Abstract
Objective
Obesity is associated to high insulin and glucagon plasma levels. Enhanced β–cell function and β–cell expansion are responsible for insulin hypersecretion. It is unknown whether hyperglucagonemia is due to α-cell hypersecretion or to an increase in α-cell mass. In this study, we investigated the dynamics of the β-cell and α-cell function and mass in pancreas of obese normoglycemic baboons.
Methods
Pancreatic β- and α-cell volumes were measured in 51 normoglycemic baboons divided into 6 groups according to overweight severity or duration. Islets morphometric parameters were correlated to overweight and to diverse metabolic and laboratory parameters.
Results
Relative α-cell volume (RαV) and relative islet α-cell volume (RIαV) increased significantly with both overweight duration and severity. Conversely, in spite of the induction of insulin resistance, overweight produced only modest effects on relative β-cell volume (RβV) and relative islet β-cell volume (RIβV). Of note, RIβV did not increase neither with overweight duration nor with overweight severity, supposedly because of the concomitant, greater, increase in RIαV. Baboons' body weights correlated with serum levels of Interleukin-6 and Tumour Necrosis Factor-α soluble Receptors (IL-6sR and sTNF-R1), demonstrating that overweight induces abnormal activation of the signaling of two cytokines known to impact differently β- and α-cell viability and replication.
Conclusion
In conclusion, overweight and insulin resistance induce in baboons a significant increase in α-cell volumes (RαV, RIαV) while have minimal effects on the β-cells. This study suggests that an increase in the α-cell mass may precede the loss of β-cells and the transition to overt hyperglycemia and diabetes.
Keywords: Obesity duration, obesity severity, α-cell volume, β-cells volume, pancreatic islet remodelling, insulin resistance
INTRODUCTION
Maintenance of normal glucose homeostasis depends mainly on the physiological balance between the effects on target tissues of insulin secreted by the β-cells and glucagon secreted by the α-cells. Paracrine interactions between β- and α-cells are also critical for this homeostatic system implying that equilibrium between β- and α-cell mass should exist to maintain normal glucose homeostasis (1–3). The mechanisms responsible for dynamic changes of the islets of Langerhans architecture are replication, neogenesis, hypertrophy and apoptosis (1–5). Previous studies on islet pathology in type 2 diabetes (T2D) were mainly focused on the failure of the β-cell to respond to an increased insulin secretory demand while the concomitant dysregulation of the α-cells function, and the disproportionately increased number of α-cells relative to β-cells of the islets of T2D subjects have received less attention (6–16). The increased α-cell volumes found in T2D subjects has been always considered as the result of the increased rate of β-cell death. Obesity is one of the main factors associated to insulin resistance and T2D. In normoglycemic obese individuals a compensatory increase in insulin secretion maintains normal glucose levels (8, 10, 17). Enhanced β-cell function and β-cell expansion are responsible for the hypersecretion of insulin observed in obese normoglycemic humans and rodents (2, 7, 18). More than thirty years ago it has been shown that normoglycemic obese subjects show also high glucagon levels but only recently this issue has received novel attention (19). Recent studies confirmed that obesity and insulin resistance are indeed associated to α-cell hypersecretion and that abnormally high fasting glucagon levels are present in normoglycemic insulin-resistant obese adults and adolescents (20–23). In the latter group, elevated fasting glucagon levels were observed in the face of elevated fasting insulin levels, suggesting that α-cells manifest insulin resistance to the suppressive effect of insulin on glucagon secretion. Data regarding the β-cell mass of normoglycemic obese humans are extremely limited and little is known about the α-cell mass (18). For instance, it is presently unknown if the hyperglucagonemia observed in obesity and T2D is exclusively due to the increased secretion of glucagon by the α-cells or also to an expansion of the α-cell mass due to an increased rate of replication. We showed previously that α-cell growth is one of the determinants, together with islet amyloidosis and reduced β-cell volume of the islets' of Langerhans remodelling of diabetic baboons (24). The aim of the present study was to learn more about the interplay between pancreatic β- and α-cell in normoglycemic baboons with different degrees of overweight severity and duration.
MATERIAL AND METHODS
Study Population
We studied normoglycemic baboons with different degrees of overweight selected from a large cohort followed during the last 14 years (from 1994 to 2007) at the Pathology database in the Southwest Foundation for Biomedical Research (SFBR). We included baboons older than 8 years with availability of pancreatic tissue, clinical and laboratory measurements, and blood samples collected during their last six months of life (2.7 ± 1.3 months) as well as the weight record during their whole life. Baboons exposed to experimental protocols that could have affected pancreatic islet morphology were excluded. 51 baboons were finally included in the present work. Animals were fed with an ad libitum standard monkey chow diet 5038 (Purina, St.Louis, MO) and housed in corrals where they performed unrestrained physical activity. Baboons were considered overweight when their weight was above the 75th percentile of the weight distribution in more than 1000 adult baboons according to sex, being 21.7 kg for females and 33.6 kg for males (24). They were stratified in 6 groups according to overweight duration (D1 = non-overweight, D2 = overweight before death for at least 3 years but not during the last 3 years of life, D3 = <5 years of overweight duration, D4 = 5–8 years of overweight duration, D5 = 9–12 years of overweight duration, and D6 = >12 years of overweight duration) and according to overweight severity (S1 = non-overweight, S2 = overweight before death for at least 3 years but not during the last 3 years of life, S3 = <8% above the 75th percentile, S4 = 8–15% above the 75th percentile, S5 = 16–30% above the 75th percentile, and S6 = >30% above the 75th percentile).
