Abstract
Objective
Low resting respiratory sinus arrhythmia (RSA) levels and blunted RSA reactivity are thought to index impaired emotion regulation capacity. Major Depressive Disorder (MDD) has been associated with abberant RSA reactivity and recovery to a speech stressor task relative to healthy controls. Whether impaired RSA functioning reflects aspects of the depressed mood state or a stable vulnerability marker for depression is unknown.
Methods
We compared resting RSA and RSA reactivity between individuals with MDD (n=49), remitted depression (RMD, n=24), and healthy controls (n=45). ECG data were collected during a resting baseline, a paced-breathing baseline, and two reactivity tasks (speech stressor, cold exposure).
Results
A group by time quadratic effect emerged (F=4.36(2,109), p=.015) for RSA across phases of the speech stressor (baseline, instruction, preparation, speech, recovery). Follow-up analyses revealed that those with MDD uniquely exhibited blunted RSA reactivity, whereas RMD and controls both exhibited normal task-related vagal withdrawal and post-task recovery. The group by time interaction remained after covariation for age, sex, waist circumference, physical activity, and respiration, but not sleep quality.
Conclusions
These results provide new evidence that abberant RSA reactivity marks features that track the depressed state, such as poor sleep, rather than a stable trait evident among asymtomatic persons.
Keywords: Major Depression, Remitted Depression, RSA, reactivity, stress
Introduction
Respiratory sinus arrhythmia (RSA) is a measure of heart rate variability that indexes the extent to which the vagus nerve exerts parasympathetic influence on the heart (1). RSA level and RSA fluctuation (task-related RSA changes) have been conceptualized as indexing emotion regulation capacity and biobehavioral flexibility (2). RSA is thus an index of autonomic flexibility, reflecting the degree to which cardiac activity is modulated to meet changing situational demands. Low RSA level and inappropriate context-related RSA fluctuation are associated with dysfunction in regulatory systems and are documented in MDD (3) and in other psychopathology (4). Findings associating MDD and resting RSA have been somewhat mixed, with some studies finding no relationship (5-6). It is important to examine both RSA level and RSA fluctuation as potentially distinct constructs that independently predict health outcomes (7). Given the mixed findings for resting RSA, RSA fluctuation may represent a more robust index of regulatory functioning.
The literature on resting RSA and depression has yet to produce a clear picture of this relationship. A meta-analysis found that major depression was associated with lower resting RSA relative to healthy controls, with a modest effect size (8). Low resting RSA was more recently associated with an MDD diagnosis in a large clinical sample (N=498 MDD, 462 healthy controls)(9). In contrast, a large study of 1075 individuals with current MDD, 774 with remitted depression, and 524 controls found associations between depression (current and remitted) and low resting RSA that were largely attribuable to antidepressant use (10), and longitudinal designs have also found that initiation of antidepressant treatment predicted reduced RSA 2 years later (9). In another meta-analysis, lower resting RSA was associated with higher depression severity and with tricyclic antidepressant, but not SSRI, use (11). Finally, other data has suggested MDD is associated with resting RSA, secondary to co-morbid anxiety disorders (12-13), and some studies find no association between MDD and resting RSA (5-6).
The smaller body of work on RSA fluctuation has been more consistent in demonstrating MDD-related effects. We have observed blunted RSA fluctuation in MDD persons relative to healthy controls during laboratory stressor tasks (14) and during intense emotional reactions, such as crying (15). RSA fluctuation abnormalities have also been reported among dysphoric individuals with elevated depression symptom levels (16).
The course and severity of depression have been associated with abnormal RSA levels and fluctuation. Increases in RSA level over time have been associated with a clinically significant response to depression treatment (17). Other studies have not observed this pattern (18) and a meta-analytic review found that symptomatic improvement after antidepressant treatment was not associated with increased RSA levels (19). However, higher vagal withdrawal in response to negative emotional stimuli has predicted subsequent recovery from depression 6 months later (3).
Importantly, low resting RSA and abnormalities in RSA fluctuation may have deleterious physical health consequences. Several meta-analyses have demonstrated a link between depression and increased risk for cardiovascular disease CVD morbidity and mortality (19-21). Low RSA may be a plausible mechanisms by which depression increases risk for CVD, as low RSA levels have been associated with increased CVD risk, more severe symptoms, poorer prognosis, and mortality (22-25). The relationship between RSA fluctuation and CVD is less clear because of a lack of empirical work. One study found that greater reductions in RSA to a stress task were associated with greater subclinical signs of CVD (26), consistent with the hypothesis that heightened cardiovascular reactivity to psychological stressors has been proposed as a key factor in increased CVD risk (22). Nevertheless, more recent research suggests that low cardiovacular reactivity has serious adverse health consequences, and that moderate responses may be optimal (27). Blunted reactivity has been associated with both depression and obesity, and each confers risk for CVD (27), leaving open the possibility that blunted RSA fluctuation may relate to CVD risk in some circumstances.
Despite the potential significance of low resting RSA and abnormalities in RSA reactivity for mental and physical health, the description of these effects in MDD is underspecified. Perhaps most critically, it is unclear whether these RSA patterns are transient and reflect some aspect of the depressed mood state, or whether these patterns represent a stable vulnerability marker for depression. Heretofore researchers have rarely tested a critical group that bears on this issue: Asymptomatic people who have a well-defined history of depression. Consistent with state-dependence, one study of resting RSA level in remitted depressed persons found no difference relative to controls (28). No studies, to our knowledge, have examined RSA reactivity in remitted depression.
