Abstract
In translational models of pulmonary arterial hypertension (PAH), spironolactone improves cardiopulmonary hemodynamics by attenuating the adverse effects of hyperaldosteronism on endothelin type-B receptor function in pulmonary endothelial cells. This observation suggests that coupling spironolactone with inhibition of endothelin type-A receptor—mediated pulmonary vasoconstriction may be a useful treatment strategy for patients with PAH. We examined clinical data from patients randomized to placebo or the selective endothelin type-A receptor antagonist ambrisentan (10 mg/day) and in whom spironolactone use was reported during ARIES-1 and -2, which were randomized, double-blind, placebo-controlled trials assessing the effect of ambrisentan for 12 weeks on clinical outcome in PAH. From patients randomized to placebo (n = 132) or ambrisentan (n = 67), we identified concurrent spironolactone use in 21 (15.9%) and 10 (14.9%) patients, respectively. Compared with patients treated with ambrisentan alone (n = 57), therapy with ambrisentan D spironolactone improved change in 6-minute walk distance by 94% at week 12 (mean ± SE, +38.2 ± 8.1 vs +74.2 ± 27.4 m, p = 0.11), improved plasma B-type natriuretic peptide concentration by 1.7-fold (p = 0.08), and resulted in a 90% relative increase in the number of patients improving ≥1 World Health Organization functional class (p = 0.08). Progressive illness, PAH-associated hospitalizations, or death occurred as an end point for 5.3% of ambrisentan-treated patients; however, no patient treated with ambrisentan + spironolactone reached any of these end points. In conclusion, these pilot data suggest that coupling spironolactone and endothelin type-A receptor antagonism may be clinically beneficial in PAH. Prospective clinical trials are required to further characterize our findings.
The potential contribution of aldosterone to the pathobiology of pulmonary artery hypertension (PAH) has been suggested by several groups,1–3 although the clinical consequences of aldosterone inhibition with spironolactone in patients with PAH remain unresolved.4,5 We have demonstrated recently that aldosterone levels are increased significantly in the pulmonary arterial circulation of patients with PAH; and in experimental animal models of PAH, this modulates pulmonary vascular dysfunction by inhibiting endothelin type-B receptor—dependent synthesis of nitric oxide.6 This observation suggests that coupling therapies that inhibit endothelin type-A receptor—mediated pulmonary vasoconstriction with spironolactone may be a useful strategy to treat PAH (Figure 1). To test this hypothesis, we retrospectively analyzed the Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Study 1 (ARIES-1) and Study 2 (ARIES-2)7 to determine if treatment with spironolactone influenced the efficacy of the selective endothelin type-A receptor antagonist ambrisentan on the predetermined study end points.
Figure 1.
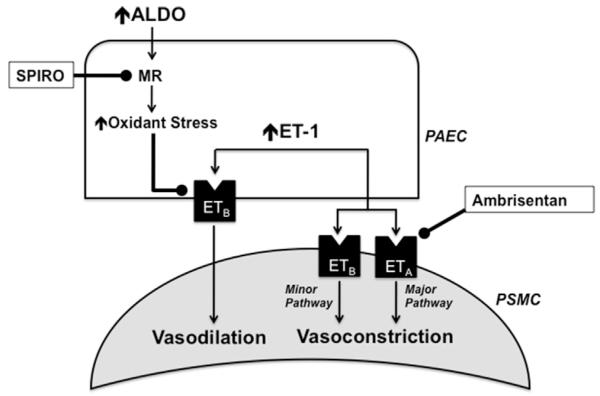
Schematic representation of the biological hypothesis supporting combination spironolactone (SPIRO) plus endothelin type-A receptor (ETA) antagonism for the treatment of PAH. Stimulation of pulmonary smooth muscle cells (PSMC) ETA and endothelin type-B receptor (ETB) are the major and minor signaling pathways, respectively, that modulate endothelin-1 (ET-1)-dependent pulmonary vasoconstriction in PAH. In contrast, pulmonary artery endothelial cell (PAEC) ETB stimulation by ET-1 promotes pulmonary vasodilation. In PAH, hyperaldosteronism is associated with a pulmonary vasculopathy that is due, in part, to increased oxidant stress levels that inhibits ETB function. Therefore, 2 potential treatment targets within the aldosterone-endothelin receptor axis are exposed: ETA to inhibit the major pulmonary vasoconstrictor pathway and the mineralocorticoid receptor (MR) to preserve ETB-dependent vasodilation.