A separate group of 80 living baboons was also studied to evaluate the relationship between weight and IL6sR and sTNFα R1 circulating levels. For the purpose of this study, we analyzed the data of those animals that were normoglycemic (n=40).
Tissue Processing
Complete necropsies were performed on all the baboons. Pancreatic tissue from the body-tail region was fixed in 10% neutral-buffered formalin, processed conventionally and embedded in paraffin blocks. Pancreases were cut into sections of 5μm of thickness that were stained for H&E, Congo red, insulin and glucagon for evaluation of islets, amyloid deposits, β- and α-cells morphometry.
Immunohistochemistry
Immunohistochemistry stains were done using Ventana Predulite (insulin) and Dako predulite (glucagon) antibodies; all of them were run on the Ventana Benchmark XT automated immunostainer using the Enhanced V-RED detection kit.
Morphological Measurements
The Computer Assisted Stereology Toolbox (CAST) 2.0 system from Olympus (Ballerup, Denmark) was used to perform all the microscopical measurements, using the stereology fundaments, which have been previously described and validated (25, 26). For the purpose of the present work we used the point counting method. Each field of view was found systematically and randomly using the CAST meander sampling facility covering the entirety of the section, such that in average we evaluated 370 fields and 100 islets per section, and placed 20720 points per section. In each field of view, point counting of total pancreatic tissue, islets, amyloid deposits, β- and α- cells at 100X were used to estimates the relative volume of each component. As pancreases weights were not available it was impossible to calculate the relative masses. Counts were summed over all fields per slice and then ratios of: - total islets points over total pancreatic tissue points was used to get the relative islets volume and, - total amyloid deposit points over total pancreas points and over total islets points were used to get the relative volume of pancreas and islets occupied by amyloid deposits, respectively. In the same way, we calculated the relative β- and α- cells volume. For this purpose we used the following formula: Volume density (Vv) or Relative volume = (PP/PT) × 100, where PP = points that hit a specific structure and PT = total points (44341 points evaluated per slice/baboon). Islets size area was estimated inserting a unique point inside the islet and several probe lines and marking the intersections between the islets' border and the probe lines. Non-acinar tissue such as ducts, vascular bundles and adipose tissue were not included as part of the tissue area.
The person who performed microscopical measurements was blinded to the metabolic status of the baboons and the reproducibility of the measurements was estimated twice in five specimens with a coefficient of variation less than 5%.
For clarity purposes in the text and figures are used the following abbreviations:
-
-
Relative islet volume/relative pancreas volume (Relative Islet Volume): RIV
-
-
Relative amyloid volume/relative pancreas volume (Relative Amyloid Volume): RAV
-
-
Relative amyloid volume/relative islet volume (Relative Islet Amyloid Volume): RIAV
-
-
Relative β-cell volume/relative pancreas volume (Relative β-cell Volume): RβV
-
-
Relative β-cell volume/relative islet volume (Relative Islet β-cell volume): RIβV
-
-
Relative α-cell volume/relative pancreas volume (Relative α-cell Volume): RαV
-
-
Relative α-cell volume/relative islet volume (Relative Islet α-cell Volume): RIαV
Analytical Measurements
Blood glucose was measured by the glucose oxidase method with the SYNCHRON CX System (Beckman Coulter, Inc), insulin levels by RIA (Linco Research, St. Louis, MO), glucagon by RIA (Euro-Diagnostica AB, Malmö, Sweden) and Free fatty acid concentrations were determined using the fluorometric method. All these measurements were performed on blood samples collected during the last 2.7 ± 1.3 months (last 6 months) of baboons' life and the baboons included in this study were normoglycemic. Obese hyperglycemic baboons were excluded from this study.
A quantitative sandwich-based ELISA was performed to measure serum levels of soluble receptor of the cytokines interleukin 6 (IL-6 sR) and Tumor necrosis factor α (sTNF R1) (R&D Systems, Minneapolis, MN, USA) in a separate group of 40 living normoglycemic baboons to evaluate the relationship between weight and cytokines levels. The mean minimum detectable dose (MDD) was 6.5pg/mL for IL-6 sR and 0.77pg/mL for sTNF R1.