To comprehensively assess these issues, the present study included participants with current depression (MDD), fully remitted depression (RMD), and healthy non-psychiatric controls. Resting RSA levels were assessed with well-established baselines (rest, paced breathing). To examine RSA reactivity in distinct contexts, we used a speech task, which elicits vagal withdrawal in normative samples, as well as a cold exposure stressor known to elicit vagal activation. We also controlled for variables known to impact RSA, including age, gender, waist circumference, sleep quality, physical activity level, and medication use.
Method
This study was approved the the Institutional Review Board of the University of South Florida. Data collection occurred from July 2008 to June 2010.
Participants
Recruitment and Clinical Assessment
Participants were recruited from fliers and online postings in the Tampa Bay metropolitan area. Respondants were initially screened by phone to determine eligibility. Screening items were based on diagnostic questions from the Structured Clinical Interview for DSM-IV (SCID; 29). A total of 820 individuals were screened. Of those, 271 were scheduled to complete SCID diagnostic interviews, which were administered by clinical psychology doctoral students to determine final diagnoses for study inclusion. A total of 240 participants completed the SCID interview session (31 individuals failed to attend their interview appointment and were unable to be rescheduled or decided against further participation). Participants were excluded at the phone pre-screen or SCID interview if they reported diagnosed CVD (n=20), diagnosed hypertension or hypotension (n=39), or insulin-dependent diabetes (n=2). Individuals were also excluded who used beta blockers or antihistamines, had a history of a major head injury, hearing impairment, diagnosis of bipolar disorder, substance abuse occurring within 6 months prior to study entry, or history of psychotic symptoms. Participants were not excluded for regular antidepressant use; however antidepressant use was relatively uncommon (see Table 2).
Table 2.
Clinical Characteristics of the Final Sample.
| MDD (n=49) | RMD (n=24) | Control (n=45) | Post Hoc Group Comparisons (ps<.05) |
|
|---|---|---|---|---|
| Antidepressants Past Month |
18.4% | 12.5% | 0% | MDD>CTRL RMD>CTRL |
| BDI-II | 31.33(9.63) | 6.58(5.75) | 2.80(4.37) | MDD>RMD>CTRL |
| HDRS | 17.96(4.79) | 1.46(2.11) | .84(1.24) | MDD>CTRL MDD>RMD |
| BAI | 18.96(9.06) | 7.04(8.31) | 2.02(3.14) | MDD>RMD>CTRL |
| PANAS-PA | 23.45(6.30) | 34.63(6.00) | 34.33(6.97) | MDD<CTRL MDD<RMD |
| PANAS-NA | 25.19(7.78) | 15.83(5.17) | 12.22(2.46) | MDD>RMD>CTRL |
| BMI | 28.45 (9.03) | 26.49 (7.08) | 24.65 (4.82) | MDD>CTRL MDD>RMD |
| PPAQ Total Kcal/Day |
476.95(1194.23) | 1197.67(1418.08) | 1430.12(1946.6 2) |
MDD<CTRL MDD<RMD |
| PSQI1 | 10.35(3.53) | 6.09(3.02) | 3.87(2.34) | MDD>RMD>CTRL |
Note: Higher scores on the PSQI indicate poorer sleep and scores of >5 are generally considered indicative of poor sleep quality.
Of those participants completing a SCID, 99 did not meet one or more inclusion/exclusion criteria, leaving 143 eligible participants. Of those, 23 failed to complete the psychophysiological assessment, primarily due to scheduling difficulties. Participants completing the psychophysiology assessment fell into 3 groups: MDD with a current episode (n=51), History of MDDwithout current symptoms (RMD, n=25), and no history of any Axis-I disorder (healthy controls, n=45). Strict remission criteria required RMD individuals to achieve full remission for 4+ weeks with no more than one subthreshold symptom and no subthreshold depressed mood or anhedonia over that interval. Sample demographic and clinical characteristics are provided in Table 1.
Table 1.
Demographic Characteristics of the Eligible Recruited Sample.
| MDD (n=49) | RMD (n=24) | Control (n=45) | |
|---|---|---|---|
| Age | 31.20 (11.46) | 29.33 (10.18) | 29.82 (12.25) |
| % Caucasian | 59.2% | 66.7% | 68.9% |
| % Female | 83.7% | 66.7% | 71.1% |
| % Completed at least some college | 89.8% | 87.5% | 91.1% |
| Median Income | $20-24,999 | $25-34,999 | $25-34,999 |
| % Married or Domestic Partner | 26.5% | 16.7% | 22.2% |
| % With Children | 38.8% | 25% | 20% |
Note: All group comparisons non-significant (ps>.05).
Clinical symptom severity measures
Participants were administered the 17-item Hamilton Rating Scale for Depression (HRSD), a well-validated, clinician-rated depression severity measure (30). The Beck Depression Inventory (BDI-II) and the Beck Anxiety Inventory (BAI) assessed depression and anxiety symptom severity (31-32). We assessed the dimensions of state positive and negative affect (PA and NA) with the state version of the Positive and Negative Affect Scale (PANAS), a well-validated 20-item inventory (33).