Methods
Patients with World Health Organization (WHO) group I PAH who had received treatment in the randomized, placebo-controlled phase III trials ARIES-1 (n = 201) and ARIES-2 (n =;192) were eligible for analysis. The full methods and results for both studies have been reported previously.7,8 Patients received for 12 weeks either placebo or the selective endothelin type-A receptor antagonist ambrisentan at 5 or 10 mg/day orally in ARIES-1 or 2.5 or 5 mg/day orally in ARIES-2. The primary end point for both studies was the change from baseline in the 6-minute walk distance (6-MWD) at week 12. The secondary end points included in the study that were relevant to the current analysis included plasma B-type natriuretic peptide (BNP) concentration, change in WHO functional class, and total clinical worsening events, which were a composite of PAH-associated hospital admissions, early study termination due to rapidly progressive illness (see the study by Galiè et al7 for early escape criteria) and death.
To determine retrospectively the effect of ambrisentan + spironolactone on outcome in PAH, we accessed the ARIES-1 and ARIES-2 database to identify patients randomized to either placebo or ambrisentan and reported concurrent spironolactone use at any dose. Consideration of patients for inclusion in the spironolactone treatment groups in the current analyses was based on the following criteria achieved during the course of the original ARIES trial: (1) the duration of spironolactone use was for ≥28 days, (2) spironolactone therapy was initiated before study enrollment or ≤28 days after the first dose of ambrisentan/placebo, and (3) discontinuation of spironolactone did not occur within 28 days before the final ambrisentan/placebo dose. We elected to study patients receiving the maximum trial dose of ambrisentan (10 mg/day, n = 67) to test our hypothesis, because it was at this treatment dose that ambrisentan induced the strongest clinical and hemodynamic effects in the ambrisentan development program.7,9
The demographic and baseline characteristics were summarized by treatment group (placebo, spironolactone, ambrisentan [10 mg/day, without concurrent spironolactone use], ambrisentan [10 mg/day] + spironolactone) and differences among treatment groups were analyzed using the Student t test for most continuous variables. The Wilcoxon rank sum test was used to analyze differences among treatment groups for WHO functional class as well as study drug and spironolactone dosing comparisons, and Fisher’s exact test was used for categorical variables. Plasma BNP concentrations were summarized with logarithmic transformation. The change from baseline in WHO functional class is presented categorically and was analyzed with a 7-point scale: −3 to −1 is improved, 0 is no change, and +1 to +3 is deterioration as reported previously.7 The last observation carried forward approach was used for 6-MWD and WHO functional class. Only the patients with both a baseline and week-12 value were included in the BNP geometric mean ratio (GMR) analysis, which is a comparison of the geometric mean BNP at week 12 relative to the geometric mean BNP at baseline. To determine if accounting for spironolactone use in this study influenced the effect of ambrisentan on the primary and secondary end points as observed in the original ARIES trial, differences between placebo versus ambrisentan or ambrisentan + spironolactone were assessed before analyzing differences between the ambrisentan and ambrisentan + spironolactone treatment groups. For all analyses, p <0.05 was considered significant. No adjustments were made for multiple comparisons.
Results
Of patients randomized to placebo (n = 132) or ambrisentan 10 mg/day (n = 67), concurrent spironolactone use was identified for 21 (15.9%) and 10 (14.9%) patients, respectively. Compared with ambrisentan alone (n = 57), patients in the ambrisentan + spironolactone group (n = 10) were more likely to have WHO functional class III/IV and tended to have worse baseline 6-MWD, pulmonary vascular resistance, and plasma BNP concentration, although no clinically meaningful differences between treatment groups were observed with respect to patient demographics, Borg Dyspnea index, cardiac index, or right atrial pressure (Table 1).
Table 1.