Calculations
Insulin resistance measured by homeostasis model (HOMA-IR) was calculated as FPI (μU/ml) × FPG (mmol/l) / 22.5. HOMA β-cell function (HOMA-β) was estimated as FPI (μU/ml) × 20 / FPG (mmol/l) - 3.5, as reported by Matthews et al. (27). The insulin resistance index in the adipose tissue was calculated by multiplying serum insulin × FFA serum levels.
Statistical Analysis
Data are presented as mean ± SEM. Student's t test was used for comparisons between two groups and ANOVA with a Bonferroni as a post hoc test for comparisons between more than two groups when normal distribution was confirmed. Log transformed values were used for those with a skewed distribution, confirming a normal distribution after the log transformation. A p value less than 0.05 was considered statistically significant.
RESULTS
Biochemical characteristics
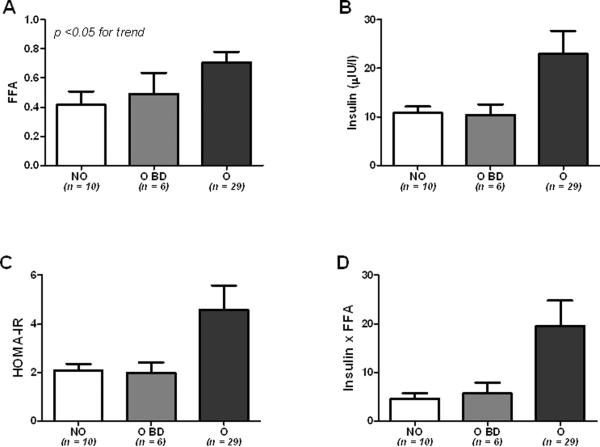
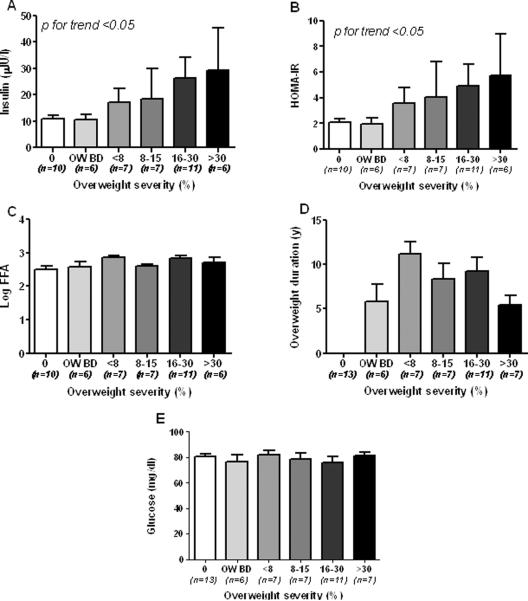
To establish whether there was any difference between overweight and non-overweight baboons, in a primary analysis the animals were divided only in 3 groups: non overweight (NO), overweight before death (O BD), and overweight (O). In these 3 groups, FFA and insulin levels were increased in obese vs non-obese and obese before death baboons (Figure 1A and B, p<0.05 for trend) as well as the HOMA-IR and adipose tissue insulin resistance index (Figure 1C and D). Table 1 shows biochemical and anthropometrical data of the baboons according to overweight duration. Non-significant statistical differences were observed in FPG (p >0.05). Insulin and total cholesterol levels, Ln_HOMA-B and Ln_HOMA-IR, were also not statistically different between groups, although tended to be higher in baboons with overweight duration between 5 and 8 years. Body weight and Ln-FFA were significantly higher in baboons according to overweight duration (p<0.05). Insulin levels and HOMA-IR progressively increased according to overweight severity (Figures 2A–B, p<0.05). In contrast, Log-FFA, overweight duration and glucose did not show any significant correlation with overweight severity (Figure 2C, D and E).
Fig. 1.
Biochemical profile in non-overweight (NO), overweight before death (O BD) and overweight normoglycemic baboons (O). FFA (A), insulin (B), HOMA-IR (C) and Insulin × FFA (D) according to overweight status.
Table 1.
