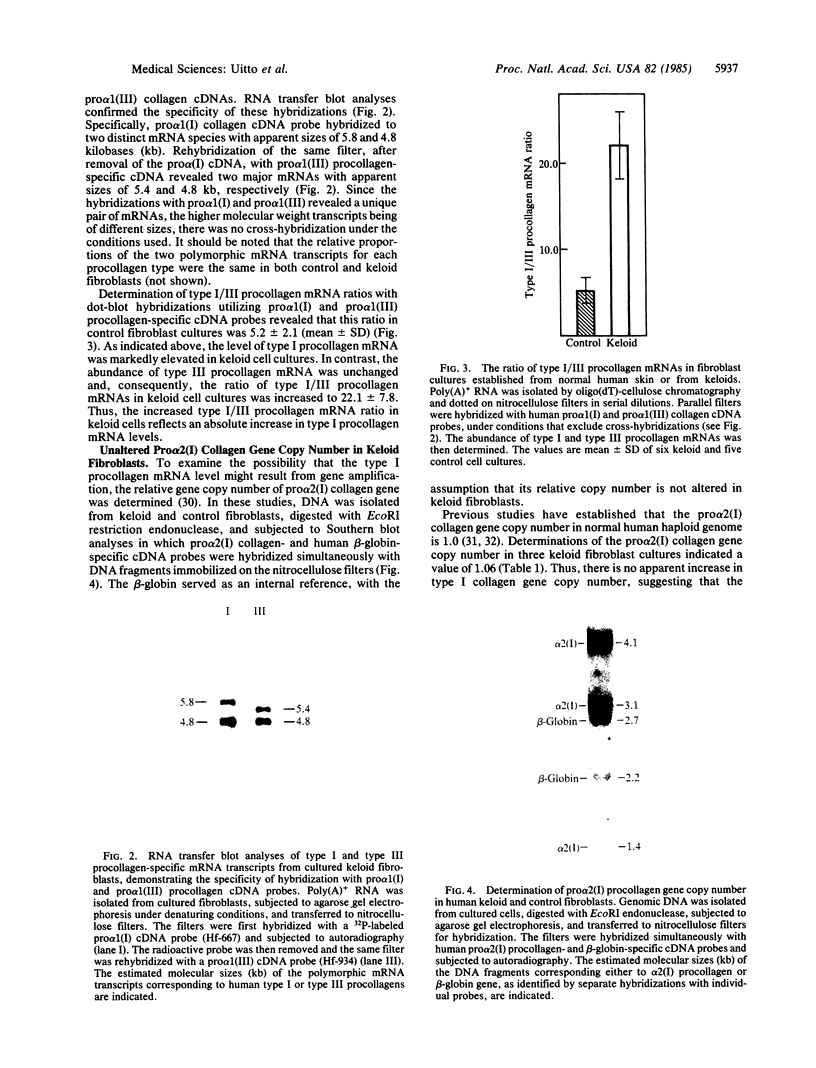
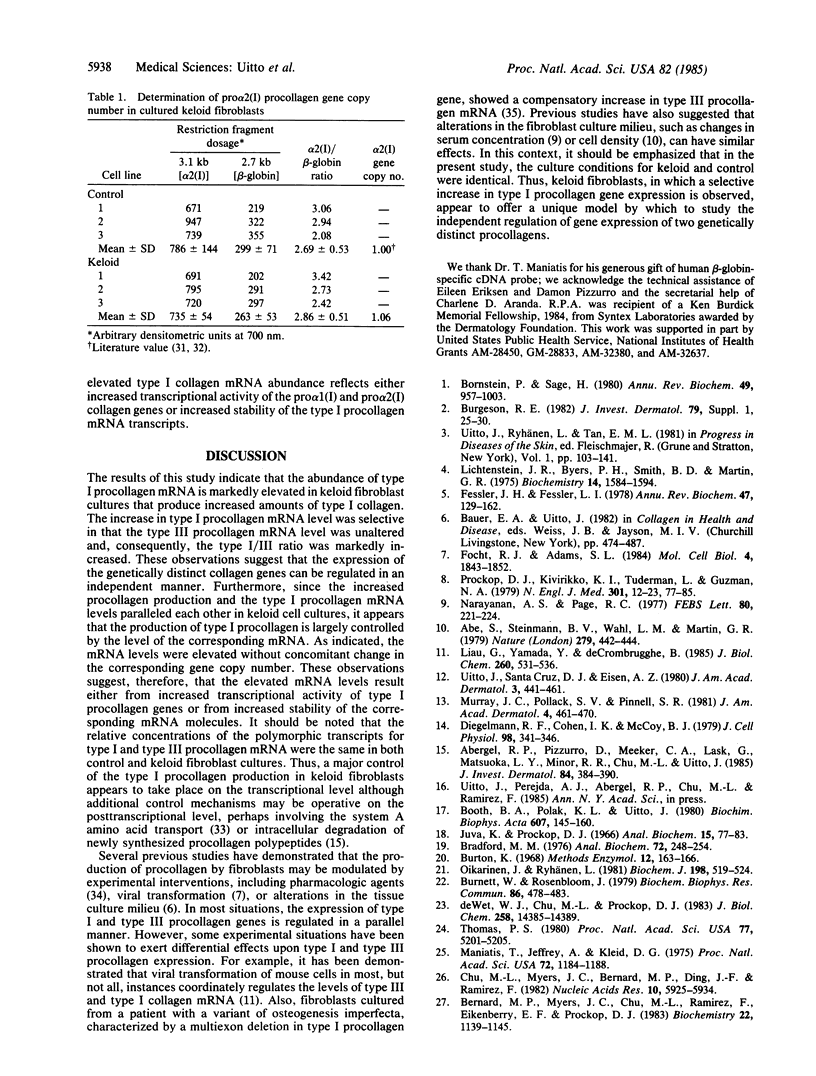
Regulation of collagen gene expression was studied in fibroblast cultures established from patients with keloids, fibrotic lesions of the skin. In selected keloid fibroblast cultures, an overproduction of type I procollagen was observed. This increase was accompanied by a parallel increase in type I procollagen-specific mRNA levels, as detected by dot-blot and RNA transfer hybridizations, without concomitant change in type I procollagen gene copy number. At the same time, type III procollagen mRNA levels were unaltered, resulting in markedly elevated type I/III procollagen mRNA ratios. Thus, keloid fibroblasts offer a unique model to study the independent regulation of the gene expression of two genetically distinct procollagens, type I and type III.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Steinmann B. U., Wahl L. M., Martin G. R. High cell density alters the ratio of type III to I collagen synthesis by fibroblasts. Nature. 1979 May 31;279(5712):442–444. doi: 10.1038/279442a0. [DOI] [PubMed] [Google Scholar]
- Abergel R. P., Pizzurro D., Meeker C. A., Lask G., Matsuoka L. Y., Minor R. R., Chu M. L., Uitto J. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985 May;84(5):384–390. doi: 10.1111/1523-1747.ep12265471. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Booth B. A., Polak K. L., Uitto J. Collagen biosynthesis by human skin fibroblasts. I. Optimization of the culture conditions for synthesis of type I and type III procollagens. Biochim Biophys Acta. 1980 Mar 28;607(1):145–160. doi: 10.1016/0005-2787(80)90228-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnett W., Rosenbloom J. Isolation and translation of elastin mRNA from chick aorta. Biochem Biophys Res Commun. 1979 Feb 14;86(3):478–484. doi: 10.1016/0006-291x(79)91739-x. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Gargiulo V., Williams C. J., Ramirez F. Multiexon deletion in an osteogenesis imperfecta variant with increased type III collagen mRNA. J Biol Chem. 1985 Jan 25;260(2):691–694. [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Weil D., de Wet W., Bernard M., Sippola M., Ramirez F. Isolation of cDNA and genomic clones encoding human pro-alpha 1 (III) collagen. Partial characterization of the 3' end region of the gene. J Biol Chem. 1985 Apr 10;260(7):4357–4363. [PubMed] [Google Scholar]
- Dalgleish R., Trapnell B. C., Crystal R. G., Tolstoshev P. Copy number of a human type I alpha 2 collagen gene. J Biol Chem. 1982 Nov 25;257(22):13816–13822. [PubMed] [Google Scholar]
- Diegelmann R. F., Cohen I. K., McCoy B. J. Growth kinetics and collagen synthesis of normal skin, normal scar and keloid fibroblasts in vitro. J Cell Physiol. 1979 Feb;98(2):341–346. doi: 10.1002/jcp.1040980210. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Focht R. J., Adams S. L. Tissue specificity of type I collagen gene expression is determined at both transcriptional and post-transcriptional levels. Mol Cell Biol. 1984 Sep;4(9):1843–1852. doi: 10.1128/mcb.4.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junien C., Weil D., Myers J. C., Van Cong N., Chu M. L., Foubert C., Gross M. S., Prockop D. J., Kaplan J. C., Ramirez F. Assignment of the human pro alpha 2(I) collagen structural gene (COLIA2) to chromosome 7 by molecular hybridization. Am J Hum Genet. 1982 May;34(3):381–387. [PMC free article] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Liau G., Yamada Y., de Crombrugghe B. Coordinate regulation of the levels of type III and type I collagen mRNA in most but not all mouse fibroblasts. J Biol Chem. 1985 Jan 10;260(1):531–536. [PubMed] [Google Scholar]