Baseline patient characteristics
| Variable | Placebo |
Ambrisentan (10 mg/day) |
p | ||
|---|---|---|---|---|---|
| −SPIRO (n = 111) | +SPIRO (n = 21) | −SPIRO (n = 57) | +SPIRO (n = 10) | ||
| Age (yrs) | 50 (18–81) | 47 (27–69) | 50 (18–78) | 47 (24–78) | 0.58 |
| Women | 87 (78.4%) | 16 (76.2%) | 45 (78.9%) | 8 (80.0%) | 1.00 |
| Race | |||||
| Caucasian | 84 (75.7%) | 16 (76.2%) | 35 (61.4%) | 9 (90.0%) | 0.15 |
| Black | 4 (3.6%) | 0 | 2 (3.5%) | 1 (10.0%) | |
| Asian | 4 (3.6%) | 0 | 1 (1.8%) | 0 | |
| Hispanic | 19 (17.1%) | 5 (23.8%) | 17 (29.8%) | 0 | |
| Other | 0 | 0 | 2 (3.5%) | 0 | |
| Pulmonary arterial hypertension origin | |||||
| Primary pulmonary hypertension | 66 (59.5%) | 19 (90.5%) | 34 (59.6%) | 7 (70%) | 0.73 |
| Nonprimary pulmonary hypertension (CTD; HIV; Anorexigen) |
45 (40.5%) | 2 (9.5%) | 23 (40.4%) | 3 (30%) | |
| Baseline WHO functional class | |||||
| I | 4 (3.6%) | 0 | 2 (3.5%) | 0 | 0.31 |
| II | 40 (36.0%) | 7 (33.3%) | 20 (35.1%) | 2 (20%) | |
| III | 55 (58.6%) | 13 (61.9%) | 31 (54.4%) | 5 (50%) | |
| IV | 2 (1.8%) | 1 (4.8%) | 4 (7.0%) | 3 (30%) | |
| Borg dyspnea index | 3.8 ± 2.1 | 4.3 ± 2.4 | 3.8 ± 2.1 | 3.8 ± 2.1 | 0.95 |
| 6-MWD (m) | 346.7 ± 75.3 | 318.9 ± 97.7 | 344.8 ± 79.0 | 322.3 ± 74.9 | 0.41 |
| Cardiac index (L/min/m2) | 2.4 ± 0.8 | 2.4 ± 0.8 | 2.6 ± 0.7 | 2.4 ± 0.8 | 0.44 |
| Pulmonary vascular resistance (Wood units) | 11.3 ± 6.8 | 12.6 ± 7.2 | 10.8 ± 5.7 | 14.5 ± 6.0 | 0.07 |
| Mean pulmonary artery pressure (mm Hg) | 49.7 ± 14.2 | 57.0 ± 12.7 | 50.4 ± 15.5 | 57.4 ± 19.0 | 0.21 |
| Mean right atrial pressure (mm Hg) | 7.5 ± 4.8 | 9.4 ± 5.9 | 8.8 ± 5.0 | 11.2 ± 8.7 | 0.22 |
| Plasma B-type natriuretic peptide (ng/L) | 130.8 (99.1–172.7) | 142.4 (78.9–257.2) | 131.7 (88.0–197.2) | 236.7 (81.5–687.4) | 0.24 |
Values are mean ± SD as appropriate, except for age, which is reported as mean and range. Values for plasma B-type brain natriuretic peptide are geometric mean (95% CI). p Values compare ambrisentan (n = 49 for BNP, n = 54 for pulmonary vascular resistance and mean right atrial pressure, n = 55 for cardiac index, and n = 57 for other variables) and ambrisentan + spironolactone (n = 10) treatment groups. p Value for race compares Caucasian and non-Caucasian. p Value for WHO functional class compares I/II and III/IV.
CTD = connective tissue disease; HIV = human immunodeficiency virus; SPIRO = spironolactone; WHO = World Health Organization.