Clinical and biochemical characteristics of the different groups according to overweight duration
| Variable (n) | Group 1 Control |
Group 2 Overweight before death |
Group 3 <5 years |
Group 4 5 – 8 years |
Group 5 9–12 years |
Group 6 >12 years |
p |
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=51 | 13 | 6 | 7 | 10 | 6 | 9 | |
| Age (years) | 19.8 ± 0.7 | 23.0 ± 0.9 | 13.0 ±0.9ab | 17.3 ± 1.0b | 18.2 ± 1.5c | 24.3±1.2acde | 0.004 |
| Sex (F/M) | 8/5 | 4/2 | 3/4 | 3/7 | 4/2 | 5/4 | 0.910 |
| Weight (kg) | |||||||
| - Females (n) | 14.8 ± 0.5 | 16.8 ± 1.0 | 24.3 ± 0.3 | 26.1 ± 2.4 | 26.2 ± 2.0 | 25.9 ± 1.2 | 0.001 |
| - Males (n) | 26.9 ± 1.7 | 28.3 ± 1.7 | 36.0 ± 0.5 | 40.5 ± 1.4 | 41.0 ± 6.3 | 41.0 ± 1.0 | 0.001 |
| Overweight severity % | 0 | 10.1±1.9 | 28.8±5.1 | 20.7±4.8 | 20.4±8.6 | 13.1±2.4 | <0.001 |
| FPG (mg/dl) | 80.2 ± 2.0 | 76.7 ± 5.3 | 73.0 ± 5.3 | 82.8 ± 3.5 | 79.0± 3.8 | 79.8±3.6 | 0.554 |
| Ln_FFA (mEq/L) | 2.5 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.05 | 2.9±0.07 | 0.006* |
| Insulin (μIU/L) | 10.8 ± 1.3 | 10.5 ± 2.1 | 24.5 ± 8.6 | 32.3 ± 10.2 | 12.9 ± 7.7 | 18.3±8.6 | 0.233 |
| Cholesterol (mg/dl) | 95.6 ± 9.0 | 79.8 ± 3.6 | 124.6 ± 22.1 | 113.0 ± 8.0 | 81.8 ± 9.3 | 97.4±8.9 | 0.098 |
| Ln_HOMA-B | 5.6 ± 0.1 | 5.5 ± 0.1 | 5.6 ± 0.2 | 5.7 ± 0.1 | 5.4 ± 0.2 | 5.3 ± 0.1 | 0.570 |
| Ln_HOMA-IR | 3.4 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.2 | 3.7 ± 0.1 | 3.3 ± 0.2 | 3.2 ± 0.1 | 0.299 |
F=female, M=male, FPG=fasting plasma glucose, FFA=free fatty acids.
p <0.05 vs Group 1,
p <0.05 vs Group 2,
p<0.05 vs Group 3,
p<0.05 vs Group 4.
Fig. 2.
Insulin values (A), Log FFA (B), glucose (C), overweight duration (D) and HOMA-IR according to overweight severity in normoglycemic baboons.
Islet morphology
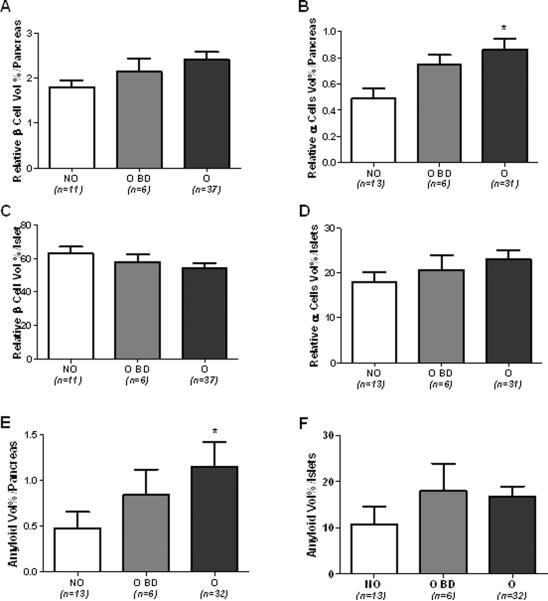
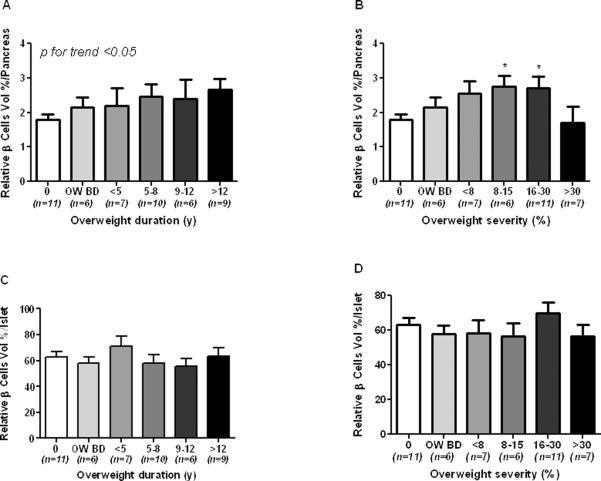
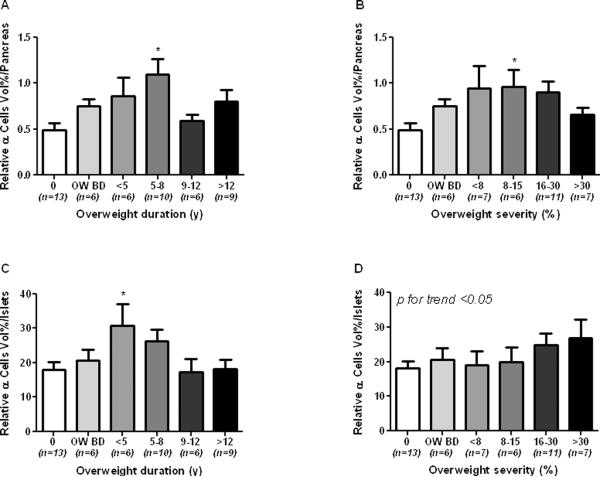
In a first series of analysis the baboon population was divided in the same 3 groups already described for the analysis of the biochemical profile. As compared to NO baboons, relative β-cell volume (RβV) was increased by 20 and 33 % in O BD and O baboons, respectively, (1.79 ± 0.15 vs 2.14 ± 0.29 and 2.40 ± 0.17%) (Figure 3A). Relative α-cell volume (RαV) was also increased by 55 and 80 % in O BD and O, respectively, as compared to NO baboons (fig. 3B). Interestingly, non significant differences were found between the three groups when the relative β- and α-cell volumes were adjusted for the total islet volume (RIβV, RIαV, Fig. 3C,D), although there was a slight increase in RIαV in the last two groups (11 and 27%, respectively). Relative amyloid volume (RAV) was higher in the O group (> 100% vs NO group, p <0.05, Figure 3E), but relative islets amyloid volume (RIA) was not statistically different among the 3 groups (Figure 3F). We then proceed to describe the data according to overweight duration and severity. Islet volume/pancreas, islet size and islet density (islets × mm2), tend to be higher according to overweight duration (Suppl. Figure 1A, C and E). In contrast, when plotted according to overweight severity all these parameters showed a progressive decrease form the lowest to the highest degree of obesity (Suppl. Figure 1B, D and F) even though statistical significance was not achieved. RβV progressively increased according to overweight duration (Figure 4A, p<0.05) and overweight severity (Figure 4B, p<0.05) with a significant