- Lichtenstein J. R., Byers P. H., Smith B. D., Martin G. R. Identification of the collagenous proteins synthesized by cultured cells from human skin. Biochemistry. 1975 Apr 22;14(8):1589–1594. doi: 10.1021/bi00679a007. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. C., Pollack S. V., Pinnell S. R. Keloids: a review. J Am Acad Dermatol. 1981 Apr;4(4):461–470. doi: 10.1016/s0190-9622(81)70048-3. [DOI] [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Serum modulates collagen types in human gingiva fibroblasts. FEBS Lett. 1977 Aug 1;80(1):221–224. doi: 10.1016/0014-5793(77)80444-4. [DOI] [PubMed] [Google Scholar]
- Oikarinen J., Ryhänen L. Cortisol decreases the concentration of translatable type-I procollagen mRNA species in the developing chick-embryo calvaria. Biochem J. 1981 Sep 15;198(3):519–524. doi: 10.1042/bj1980519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Russell S. B., Russell J. D., Trupin J. S. Hydrocortisone induction of system A amino acid transport in human fibroblasts from normal dermis and keloid. J Biol Chem. 1984 Sep 25;259(18):11464–11469. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Myers J. C., Ramirez F., Prockop D. J. Restriction fragment length polymorphism associated with the pro alpha 2(I) gene of human type I procollagen. Application to a family with an autosomal dominant form of osteogenesis imperfecta. J Clin Invest. 1983 Oct;72(4):1262–1267. doi: 10.1172/JCI111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Ryhänen L., Tan E. M., Oikarinen A. I., Zaragoza E. J. Pharmacological inhibition of excessive collagen deposition in fibrotic diseases. Fed Proc. 1984 Oct;43(13):2815–2820. [PubMed] [Google Scholar]
- Uitto J., Santa Cruz D. J., Eisen A. Z. Connective tissue nevi of the skin. Clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980 Nov;3(5):441–461. [PubMed] [Google Scholar]
- de Wet W. J., Chu M. L., Prockop D. J. The mRNAs for the pro-alpha 1(I) and pro-alpha 2(I) chains of type I procollagen are translated at the same rate in normal human fibroblasts and in fibroblasts from two variants of osteogenesis imperfecta with altered steady state ratios of the two mRNAs. J Biol Chem. 1983 Dec 10;258(23):14385–14389. [PubMed] [Google Scholar]