The dose, duration, and clinical indication for spironolactone therapy are listed in Tables 2 and 3. Spironolactone therapy was initiated after the start of the ARIES trial date for 2 patients in the ambrisentan + spironolactone group (day 15 and 21) and for 2 patients in the spironolactone group (day 9 and 29), and the start date of spironolactone therapy was before ARIES but unknown for 2 patients in the spironolactone group. Spironolactone therapy initiation for the remaining patients in the spironolactone (n = 17) and ambrisentan + spironolactone (n = 8) groups occurred well in advance of patients’ first ARIES trial date and did not differ significantly between groups. In both placebo (n = 21) and ambrisentan (n = 10) conditions, spironolactone therapy was reported for ≥96% of ARIES drug days, and no significant difference was observed for mean ± SD daily spironolactone dose between groups. Of patients in the ambrisentan-only condition (n = 57), 6 patients (10.5%) identified as spironolactone users did not meet the criteria for inclusion in the ambrisentan + spironolactone condition. For these patients, the first dose of spironolactone therapy occurred at a mean ± SD of 38 ± 13 days after ARIES drug initiation, and the mean ± SD duration of spironolactone use in these patients was 21 ± 22 days, which spanned 29 ± 24% of the ARIES drug days.
Table 2.
Spironolactone therapy characteristics
| Treatment Characteristic | Spironolactone Treatment Condition |
p | |
|---|---|---|---|
| Placebo, n = 21 (SD) | Ambrisentan, n = 10 (SD) | ||
| Number of days on SPIRO therapy before ARIES drug initiation | 294 (352)* | 280 (312)† | 0.68 |
| Number of ARIES drug days | 80 (15) | 85 (3) | 0.93 |
| Number of days on study drug in ARIES in which SPIRO was prescribed | 78 (16) | 81 (8) | 0.93 |
| % ARIES days that patients were prescribed SPIRO | 97 (8) | 96 (9) | 0.64 |
| Daily SPIRO dose (mg/day) | 39 (15) | 31 (12) | 0.17 |
Values are provided as mean (SD).
SPIRO = spironolactone.
n = 17.
n = 8.
Table 3.
Clinical indications reported for spironolactone use during the ARIES trial
| Clinical Indication for Spironolactone* |
Spironolactone Treatment Condition |
|
|---|---|---|
| Placebo (n = 21) |
Ambrisentan (n = 10) |
|
| Pulmonary hypertension | 14 | 7 |
| Edema or prevention of edema | 6 | 5 |
| RV failure | 4 | 3 |
| Electrolyte imbalance | 1 | 0 |
RV = right ventricle.
Patients may report >1 indication.
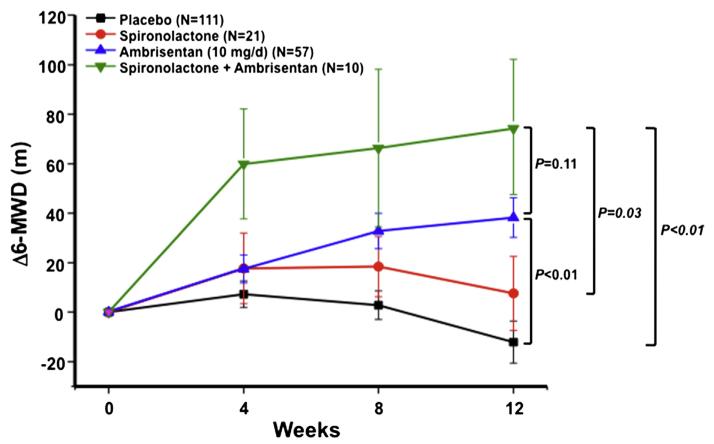
We first analyzed the change in 6-MWD from baseline between placebo and ambrisentan-treated patients. We observed that compared with placebo, ambrisentan improved 6-MWD at week 4 (mean ± SE: +17.5 ± 5.6 m), week 8 (+32.8 ± 7.1 m), and week 12 (+38.2 ± 8.1 m), whereas a −12.1 ± 8.5 m decrease in 6-MWD was observed at week 12 for placebo-treated patients (p <0.01 at week 12). Similarly, we observed that compared with placebo, patients treated with ambrisentan + spironolactone demonstrated a significantly improved change in 6-MWD from baseline at week 4 (mean ± SE: +59.9 ± 22.2 m), week 8 (+66.3 ± 31.9 m), and week 12 (+74.2 ± 27.4 m, p <0.01). Collectively, these trends agree with findings reported in the ARIES-1 and ARIES-2 trials, and indicate that the effect of ambrisentan on the predetermined primary end point was not contingent on concurrent treatment with spironolactone. We next analyzed the effect of spironolactone on change in 6-MWD from baseline in ambrisentan-treated patients. We observed that ambrisentan + spironolactone therapy patients outperformed ambrisentan-treated patients at each measured time point, ultimately achieving a peak change in 6-MWD distance at week 12 (mean ± SE, +59.9 ± 22.2 vs +17.5 ± 5.6 m [week 4], +66.3 ± 31.9 vs +32.8 ± 7.1 m [week 8], +74.2 ± 27.4 vs +38.2 ± 8.1 m [week 12], p = 0.11 at week 12; Figure 2), which was associated with a +13.6 m difference in the absolute 6-MWD at week 12 (383.0 ± 12.3 vs 396.6 ± 26.3 m).