decrement in the group with >30% of overweight severity. On the other hand, RIβV was not different between groups (Figures 4C and D). RαV progressively increased in groups with overweight duration between 5 and 8 years and with overweight severity between 8–15%, showing a slight decrease in baboons with overweight duration >9 years and severity >15% (Figures 5A and B, p<0.05). RIαV according to overweight duration showed a similar inverted U shape profile (Figure 5C, p<0.05). However, interestingly, RIαV progressively increased according to overweight severity to reach the highest value in baboons with >30% of overweight severity (Figure 5D, p<0.05).
Fig. 3.
Relative β cell vol/pancreas (A), relative α cell vol/pancreas (B), relative β cell vol/islet (C), relative α cell vol/islet (D), amyloid vol/pancreas (E), and amyoid vol/islet according to the overweight status in normoglycemic baboons.
Non-overweight (NO), overweight before death (O BD), and overweight (O) normoglycemic baboons. *p<0.05 vs NO group.
Fig. 4.
Relative beta cell vol/pancreas according to overweight duration (A) and severity (B), and relative beta cell vol/islet according to overweight duration (C) and severity (D) in normoglycemic baboons.
0 = control group, OW BD=overweight before death
*p <0.05 vs. control group
Fig. 5.
Relative alpha cell vol/pancreas according to overweight duration (A) and severity (B), and relative alpha cell vol/islet according to overweight duration (C) and severity (D).
0 = control group, OW BD = overweight before death
*p<0.05 vs. control group
Serum levels of soluble IL-6 and TNF-α receptors
Serum levels of soluble IL-6 and TNF-α receptors (IL-6 sR and sTNF-R1) positively correlated with body weight in a subgroup of 40 living normoglycemic baboons (r=0.3702, p=0.0102 and r=0.5683 p=0.0001, respectively) (Suppl. Figures 2A–B); in these group of baboons there was a 22.5 % prevalence of overweight and there were not significant differences in age, glucose, insulin and HOMA-IR between females and males (Suppl. Table 1); thereby demonstrating the induction of abnormal activation of the pro-inflammatory IL-6 and TNF-α cytokines signaling systems by the overweight.
DISCUSSION
By sharing a 97% genetic similarity to the humans, baboons (Papio Hamadryas) represent an ideal model for the study of molecular and pathogenesis of human obesity, insulin resistance and T2D. We have previously reported the correlation between insulin resistance, body fat and waist circumference in this model, and that insulin resistance is characterized by the molecular alterations reminiscent to what has been previously described in rodents and humans (28, 29, 30, 31). We also demonstrated that amyloid deposition is a critical determinant of beta-cell failure and islet remodeling in non-human primates with T2D, similar to T2D patients (24, 32). In the present study we determined the impact of overweight severity and duration on clinical and biochemical data and pancreatic β- and α-cell volumes in normoglycemic baboons.
We divided the baboons in 3 groups exclusively based on : (i) the absence of overweight (NO), (ii) the presence of overweight before death but not in the last 3 years of life (OBD) and (iii) the presence of overweight (O), and we observed that the latter group showed significantly higher fasting FFAs and insulin levels, HOMA-IR and adipose tissue insulin resistance index (insulin × FFA) than the former two groups.