Health, sleep, and physical activitiy measures
On the day of the psychophysiological session, participants completed health, sleep, and physical activity measures. Waist circumference was measured at the level of the umbilicus in cm. The Paffenbarger Physical Activity Questionnaire (PPAQ), a well-validated self-report instrument of physical activity was included to measure physical activity (34-35). The Pittsburgh Sleep Quality Index (PSQI) was also administered, which is a well-validated measure sleep quality, with higher global scores indicating poorer sleep quality (36).
Diagnostic reliability
Diagnostic reliability was monitored on an ongoing basis. Diagnostic agreement was assessed by having a second rater independently code 15 cases based on audiotaped SCID responses. For classification of current MDD, the raters agreed in all cases, (k =1.00); for RMD, the raters agreed in 14/15 cases (k =.81); and for healthy control subjects, both raters agreed in all cases, (k=1.00).
Procedure for Psychophysiological Assessment
Psychophysiological assessments were conducted within 3 weeks of the clinical assessment. If more time had passed due to scheduling difficulties, participants were re-administered the SCID mood modules. One person was deemed ineligible because of a changed diagnostic status.
After obtaining informed consent, participants completed questionnaires and were assessed for height, weight, and waist circumference. Next, the experimenter attached cardiovascular sensors, and participants were seated comfortably in a small recording room. The experimenter noted the presence of a video camera and informed the participants that they would be continously monitored. Participants then viewed a neutral travelogue film for a 10-minute acclimation and assessment of resting RSA, followed by a 4-minute paced breathing baseline where participants were instructed to pace their breathing to a rising and falling tone. The two RSA reactivity tasks were then administered in counterbalanced order. The speech stressor required participants to prepare and deliver a speech on a specific topic (i.e., defending themselves against a traffic ticket). The preparation and delivery phases of the speech task were each 3 minutes. To increase evaluation apprehension during the speech task, participants were made aware of the camera recording their speech, and an experimental observer was present and silently pretending to take notes on the participant’s behavior. The second RSA reactvity task was a forehead cold pressor, which required participants to have a cold icepack placed on their foreheads for 2 minutes. This task elicits vagal activation and increased RSA (37) via the “dive reflex,” characterized by reduced heart rate in response to cold exposure (38). Each task was separated by a 5-minute recovery, during which participants rested, and a 5-minute buffer period, where participants watched more of the travelogue film. Finally, sensors were removed, and participants were paid and debriefed.
Data Recording, Reduction, and Processing
Heart rate values were obtained continuously via electrocardiogram (ECG) according to published guidelines (39). ECG acquisition used a PC with AcqKnowledge 3.7.2 software, and recorded continuously during baseline, task, and recovery phases. Cleartrace LT disposable Ag/AgCl electrodes (Conmed Andover Medical, Haverhill, MA) were placed in a modified Lead-II configuration on the chest, and ECG was amplified using a Biopac MP150 system with an ECG100 amplifier (Biopac Instruments Inc., Goleta, CA). Respiration Rate (RR) was measured with two RSP100C amplifiers, each with an TSD100C respiratory transducer. One transducer was placed around the abdomen, the other was placed around the chest, crossing under the armpits and atop the breastbone. All signals were sampled at 1000Hz. Impedence caridiography and blood pressure were also recorded and reported elsewhere (40). We collected systolic and diastolic blood pressure measurements at rest and throughout the protocol every 1-2 minutes using an Accutorr Plus blood pressure monitor (Datascope Corp., Mahwah, NJ).
Computation of RSA
RSA was calculated using MindWare HRV 2.51 software (MindWare Technologies, Ltd., Gahanna, OH). R-wave markers in the ECG signal were processed with the MAD/MED artifact detection algorithm (41) implemented in the MindWare software. The signals were also manually inspected and suspected artifacts were corrected. Our approach accords with current guidelines for frequency domain methods to determine heart rate variability and is well suited for short-term (~5 min) recordings (42). To compute minute-by-minute estimates of heart rate and RSA during baselines and tasks, a 60s time series of interbeat intervals (IBIs: the time in milliseconds between sequential ECG R-spikes) was created from an interpolation algorithm that has a 250ms sample time. This 60s IBI time series was (a) linearly-detrended, (b) mean-centered, and (c) tapered using a Hanning window. Spectral-power values were determined (in ms2/Hz) with fast Fourier transformations, and the power values in the 0.15-0.50 Hz spectral bandwidth were integrated (ms2). These spectral-power values were natural-log transformed prior to statistical analyses because of distributional violations, and the natural-logged (ln) spectral-power value in the high frequency (HF) 0.15-0.50 Hz bandwidth was the indicator of RSA for each epoch. The HRV software also provided calculation of respiration rate using the signal from the abdominal respiration band.
Computation of Task Mean and Recovery Mean Scores
Baseline, task, and recovery values for each measure (RSA, RR) were computed by averaging the available epochs for each phase. Recovery scores were calculated as arithmetic differences between recovery and resting baseline values, such that smaller values indicate greater recovery.
Statistical Analysis
Of 121 eligible participants, RSA data from 3 were unusable because of suspected heart arrhythmias (1 MDD), equipment failure (1 MDD), or excessive noise (1 RMD). Therefore, the final sample used in data analyses included 49 MDD, 24 RMD, and 45 controls.
To statistically control the impact of RR on RSA, we regressed average RR on RSA for the baselines and each task phase and saved the unstandardized residuals (43). These residuals were used in subsequent analyses and will be referred to as “Adjusted RSA.”