Figure 2.
Change frombaseline in the 6-MWD. Mean±SE change from baseline in the 6-MWD at week 4, 8, and 12 in the placebo (n = 111), spironolactone (n = 21), ambrisentan (10 mg/day, n = 57), and ambrisentan + spironolactone (n = 10) treatment groups. p Values reflect comparisons among groups at week 12.
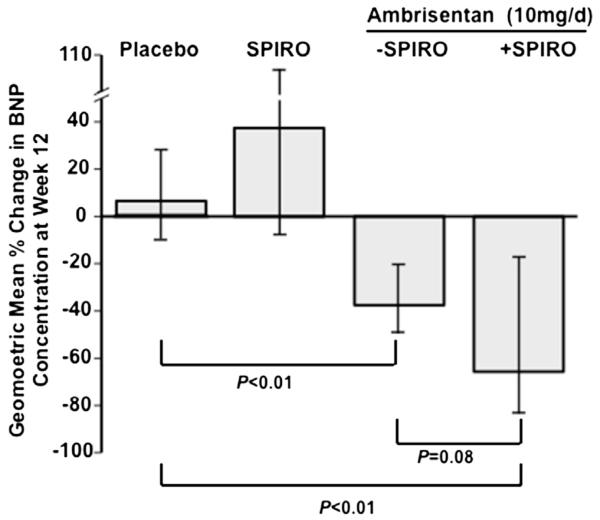
Compared with the placebo group (n = 83), in which a 7% increase in the GMR for week 12 relative to baseline BNP levels was observed, we found a 39% decrease in GMR (p <0.01) and 66% decrease in GMR (p <0.01) for ambrisentan patients (n = 43) and ambrisentan + spironolactone therapy patients (n = 10), respectively. Although total plasma BNP levels were similar at week 12 between the ambrisentan and ambrisentan + spironolactone treatment groups (geometric mean [95% confidence interval, CI] 70.4 [49.6, 99.9] vs 80.9 [33.6, 194.4]), we attributed this finding largely to the substantially different BNP levels between these 2 groups at baseline (geometric mean (95% CI) 114.9 [74.8, 176.5] vs 236.7 [81.5, 687.4]). Thus, we next investigated if ambrisentan + spironolactone therapy was associated with a change from baseline in BNP concentration that was directionally similar to improvements mediated by ambrisentan + spironolactone in change from baseline 6-MWD. Indeed, we observed a trend at week 12 in which ambrisentan + spironolactone induced a 1.7-fold improvement in BNP levels compared with ambrisentan alone, with a decrease in the geometric mean by 39% for ambrisentan (GMR (95% CI) 0.61 [0.48, 0.79] compared with a 66% decrease for ambrisentan + spironolactone [GMR (95% CI)] 0.34 [0.13, 0.89], p = 0.08; Figure 3). Taken together, these data suggest a potential treatment advantage in PAH with ambrisentan + spironolactone therapy compared with ambrisentan alone as assessed by improvements to exercise tolerance and biochemical evidence of pulmonary hypertension severity.
Figure 3.