Islet morphometric analysis revealed some unexpected findings. In fact, overweight and obesity induced only modest positive effects on RβV (increased by 20 and 34% in the O BD and O group, respectively), and RIβV tended to decrease in normoglycemic overweight baboons (Fig. 3A,C) possibly as the result of the slight increase in the relative amyloid volume (RAV) and RIαV. Surprisingly, the impact of obesity was greater for RαV and RIαV which showed a significant increase (Fig. 3B and D). Of note, overweight baboons showed also a higher RAV (Fig. 3E), confirming our previous observation that amyloid deposition precedes hyperglycemia and is likely to play an important role in β-cell failure in non-human primates as well as in human with T2D (24, 32).
A significant positive trend was observed between RβV and overweight duration (Fig. 4A), and only partially between RβV and overweight severity (Fig. 4B). In contrast, RIβV did not change neither with overweight duration nor with overweight severity (Fig. 4C and D). Lack of correlation between overweight severity and RIβV might be explained by a concomitant, and greater, increase in the RIαV. Obesity is associated to β-cell mass increase in rat and mice (33–35). Similar results were observed in larger mammals but data on humans are few and controversial (18, 36). Some have reported an increased β-cell mass in nondiabetic obese humans while other investigators have not, consistent with the fact that the rate of replication of the human β-cells is much lower than that of rodents and decrease with age (7, 11, 14, 18, 37, 38). The genetic and physiological similarity to humans, might also explain the observed modest effect that overweight exerts on the baboon pancreatic β-cell volume.
The major, unexpected, finding of this study is that RαV and RIαV increased together with overweight duration and severity (Fig. 3B, D; Fig. 5). In fact, overweight-induced increase in RαV and RIαV was significantly greater than that in RβV and RIβV. The steepest increase in RαV and RIαV was observed during the first 5–8 years of overweight duration indicating that is an early event (Fig. 5A and C). Only at the highest degree of overweight duration, perhaps due to aging, there was a reduction of RαV and RIαV which, however, remained similar to those of the control group. The impact of overweight severity on α-cell volumes was different as RIαV increased further even at the highest degree of overweight severity (Fig. 5D). Despite the early recognition, the role of the α-cell in the pathogenesis of T2D has returned to receive the adequate attention only in recent years (1, 39, 40). The correlation between body weight and α-cell mass has never been studied, nevertheless, a progressive increase in the α-cell number, leading to an imbalance between β- and α-cell mass, might occur for several reasons during obesity.
Interleukin-6 (IL6) is a pleiotropic cytokine that influences metabolic regulation and whose levels are chronically elevated in obesity and higher levels are present in T2D (41–43). IL6 is also a potent regulator of cellular proliferation and survival and the pancreatic α-cell is a primary target of IL6 which increases glucagon secretion and stimulates α-cell proliferation (44). We also show that both IL-6 sR and sTNF-R1 (Suppl. Figure 2 A–B) positively correlated with baboons body weight, thereby suggesting that the induction of an abnormal activation of IL-6 and TNF-α signaling systems could be associated with increased α-cell mass.
Overweight-induced increase in α-cell mass and function may directly challenge neighbouring β-cells via an increased glutamate extracellular concentration in the islet microenvironment. Glutamate is co-secreted with glucagon by the α-cells, and acute stimulation of glutamate receptors modulates the secretion of either glucagon, insulin and somatostatin (45–52). We have recently shown that β-cells are selectively vulnerable to glutamate-induced cytotoxicity and that the glial glutamate transporter 1 (GLT1), present on the β-cell membrane, promotes β-cell survival by controlling extracellular glutamate levels (52). Changes in the islet's β- to α-cell ratio in favour of the α-cells may thereby perturb islet glutamate homeostasis and cause β-cell death.
The increased α-cell volumes in the face of the stability of the β-cells, and the weight-related increase in IL6 strongly suggest that overweight-induced islet remodelling is in favour of the α-cells, possibly through proliferation (1, 13, 23, 24, 32, 44, 53).
We also found a significant increase of amyloid deposits into islets of Langerhans in obese baboons. These findings are consistent with previous data showing that islet amyloid deposition is strongly associated with hyperglycemia and hyperinsulinemic compensatory response and that amylin hypersecretion and its incomplete processing might negatively affect β-cell function and survival (9, 24, 32).
In conclusion, we believe that obesity-induced modest changes in the β-cell volume in normoglycemic baboons concomitant with increased α-cell volumes and amyloid deposits might have have important implications in the natural history of obesity and T2D. Obesity-mediated increase in α-cell number and secretion of glucagon might exacerbate insulin resistance (54). On the other hand, increased secretion of glutamate by α-cells, might directly impair β-cell function and survival via glutamate-induced β-cell death, possibly preceding the β-cell failure of overt T2D (6,7,11,52,55).
Supplementary Material
Islet morphology according to overweight duration and severity in normoglycemic baboons. Islet volume (A and B), islet size in μm2 (C and D), and islet density (islets × mm2 ) (E and F), according to overweight duration and severity.