To examine baseline, we used adjusted RSA levels for the two baseline tasks (resting and paced-breathing) utilizing two one-way ANOVAs with group (MDD, RMD, Control) as a between-subjects factor. Follow-up Tukey’s LSD t-tests were planned to explore any group effects. Secondary analyses were planned adding covariates known to affect RSA to models (age, gender, BMI, physical activity).
For each of the two stressor tasks (speech and cold pressor), we conducted an omnibus repeated measures ANOVA on adjusted RSA values during each segment of the tasks using group as the between-subjects factor. Follow-up Tukey’s LSD t-tests were planned to explore any group effects. For analyses demonstrating a significant group effect, secondary analyses were planned to address whether group differences in RSA fluctuation were accounted for by demographic and physical health variables, with age, gender, BMI, physical activity, and sleep quality added to models as covariates. Finally, we performed exploratory correlational analyses to measure the strength of association between RSA (level and reactivity) and clinical variables (i.e., depression and anxiety severity, affect, antidepressant use).
Results
Demographic, Physical, and Clinical Variables
There were no differences among the three groups on any demographic variable (all Cramer V’s>.05; see Table 1). Clinical and physical characteristics are presented in Table 2. The groups differed on clinical symptom measures in expected ways, with the MDD group reporting the highest scores on symptom severity measures, and the RMD group falling between the MDD group and healthy controls on these measures. As anticipated, the MDD group was also less physically active, had higher BMIs, and reported poorer sleep quality, with the RMD group again falling between the other two groups. Although a larger percentage of MDD participants (18.4%) were taking antidepressants relative to the RMD group (12.5%), this difference was not statistically significant. Groups also did not differ in resting blood pressure, with a mean systolic blood pressure of 113 and mean diastolic blood pressure of 68 (systolic range=82-148; diastolic range=48-95). Preliminary exploratory analyses examined whether antidepressant use impacted resting RSA. One-way ANOVAs were conducted within the MDD and RMD participants (healthy controls were excluded from this analysis as none were using antidepressants) with antidepressant use dummy-coded (1=used antidepressants regularly in past month, 0=did not use antidepressants regularly in past month). Results revealed that antidepressant use was not significantly related to RSA levels (resting or paced breathing) or reactivity (speech or cold pressor) and this variable was not considered further (all ps>.05).
Resting RSA Levels
RSA and RR means for both baseline tasks by group are shown in Table 3. No group differences in RSA were observed for the resting baseline, F(2,114)=1.78, p=.17. However, analyses of paced breathing revealed a marginal group effect on RSA, F=2.82(2,113), p=.064. Follow-up LSD analyses indicated that the MDD group exhibited lower RSA relative to the healthy controls (p=.020), while the RMD group did not differ from the MDD or the controls (ps>.05). However, group effects for paced breathing were no longer significant when age, gender, waist circumference, and physical activity were covaried, F=1.93(2,116), p=.150. Age was strongly negatively related to paced-breathing RSA values, F=42.69(1,116), p<.001, with higher age being associated with lower paced-breathing baseline RSA, but no other covariates had a significant relationship with baseline RSA (ps>.05).
Table 3.
Interbeat interval (IBI), respiratory rate (RR), and unajdusted respiratory sinus arrhythmia (RSA) mean (SD) values for each task by group.
| Measure | MDD | RMD | Control | Post Hoc Group Comparisons (ps<.05) |
|---|---|---|---|---|
| IBI (ms) | ||||
| Baseline | 830.87 (112.35) | 857.06 (110.58) | 881.22 (111.25) | |
| Paced breathing | 819.73 (109.77) | 827.11 (100.40) | 866.50 (103.15) | MDD<CTRL |
| Speech instructions | 787.61 (121.70) | 762.94 (107.38) | 680.61 (110.84) | |
| Speech preparation | 782.69 (116.11) | 752.68 (110.30) | 786.65 (118.74) | |
| Speech delivery | 731.94 (108.44) | 683.37 (124.61) | 708.75 (121.01) | |
| Speech recovery | 819.84 (118.09) | 829.57 (110.70) | 872.36 (113.55) | MDD<CTRL |
| Cold pressor | 889.58 (140.23) | 886.98 (136.89) | 924.13 (146.26) | |
| Cold pressor recovery | 839.05 (122.76) | 840.21 (110.82) | 887.15 (118.33) | |
| RR (breaths/minute) | ||||
| Baseline | 14.46 (3.24) | 13.00 (4.62) | 14.53 (4.12) | |
| Paced breathing | 9.16 (.80) | 8.99 (.14) | 9.14 (1.06) | |
| Speech instructions | 15.53 (3.98) | 16.28 (5.20) | 17.11 (4.54) | |
| Speech preparation | 14.10 (4.24) | 14.52 (5.60) | 16.21 (4.37) | MDD<CTRL |
| Speech delivery | 14.11 (3.60) | 13.87 (4.41) | 14.54 (3.97) | |
| Speech recovery | 12.48 (2.64) | 12.31 (4.19) | 13.08 (3.80) | |
| Cold pressor | 12.57 (4.31) | 12.38 (4.16) | 13.81 (4.46) | |
| Cold pressor recovery | 12.89 (3.32) | 12.69 (3.80) | 13.99 (3.83) | |
| RSA (ln HF Power) | ||||
| Baseline | 5.99(1.28) | 6.23(1.48) | 6.52(1.41) | |
| Paced breathing | 6.71(1.27) | 6.89(1.45) | 7.38(1.44) | MDD<CTRL |
| Speech instructions | 5.81(1.19) | 5.74(1.71) | 5.82(1.39) | |
| Speech preparation | 5.93(1.08) | 5.79(1.37) | 6.20(1.25) | |
| Speech delivery | 5.62(1.14) | 5.52(1.49) | 5.82(1.18) | |
| Speech recovery | 6.04(1.32) | 6.27(1.24) | 6.61(1.32) | MDD<CTRL |
| Cold pressor | 6.54(1.34) | 6.60(1.58) | 6.77(1.41) | |
| Cold pressor recovery | 6.10(1.19) | 6.10(1.26) | 6.50(1.36) |
Manipulation Checks
To ensure that the stress tasks elicited RSA changes, we next conducted manipulation checks. Paired-sample t-tests found the task RSA means for the cold pressor, speech instructions, speech prepartion, and speech delivery all differed from resting baseline RSA means (unadjusted for respiration), with increased RSA in response to the cold task and decreased RSA for speech preparation and delivery, as expected (all ps<.001).