The effect of treatment on BNP concentrations. The percent change at week 12 from baseline in geometric mean plasma BNP concentration with 95% CIs are presented for patients in the placebo (n = 83), spironolactone (SPIRO, n = 12), ambrisentan (10 mg/day, n = 43), and ambrisentan + spironolactone (n = 10) treatment groups.
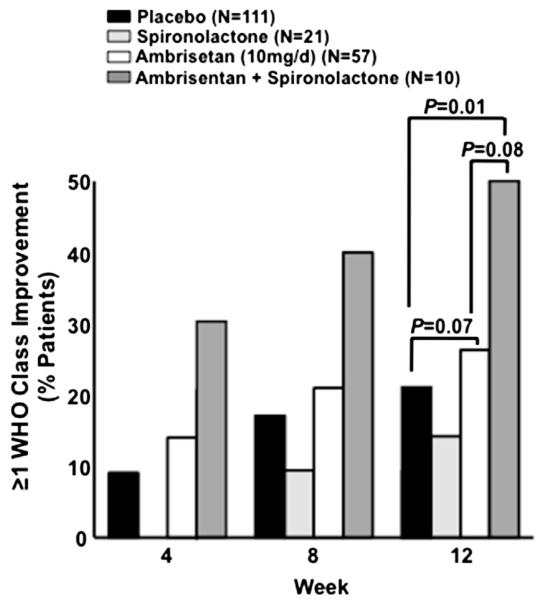
Improvements in 6-MWD and plasma BNP levels in the ambrisentan + spironolactone therapy group were associated with an attendant improvement in WHO functional classification. Compared with placebo (in which 21.6% of patients improved by ≥1 WHO functional class at week 12), we observed an improvement in functional status at week 12 for 50.0% (p = 0.01) of patients in ambrisentan + spironolactone therapy group (n = 10) and 26.3% (p = 0.07) of patients in the ambrisentan group (n = 57; Figure 4). Importantly, a 90.1% relative increase in the number of patients that improved by ≥1 WHO functional class at week 12 was observed for ambrisentan + spironolactone compared with ambrisentan therapy alone (p = 0.08), which is consistent with our observations for 6-MWD and change in BNP level. Moreover, in contrast to the ambrisentan-treated patients, in which clinical worsening was observed by virtue of total predetermined clinical worsening events (5.3%), PAH-associated hospitalizations (3.5%), escape criteria (3.5%), study withdrawal because of addition of other PAH therapeutic agents (1.8%), and death (1.8%), no patient in the ambrisentan + spironolactone group met criteria for any of these predetermined end points.
Figure 4.
The effect of treatment on World Health Organization (WHO) functional class. The percentage of patients in whom an improvement by ≥1 WHO functional class is reported at week 4, 8, and 12 for the placebo (n = 111), spironolactone (n = 21), ambrisentan (10 mg/day, n = 57), and ambrisentan + spironolactone (n = 10) treatment groups.
Discussion
Despite increasing evidence implicating the involvement of vasoactive hormones such as aldosterone in the pathophysiology of PAH,2,6,10–12 reports on the clinical application of aldosterone antagonists in this disease are limited to only individual patients.3,13 This study represents the first report describing data collected systematically in patients with PAH for whom spironolactone was identified as part of the therapeutic profile.
Akin to ARIES-1 and ARIES-2, we observed that ambrisentan treatment improved 6-MWD and other clinical outcome measures in patients with PAH, which was not contingent on concomitant therapy with spironolactone. Our findings demonstrate a trend toward further improvement in 6-MWD and BNP concentration in patients randomized to ambrisentan (10 mg/day) and in whom spironolactone therapy was reported during the ARIES trial. Importantly, this trend was internally consistent across all of the clinical outcome measures available for analysis and occurred despite evidence to suggest that at baseline, patients in the ambrisentan + spironolactone therapy group tended to have a greater functional capacity limitation, biochemical evidence of worse congestive heart failure, and more severe pulmonary vascular remodeling. In contrast, spironolactone treatment was not associated with a clinically meaningful improvement for any outcome measure in the placebo condition. This finding is consistent with the contemporary hypothesis that (1) clinical expression of PAH is because of numerous aldosterone-independent pathobiologic mechanisms and (2) single-drug regimens are often insufficient for the treatment of PAH in clinical practice.14,15 In contrast, these data provide a series of pilot clinical observations in support of our earlier biological hypothesis identifying the aldosterone-endothelin-receptor axis as a modifiable contributor to PAH pathobiology (Figure 1).