Correlation between IL-6 sR (A) and sTNF-RI (B) with weight in living normoglycemic baboons.
ACKNOWLEDGMENTS
Dr. Rodolfo Guardado Mendoza was supported by SANOFI endocrine fellowship. Dr. Teresa Vanessa Fiorentino was supported in part by fellowship from FO.RI.SID This work was partially supported by NIH RO1 DK080148 (Dr. Franco Folli)
Footnotes
CONFLICT OF INTERST The authors declare no conflict of interest.
Supplementary information is available at IJO's website
REFERENCES
- 1.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A. 2010;107(37):16009–12. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn AR, Blanco C, Perego C, Finzi G, La Rosa S, Capella C, Guardado-Mendoza R, Casiraghi F, Gastaldelli A, Johnson M, Dick E, Folli F. The Ontogeny of the Endocrine Pancreas in the Fetal/Newborn Baboon. J Endocrinol. 2012 doi: 10.1530/JOE-12-0070. e-pub ahead of print 21 Jun 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85(4):1255–70. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 2000;141(6):1926–9. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 5.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–8. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folli F, Okada T, Perego C, Gunton J, Liew CW, Akiyama M, D'Amico A, La Rosa S, et al. Altered insulin receptor signalling and β-cell cycle dynamics in type 2 diabetes mellitus. PLoS One. 2011;6(11):e28050. doi: 10.1371/journal.pone.0028050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 8.Camastra S, Manco M, Mari A, Baldi S, Gastaldelli A, Greco AV, et al. beta-cell function in morbidly obese subjects during free living: long-term effects of weight loss. Diabetes. 2005;54(8):2382–9. doi: 10.2337/diabetes.54.8.2382. [DOI] [PubMed] [Google Scholar]
- 9.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9(4):151–9. [PubMed] [Google Scholar]
- 10.Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D'Alessio DA. Beta-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab. 2006;91(1):185–91. doi: 10.1210/jc.2005-0853. [DOI] [PubMed] [Google Scholar]
- 11.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4(2):110–25. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 12.Meier JJ. Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia. 2008;51(5):703–13. doi: 10.1007/s00125-008-0936-9. [DOI] [PubMed] [Google Scholar]
- 13.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24(5):366–71. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 14.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45(1):85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 16.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88(5):2300–8. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 17.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 18.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell Mass and Turnover in Humans: Effects of obesity and aging. Diabetes Care. 2012 doi: 10.2337/dc12-0421. epub ahead of print 8 Aug 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starke AA, Erhardt G, Berger M, Zimmermann H. Elevated pancreatic glucagon in obesity. Diabetes. 1984;33(3):277–80. doi: 10.2337/diab.33.3.277. [DOI] [PubMed] [Google Scholar]
- 20.Asano T, Ninomiya H, Kan K, Yamamoto T, Okumura M. Plasma glucagon response to intravenous alanine in obese and non-obese subjects. Endocrinol Jpn. 1989;36(5):767–73. doi: 10.1507/endocrj1954.36.767. [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Muscelli E, Natali A, Gabriel R, Mitrakou A, Flyvbjerg A, et al. Association of fasting glucagon and proinsulin concentrations with insulin resistance. Diabetologia. 2007;50(11):2342–7. doi: 10.1007/s00125-007-0806-x. [DOI] [PubMed] [Google Scholar]
- 22.Solerte SB, Rondanelli M, Giacchero R, Stabile M, Lovati E, Cravello L, et al. Serum glucagon concentration and hyperinsulinaemia influence renal haemodynamics and urinary protein loss in normotensive patients with central obesity. Int J Obes Relat Metab Disord. 1999;23(9):997–1003. doi: 10.1038/sj.ijo.0801032. [DOI] [PubMed] [Google Scholar]
- 23.Weiss R, D'Adamo E, Santoro N, Hershkop K, Caprio S. Basal alpha-cell up-regulation in obese insulin-resistant adolescents. J Clin Endocrinol Metab. 2011;96(1):91–7. doi: 10.1210/jc.2010-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, et al. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106(33):13992–7. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freere RH, Weibel ER. Stereologic techniques in microscopy. J Royal Microsc Soc. 1967;87:25–34. doi: 10.1111/j.1365-2818.1967.tb04489.x. [DOI] [PubMed] [Google Scholar]
- 26.Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Cienc. 2003;75(4):469–86. doi: 10.1590/s0001-37652003000400006. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Chavez AO, Gastaldelli A, Guardado-Mendoza R, Lopez-Alvarenga JC, Leland MM, Tejero ME, et al. Predictive models of insulin resistance derived from simple morphometric and biochemical indices related to obesity and the metabolic syndrome in baboons. Cardiovasc Diabetol. 2009;8:22. doi: 10.1186/1475-2840-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkamol A, et al. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57(4):899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- 30.Folli F, Saad MJ, Backer JM, Kahn CR. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993;92(4):1787–94. doi: 10.1172/JCI116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105(3):311–20. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, Kahn SE, Hull RL. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178(6):2632–40. doi: 10.1016/j.ajpath.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gapp DA, Leiter EH, Coleman DL, Schwizer RW. Temporal changes in pancreatic islet composition in C57BL/6J-db/db (diabetes) mice. Diabetologia. 1983;25(5):439–43. doi: 10.1007/BF00282525. [DOI] [PubMed] [Google Scholar]