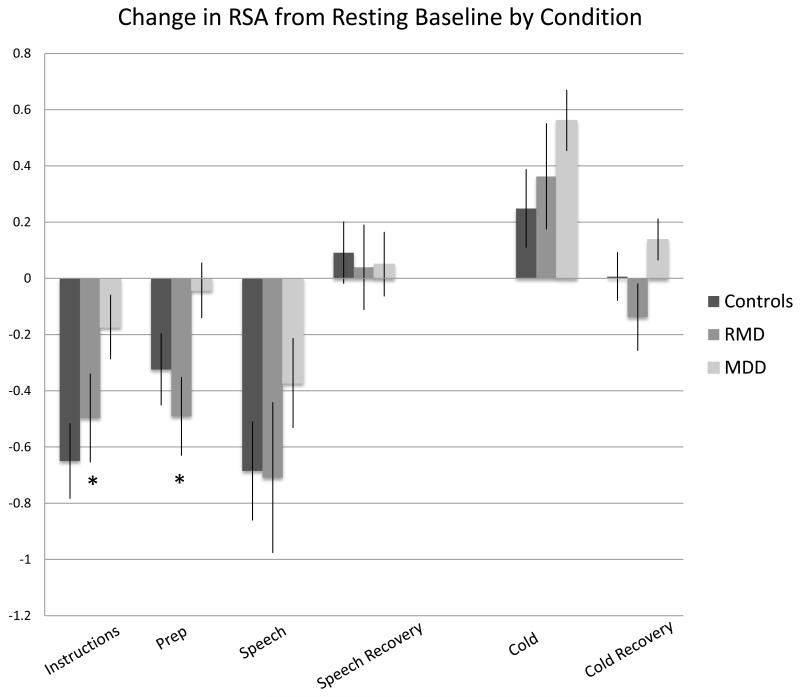
RSA Fluctuation
A significant group by task phase quadratic effect, F=4.36(2,109), p=.015 emerged for adjusted RSA during the the speech task (see Figure 1). Consistent with mood state-dependence, follow-up ANOVAs revealed that the MDD group uniquely exhibited blunted RSA fluctuation during the stressor phases of the task (preparation, delivery, and recovery), whereas RMD and healthy controls both exhibited normal task-related vagal withdrawal and post-task vagal recovery.
Figure 1.
Change in Unadjusted RSA from Resting Baseline by Condition. Error bars represent standard errors.
*ps<.05 MDD vs. Controls and RMD vs. Controls.
After entering age, sex, waist circumference, and physical activity as covariates, the significant group by task phase quadratic effect still remained, F=4.46(2,106), p=.01. However, the group by phase quadratic effect disappeared after sleep quality (PSQI) was added to the model, F(2,102)=0.87, p=.42.
There were no significant group or group by phase interaction effects for the cold pressor task. Results remained unchanged with or without co-variates (all ps>.05).
Correlations with symptom severity measures
Resting and paced-breathing adjusted RSA means were marginally correlated with depression severity, but not anxiety, such that depression severity scores were marginally negatively correlated with resting baseline RSA (BDI: r=-.175, p=.059, HRSD: r=-.172, p=.065) and paced-breathing RSA (BDI: r=-.174, p=.062, HRSD: r=-.171, p=.068). Similarly, NA, but not PA, had a trend relationship to both resting (r=-.168, p=.071) and paced-breathing baseline adjusted RSA (r=-.171, p=.067). PSQI scores (where higher scores indicate poorer sleep) were correlated with both resting baseline adjusted RSA (r=-.337, p<.001) and paced-breathing baseline adjusted RSA (r=-.336, p<.001). Average daily total kilocalories expended (PPAQ scores) was also correlated with paced-breathing baseline adjusted RSA (r=-.195, p=.036), but not resting RSA. Finally, there was no relationship between RSA reactivity for the speech task (instructions, preparation, or delivery) and other metrics of symptom severity (BDI, BAI, HRSD, PANAS, all ps>.05).
Discussion
Low RSA level and diminished context-related RSA fluctuations have been associated with MDD, although these findings have been far from unanimious. It remains unclear from prior work whether these dysfunctions reflect a trait-like vulnerability marker for depression, or transient states that wax and wane with the manifestation of depression symptoms. Our study was the first to examine RSA level and fluctuation in a sample that included individuals with current MDD, fully remitted depression, and healthy controls.