Conclusions from this work are subject to the traditional limitations inherent to all retrospective studies, particularly with respect to selection and historical biases. Additionally, several other important limitations specific to this work merit consideration. Most prominently, the number of patients eligible for analysis in the ambrisentan + spironolactone therapy group was low, thereby potentially confounding treatment effects between groups with respect to clinical event rates and continuously measured variables. However, low patient enrollment is common in PAH clinical trials because of the infrequency of this disease; in fact, sample size for each group in this study compares favorably to other published reports on PAH with similar study design.16-19 Nevertheless, due, in part, to the low number of patients in this study, sufficiently powered, prospective, randomized clinical trials are required to accurately characterize the effect of aldosterone inhibition on outcome in patients with PAH.
An additional limitation to this work was that plasma aldosterone levels were not analyzed. Therefore, the possibility exists that unmeasured factors, including drug therapies other than spironolactone, contributed to our findings. Along these lines, spironolactone promotes (mild) natriuresis, which is associated with improved functional capacity in patients with PAH and elevated BNP.15 Therefore, diuresis by spironolactone, rather than attenuation of pulmonary vascular dysfunction, cannot be excluded as a rationale by which to account for our findings. Indeed, changes in jugular venous pressure or the degree of lower extremity edema, which would be valuable for assessing the diuretic effect of spironolactone, were not available for analysis in this study. Furthermore, the potentially adverse effect of spironolactone on changes to electrolyte concentrations, particularly potassium homeostasis in patients with chronic renal insufficiency,20,21 is an important consideration for the use of spironolactone, but that was not assessed in the current analysis.
Our observation that ARIES patients who were prescribed spironolactone generally demonstrated worse baseline functional class and biochemical evidence of more severe right-sided heart failure raises the possibility that spironolactone use is potentially a marker for PAH disease severity. Thus, an important alternative explanation for our results is that the clinical response observed in patients treated with ambrisentan + spironolactone reflects enhanced therapeutic efficacy of ambrisentan in patients with more severe PAH. Consistent with the proof-of-principle rationale of this study, we elected to study patients randomized to ambrisentan at the maximally effective dose tested in the original ARIES trials. Therefore, the applicability of our observations to submaximal ambrisentan doses or to patients outside of an organized clinical trial was not tested.
Acknowledgment
The authors wish to graciously acknowledge the efforts of the ARIES trial investigators.
This work was supported by grants HL105301 to Dr. Leopold; HL61795, HL48743, HL107192, HL070819, and HL108630 to Dr. Loscalzo; 1K08HL111207-01A1 to Dr. Maron from the US National Institutes of Health (Bethesda, Maryland) and the Lerner Foundation (Boston, Massachusetts) at Brigham and Women’s Hospital (Dr. Maron) and grant 11POST6720000 to Dr. Maron from the American Heart Association (Dallas, Texas).
Footnotes
Disclosures
ABW and BAM receive grant funding to study pulmonary hypertension from Gilead. HG and CB are employed by Gilead, which sponsored the original ARIES trials.