- 34.Montanya E, Nacher V, Biarnes M, Soler J. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes. 2000;49(8):1341–6. doi: 10.2337/diabetes.49.8.1341. [DOI] [PubMed] [Google Scholar]
- 35.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47(3):358–64. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 36.Bock T, Kyhnel A, Pakkenberg B, Buschard K. The postnatal growth of the beta-cell mass in pigs. J Endocrinol. 2003;179(2):245–52. doi: 10.1677/joe.0.1790245. [DOI] [PubMed] [Google Scholar]
- 37.Reers C, Erbel S, Esposito I, Schmied B, Buchler MW, Nawroth PP, et al. Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol. 2009;160(2):185–91. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- 38.Tyrberg B, Eizirik DL, Hellerstrom C, Pipeleers DG, Andersson A. Human pancreatic beta-cell deoxyribonucleic acid-synthesis in islet grafts decreases with increasing organ donor age but increases in response to glucose stimulation in vitro. Endocrinology. 1996;137(12):5694–9. doi: 10.1210/endo.137.12.8940401. [DOI] [PubMed] [Google Scholar]
- 39.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 40.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14–6. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 41.Cardellini M, Perego L, D'Adamo M, Marini MA, Procopio C, Hribal ML, Andreozzi F, Frontoni S, Giacomelli M, Paganelli M, Pontiroli AE, Lauro R, Folli F, Sesti G. C-174G polymorphism in the promoter of the interleukin-6 gene is associated with insulin resistance. Diabetes Care. 2005;28(8):2007–12. doi: 10.2337/diacare.28.8.2007. [DOI] [PubMed] [Google Scholar]
- 42.Marques-Vidal P, Bastardot F, von Känel R, Paccaud F, Preisig M, Waeber G, Vollenweider P. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study) Clin Endocrinol (Oxf) 2012 doi: 10.1111/j.1365-2265.2012.04384.x. e-pub ahead of print 12 Mar 2012; doi: 10.1111/j.1365-2265.2012.04384.x. [DOI] [PubMed] [Google Scholar]
- 43.Browning LM, Krebs JD, Magee EC, Frühbeck G, Jebb SA. Circulating markers of inflammation and their link to indices of adiposity. Obes Facts. 2008;1(5):259–65. doi: 10.1159/000169832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A. 2008;105(35):13163–8. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertrand G, Gross R, Puech R, Loubatieres-Mariani MM, Bockaert J. Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur J Pharmacol. 1993;237(1):45–50. doi: 10.1016/0014-2999(93)90091-u. [DOI] [PubMed] [Google Scholar]
- 46.Brice NL, Varadi A, Ashcroft SJ, Molnar E. Metabotropic glutamate and GABA(B) receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2002;45(2):242–52. doi: 10.1007/s00125-001-0750-0. [DOI] [PubMed] [Google Scholar]
- 47.Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Kohler M, Fachado A, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 2008;7(6):545–54. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho JH, Chen L, Kim MH, Chow RH, Hille B, Koh DS. Characteristics and functions of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors expressed in mouse pancreatic {alpha}-cells. Endocrinology. 2010;151(4):1541–50. doi: 10.1210/en.2009-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, et al. Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem. 2003;278(3):1966–74. doi: 10.1074/jbc.M206758200. [DOI] [PubMed] [Google Scholar]
- 50.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402(6762):685–9. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 51.Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, Yatsushiro S, et al. Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by alpha-cells of islet of Langerhans. Diabetes. 2004;53(4):998–1006. doi: 10.2337/diabetes.53.4.998. [DOI] [PubMed] [Google Scholar]
- 52.Di Cairano ES, Davalli AM, Perego L, Sala S, Sacchi VF, La Rosa S, et al. The glial glutamate transporter 1 (GLT1) is expressed by pancreatic beta-cells and prevents glutamate-induced beta-cell death. J Biol Chem. 2011;286(16):14007–18. doi: 10.1074/jbc.M110.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahren B. Beta- and alpha-cell dysfunction in subjects developing impaired glucose tolerance: outcome of a 12-year prospective study in postmenopausal Caucasian women. Diabetes. 2009;58(3):726–31. doi: 10.2337/db08-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28(3):253–83. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- 55.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290–301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Islet morphology according to overweight duration and severity in normoglycemic baboons. Islet volume (A and B), islet size in μm2 (C and D), and islet density (islets × mm2 ) (E and F), according to overweight duration and severity.
Correlation between IL-6 sR (A) and sTNF-RI (B) with weight in living normoglycemic baboons.