We found fragile evidence of low resting RSA in the MDD group, where this difference did not remain once several covariates were considered, suggesting that other factors may explain this relationship. However, we did observe blunted RSA fluctuation to a speech stressor task in the MDD group when relevant covariates were controlled for, suggesting that this effect may be more reliable, though effects lost significance when an additional control was added for sleep quality. No group effects were observed for the cold pressor task, indicating that MDD-related findings may be specific to vagal withdrawal. Importantly, the RMD group, despite having prior depression history, did not differ from healthy controls in baseline RSA or RSA reactivity to the stressor tasks. These results mirror Chang and colleagues (28), who found essentially normal resting RSA levels among remitted depressed individuals. Group-level results suggest that RSA abnormalities may co-vary with mood state or other depression-related symptoms rather than being trait-like depression vulnerability markers. Consistent with this possibility, lower baseline RSA was marginally associated with more severe depression in continuous analyes. Although it is conceivable that RSA results are driven by the MDD group having a weaker affective response to the speech task, in an earlier study using a similar speech task, depressed individuals appraised the speech as more stressful, but still exhibited blunted RSA reactivity (44).
We found intriguing relationships between impaired sleep quality, a typical symptom of depression, and both RSA level and RSA fluctuation. Poorer sleep quality was associated with low RSA level and blunted RSA fluctuation. Further, when sleep quality was added to models, group reactivity effects were no longer significant. Although our design is correlational, these data suggest that sleep disturbance may contribute to and account for the patterns of low resting RSA and blunted RSA fluctuation repeatedly observed in MDD. These findings, along with the contribution of physical activity to the model, are also consistent with De Jong et al. (45) who found in cardiovascular disease patients that low RSA was more related to somatic symptoms of depression than to cognitive symptoms. Elsewhere, RSA has been associated with disturbed or insufficient sleep. For example, better sleep efficiency has been related to higher ambulatory resting RSA (46). Similarly, disturbed sleep predicted reduced next-day RSA, even after controlling for daily affect (47). Causal links between RSA and sleep may operate in the other direction-higher baseline RSA in children predicted better sleep duration and efficiency in subsequent days (48). Little sleep research has considered RSA fluctuation, but less RSA suppression during a reaction time task predicted increased sleep problems in subsequent days (49). Therefore, based on research on nondepressed persons, and given the importance of sleep disturbance for self-regulation and mood, (50-53) sleep disturbance may partially account for depression-related RSA abnormalities.
Our results have several treatment implications. Since changes in RSA appear to track symptoms associated with the depressed state, RSA may be an objective indicator of treatment response. At the same time, our data suggest that RSA changes may have strong relationships to particular depression symptoms, such as sleep disturbance. Elsewhere, targeting sleep disturbance has been underlined as a component in depression interventions (54-56), and disturbed sleep may be relevant to abnormal RSA findings in other conditions, such as alcohol dependence (57). Few studies have examined RSA as a treatment response indicator, and with mixed results (11,18,58), and treatment studies thus far have rarely considered RSA fluctuation (17).
These results may also have physical health implications. Depression has been associated with increased risk for CVD and cardiac-related mortality (19-21), and CVD has also been associated with low RSA even in the absence of depression (22-25). Again, it may help to consider sleep disturbance as a means to integrate these findings. Indeed, Schwartz speculated that reduced RSA might be a potential pathway linking sleep problems with CVD, and sleep disruption may be part of a larger syndrome that encompasses poor health and depression that may be related to autonomic dysfunction (i.e., RSA) or other factors, such as environmental stress that increase cardiovascular risk (59). Along these lines, sleep disturbance has been associated with coronary heart disease events, even when controlling for other potential confounds (59), and longitudinal studies have demonstrated that poor sleep predicts hypertension (60-61), and heart disease mortality (62). Recent work has also shown that depressive symptomatology is also related to blunted heart rate reactivity (63-64). These authors speculate that blunted reactivity reflects dysregulated motivational systems that contribute to unhealthy states such as obesity, depression, and addiction (65). Thus, while exaggerated reactivity may reflect a direct pathway to CVD risk, blunted reactivity may contribute to CVD risk via indirect pathways, such as poor sleep or other factors related to poor health.
We acknowledge several study limitations. First, our cross-sectional design does not address how changes in RSA over time might correspond to the unfolding and remission of MDD episodes; longitudinal designs are needed. Another limitation is that some partipants were taking medications. Although preliminary analyses found no significant impact of antidepressants, these analyses were not well-powered to examine medication effects. Indeed, larger studies have found an association between antidepressants and reduced RSA levels both concurrently (10) and prospectively (9). Another limiting issue was low statistical power to examine whether comorbid diagnoses, particularly anxiety disorders, contributed to low resting RSA and abnormal RSA fluctuations (4). Future studies should attempt to untangle relative contributions of depression and anxiety symptoms. Indeed, sleep disturbance may be a common link between depression, anxiety, and deficient RSA. Finally, although we excluded individuals with insulin-dependent diabetes, in future work abnormal glycemic control should be considered since depression predicts future onset of diabetes (65) and impairments in RSA are associated with impaired insulin resistance (66).