References
- 1.Maron BA, Opotowsky AR, Landzberg MJ, Loscalzo J, Waxman AB, Leopold JA. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail. 2013;15:277–283. doi: 10.1093/eurjhf/hfs173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Man FS, Tu L, Handoko ML, Rain S, Ruiter G, François C, Schalij I, Dorfmüller P, Simmoneau G, Fadel E, Perros F, Boonstra A, Postmus PE, van der Velden J, Vonk-Noordegraaf A, Humbert M, Eddahibi S, Guignabert C. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:780–790. doi: 10.1164/rccm.201203-0411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martyniuk TV, Chazova IE, Masenko VP, Volkov VN, Belenkov Iu N. Activity of renin-angiotensin-aldosterone system (RAAS) and vasopressin level in patients with primary pulmonary hypertension. Ter Arkh. 1998;70:33–36. [PubMed] [Google Scholar]
- 4.Bansal S, Badesch D, Bull T, Schrier RW. Role of vasopressin and aldosterone in pulmonary arterial hypertension: a pilot study. Contemp Clin Trials. 2009;30:392–399. doi: 10.1016/j.cct.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elinoff JM, Rame JE, Forfia PR, Hall MK, Sun J, Gharib A, Abd-Elmoniem K, Graninger G, Harper B, Danner RL, Solomon MA. A pilot study of the effect of spironolactone therapy on exercise capacity and endothelial dysfunction in pulmonary arterial hypertension: study protocol for a randomized controlled trial. Trials. 2013 doi: 10.1186/1745-6215-14-91. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron BA, Zhang Y-Y, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126:963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro S, Pollock DM, Gillies H, Henig N, Allard M, Blair C, Anglen C, Kohan DE. Frequency of edema in patients with pulmonary arterial hypertension receiving ambrisentan. Am J Cardiol. 2012;110:1373–1377. doi: 10.1016/j.amjcard.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galié N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, Frost AE, Zwicke D, Naeije R, Shapiro S, Olschewski H, Rubin LJ. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 10.Piao L, Fang YH, Parikh KS, Ryan JJ, D’Souza KM, Theccanat T, Toth PT, Pogoriler J, Paul J, Blaxall BC, Akhter SA, Archer SL. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation. 2012;126:2859–2869. doi: 10.1161/CIRCULATIONAHA.112.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin I, McClure JD, Thomas M, Mair KM, MacLean MR. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 12.Preston IR, Sagliani KD, Warburton RR, Hill NS, Fanburg BL, Jaffe IZ. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2013 Mar 1; doi: 10.1152/ajplung.00300.2012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokubu T, Kazatani Y, Hamada M, Matsuzaki K, Ito T, Nishimura K, Ochi T, Daimon F, Joh T. Is captopril effective in primary pulmonary hypertension? Circ J. 1982;46:1095–1097. doi: 10.1253/jcj.46.1095. [DOI] [PubMed] [Google Scholar]
- 14.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simmoneau G. Bosentan therapy for pulmonary arterial hypertension. N Eng J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin W, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, American College of Cardiology Foundation Task Force on Expert Consensus Documents. American Heart Association. American College of Chest Physicians. American Thoracic Society, Inc. Pulmonary Hypertension Association ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Pui-Sze So P, Davies RA, Chandy G, Stewart D, Beanlands RSB, Haddad H, Pugliese C, Mielniczuk LM. Usefulness of beta-blocker therapy and outcomes in patients with pulmonary arterial hypertension. Am J Cardiol. 2012;109:1504–1509. doi: 10.1016/j.amjcard.2012.01.368. [DOI] [PubMed] [Google Scholar]
- 17.Cabrol S, Souza R, Jais X, Fadel E, Ali RH, Humbert M, Dartevelle P, Simonneau G, Sitbon O. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2007;26:357–362. doi: 10.1016/j.healun.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Kirshnan U, Takatsuki S, Ivy DD, Kerstein J, Calderbank M, Coleman E, Rosenzweig EB. Effectiveness and safety of inhaled treprostinil for the treatment of pulmonary arterial hypertension in children. Am J Cardiol. 2012;110:1704–1709. doi: 10.1016/j.amjcard.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamichi-Yamamoto S, Fukumoto Y, Sugimura K, Ishii T, Satoh K, Milura Y, Tatebe S, Nochioka K, Aoki T, Do EZ, Shimokawa H. Intensive immunosuppressive therapy improves pulmonary hemodynamics and long-term prognosis in patients with pulmonary arterial hypertension associated with connective tissue disorder. Circ J. 2011;75:2668–2674. doi: 10.1253/circj.cj-11-0473. [DOI] [PubMed] [Google Scholar]
- 20.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Eng J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 21.Maron BA, Leopold JA. Aldosterone receptor antagonists: effective but often forgotten. Circulation. 2010;121:934–939. doi: 10.1161/CIRCULATIONAHA.109.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]