Our investigation also has notable strengths. It was the first study to examine RSA level and RSA fluctuation in MDD and RMD persons. Second, participants were well-characterized and received comprehensive diagnostic assessments, with strict remission criteria for the RMD group. Finally, results were relatively clear cut, with group-level and correlational findings suggesting that low RSA and blunted RSA fluctuation mark features that track the depressed state, like poor sleep, rather than a stable trait observed in all depression-vulnerable individuals.
Acknowledgments
This work was supported by the National Institutes of Health (MH077669-02, PIs: Jonathan Rottenberg and Kristen Salomon)
Glossary
- BAI
Beck Anxiety Inventory
- BDI
Beck Depression Inventory
- ECG
electrocardiography
- ECI
Emotion Contet Insensitivity
- HDRS
Hamilton Depression Rating Scale
- IBI
interbeat interval
- MDD
major depressive disorder
- PA
positive affect
- PANAS
Positive and Negative Affect Schedule
- PPAQ
Paffenbarger Physical Activity Questionnaire
- PSQI
Pittsburgh Sleep Quality Index
- NA
negative affect
- RMD
remitted major depression
- RR
respiratory rate
- RSA
respiratory sinus arrythymia
Footnotes
Conflicts of Interest: None
References
- 1.Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32(4):301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 2.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42(3):277–81. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 4.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39(4):255–66. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 5.Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Jung I, Sherwood P. Heart rate variability in patients with major depression. Psychiatry Res. 1991;37(1):35–46. doi: 10.1016/0165-1781(91)90104-w. [DOI] [PubMed] [Google Scholar]
- 6.Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Liebmann P, Drnovsek B, Zapotoczky HG. Major depression and cardiac autonomic control. Biol Psychiatry. 1997;42(10):914–919. doi: 10.1016/S0006-3223(96)00494-5. [DOI] [PubMed] [Google Scholar]
- 7.Salomon K. Respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychol. 2005;24(1):68–76. doi: 10.1037/0278-6133.24.1.68. [DOI] [PubMed] [Google Scholar]
- 8.Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biol Psychol. 2007;74(2):200–11. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Chang HA, Chang CC, Chen CL, Kuo TBJ, Lu RB, Huang SY. Major depression is associated with cardiac autonomic dysregulation. Acta Neuropsychiatrica. 2012;24(6):318–327. doi: 10.1111/j.1601-5215.2011.00647.x. [DOI] [PubMed] [Google Scholar]
- 10.Licht CM, deGeus EJ, Zitman FG, Hoogendijk WJ, vanDyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatry. 2008;65(12):1358. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- 11.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol Psychiatry. 2010;67(11):1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Licht CM, deGeus EJ, vanDyck R, Penninx BW. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA) Psychosom Med. 2009;71(5):508–518. doi: 10.1097/PSY.0b013e3181a292a6. [DOI] [PubMed] [Google Scholar]
- 13.Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74(2):185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44(3):450–8. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 15.Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40(1):1–6. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- 16.Hughes JW, Stoney CM. Depressed mood is related to high-frequency heart rate variability during stressors. Psychosom Med. 2000;62(6):796–803. doi: 10.1097/00006842-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Rottenberg J, Chambers AS, Allen JJB, Manber R. Cardiac vagal control in the severity and course of depression: The importance of symptomatic heterogeneity. J Affect Disord. 2007;103(1):173–9. doi: 10.1016/j.jad.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers AS, Allen JJB. Vagal tone as an indicator of treatment response in major depression. Psychophysiology. 2003;39(6):861–4. doi: 10.1111/1469-8986.3960861. [DOI] [PubMed] [Google Scholar]
- 19.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: A meta-analysis. Psychosom Med. 2004;66(6):802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146,538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 21.Rugulies R. Depression as a predictor for coronary heart disease: A review and meta-analysis. Am J Prev Med. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 22.Hayano J, Yamada A, Mukai S, Sakakibara Y, Yamada M, Ohte N, et al. Severity of coronary atherosclerosis correlates with the respiratory component of heart rate variability. Am Heart J. 1991;121(4):1070–9. doi: 10.1016/0002-8703(91)90664-4. [DOI] [PubMed] [Google Scholar]
- 23.Martin GJ, Magid NM, Myers G, Barnett PS, Schaad JW, Weiss JS, et al. Heart rate variability and sudden death secondary to coronary artery disease during ambulatory electrocardiographic monitoring. Am J Cardiol. 1987;60(1):86–9. doi: 10.1016/0002-9149(87)90990-8. [DOI] [PubMed] [Google Scholar]
- 24.Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 25.Bigger J, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88(3):927–34. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- 26.Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH, et al. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosom Med. 2005;67(4):553–60. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips AC, Ginty AT, Hughes BM. The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int J Psychophysiol. 2013 doi: 10.1016/j.ijpsycho.2013.02.002. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Chang HA, Chang CC, Chen CL, Kuo TBJ, Lu RB, Huang SY. Heart rate variability in patients with fully remitted major depressive disorder. Acta Neuropsychiatrica. 2012 doi: 10.1111/j.1601-5215.2012.00658.x. In Press. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis-I disorders, research version, patient edition with psychotic screen (SCID-I/P W/PSY SCREEN) Biometrics Research, NY State Psychiatric Institute; NY: 2002. [Google Scholar]
- 30.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA, Brown GK. BDI-II manual. The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- 32.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 33.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 34.Paffenbarger R, Wing A, Hyde R. Paffenbarger physical activity questionnaire. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 35.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger R. Accuracy of the college alumnus physical activity questionnaire. J Clin Epidemiol. 1993;46(12):1403–11. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 36.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz JM, Uchino BN, Smith TW. Hostility and sex differences in the magnitude, duration, and determinants of heart rate response to forehead cold pressor: Parasympathetic aspects of risk. International journal of psychophysiology. 2006;60(3):274–83. doi: 10.1016/j.ijpsycho.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Heath M, Downey J. The cold face test (diving reflex) in clinical autonomic assessment: Methodological considerations and repeatability of responses. Clinical science. 1990;78(2):139. doi: 10.1042/cs0780139. [DOI] [PubMed] [Google Scholar]
- 39.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 40.Salomon KS, Bylsma LM, White KE, Panaite V, Rottenberg J. Is blunted cardiovascular reactivity in depression mood-state dependent? Int J Psychophysiol. 2013 doi: 10.1016/j.ijpsycho.2013.05.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berntson GG, Quigley KS, Jang JF, Boysen ST. An approach to artifact identification: Application to heart period data. Psychophysiology. 2007;27(5):586–98. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 42.Lombardi F, Malliani A. Task Force of the European society of cardiology and the North American society of pacing and electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 43.Ritz T, Dahme B. Implementation and interpretation of respiratory sinus arrhythmia measures in psychosomatic medicine: Practice against better evidence? Psychosom Med. 2006;68(4):617–627. doi: 10.1097/01.psy.0000228010.96408.ed. [DOI] [PubMed] [Google Scholar]
- 44.Salomon KS, Clift A, Karlsdóttir M, Rottenberg J. Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychol. 2009;28(2):157. doi: 10.1037/a0013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2007;69(8):735–739. doi: 10.1097/PSY.0b013e31815743ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker M. The role of sleep in cognition and emotion. Ann NY Acad Sci. 2009;1156(1):168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 47.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—a prefrontal amygdala disconnect. Current Biology. 2007;17(20):R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Bower B, Bylsma LM, Morris BH, Rottenberg J. Poor reported sleep quality predicts low positive affect in daily life among healthy and mood-disordered persons. J Sleep Res. 2010;19(2):323–32. doi: 10.1111/j.1365-2869.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- 49.Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition & Emotion. 2012 doi: 10.1080/02699931.2012.727783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palesh O, Zeitzer JM, Conrad A, Giese-Davis J, Mustian KM, Popek V, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. JCSM. 2008;4(5):441. [PMC free article] [PubMed] [Google Scholar]
- 51.Jackowska M, Dockray S, Endrighi R, Hendrickx H, Steptoe A. Sleep problems and heart rate variability over the working day. J Sleep Res. 2012;21(4):434–440. doi: 10.1111/j.1365-2869.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 52.Elmore-Staton L, El-Sheikh M, Vaughn B, Arsiwalla DD. Preschoolers’ daytime respiratory sinus arrhythmia and nighttime sleep. Physiol Behav. 2012;107(3):414–417. doi: 10.1016/j.physbeh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 53.El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children’s sleep problems. Dev Psychobiol. 2005;46(4):307–17. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- 54.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jindal RD, Thase ME. Treatment of insomnia associated with clinical depression. Sleep medicine reviews. 2004;8(1):19–30. doi: 10.1016/S1087-0792(03)00025-X. [DOI] [PubMed] [Google Scholar]
- 56.Riemann D, Berger M, Voderholzer U. Sleep and depression—results from psychobiological studies: An overview. Biol Psychol. 2001;57(1):67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 57.Irwin MR, Valladares EM, Motivala S, Thayer JF, Ehlers CL. Association between nocturnal vagal tone and sleep depth, sleep quality, and fatigue in alcohol dependence. Psychosom Med. 2006;68(1):159–66. doi: 10.1097/01.psy.0000195743.60952.00. [DOI] [PubMed] [Google Scholar]
- 58.Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62(5):639–47. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz S, Anderson WMD, Cole SR, Cornoni-Huntley J, Hays JC, Blazer D. Insomnia and heart disease: A review of epidemiologic studies. J Psychosom Res. 1999;47(4):313–33. doi: 10.1016/s0022-3999(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 60.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in japanese male workers. Journal of occupational health. 2003;45(6):344–50. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 61.Rod NH, Vahtera J, Westerlund H, Kivimaki M, Zins M, Goldberg M, et al. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173(3):300–9. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among chinese adults in singapore: A population-based cohort study. Am J Epidemiol. 2008;168(12):1367–73. doi: 10.1093/aje/kwn281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips AC, Hunt K, Der G, Carroll D. Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: Evidence from a large community study. Psychophysiology. 2010;48(1):142–8. doi: 10.1111/j.1469-8986.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 64.Carroll D, Phillips AC, Hunt K, Der G. Symptoms of depression and cardiovascular reactions to acute psychological stress: Evidence from a population study. Biol Psychol. 2007;75(1):68–74. doi: 10.1016/j.biopsycho.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Lovallo WR. Do low levels of stress reactivity signal poor states of health? Biol Psychol. 2011;86(2):121–8. doi: 10.1016/j.biopsycho.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and Type 2 Diabetes Over the Lifespan: A meta-analysis. Diabetes Care. 2008;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, et al. Association of hyperglycemia with reduced heart rate variability. Am J Cardiol. 2000;86(3):309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]