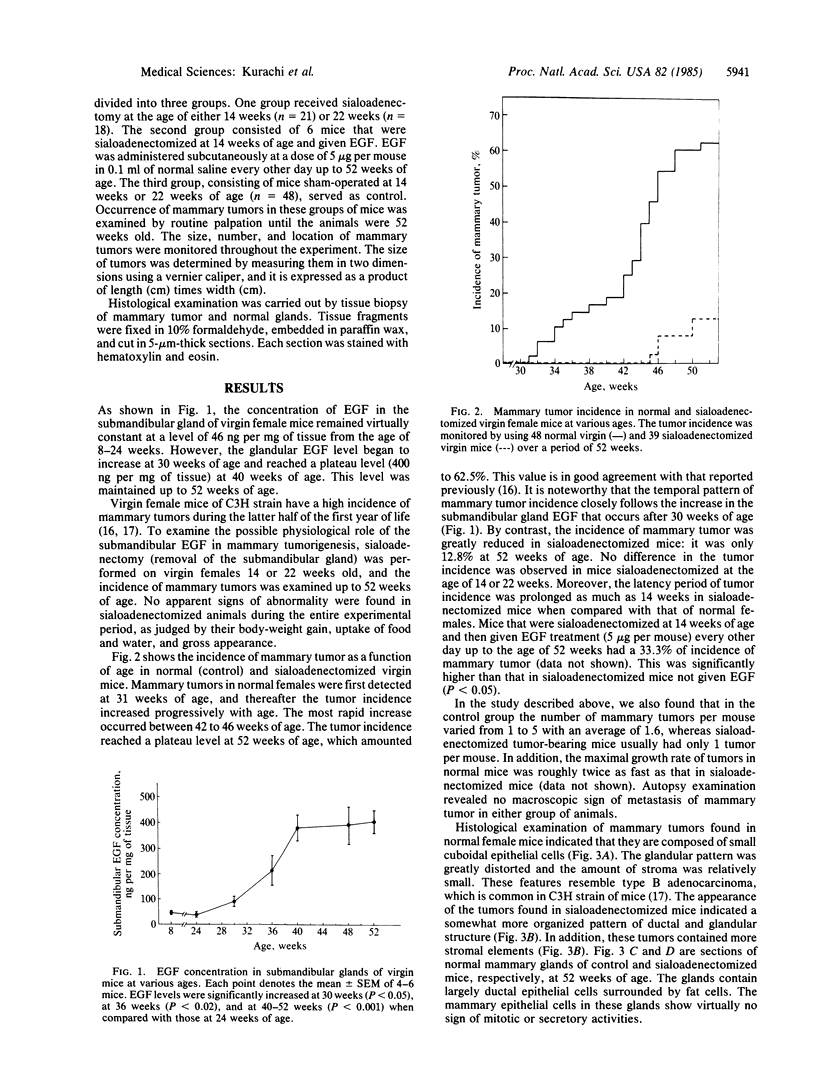
Abstract
The submandibular gland is a rich source of epidermal growth factor (EGF) in mice. The concentration of EGF in the gland of virgin female mice of C3H/HeN strain increased as much as 9-fold from the age of 30 to 52 weeks. During this period, the incidence of mammary tumor in virgin females increased markedly to a maximal level of 62.5% (n = 48) at 52 weeks of age. Removal of the submandibular gland (sialoadenectomy) of virgin mice 14-22 weeks old reduced the tumor incidence to 12.8% (n = 39) at the age of 52 weeks and also increased the latency period of mammary tumor development as much as 14 weeks when compared to that of normal mice. Long-term treatment of sialoadenectomized virgin mice with EGF (5 micrograms per mouse every other day) increased the tumor incidence to 33.3%. Moreover, sialoadenectomy of mammary tumor-bearing animals caused a rapid and sustained cessation of tumor growth, but EGF administration (5 micrograms per mouse per day) quickly restored the rate of tumor growth to a normal level. These results indicate that submandibular gland EGF plays a crucial role in mouse mammary tumorigenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERN H. A., NANDI S. Recent studies of the hormonal influence in mouse mammary tumorigenesis. Prog Exp Tumor Res. 1961;2:90–144. doi: 10.1159/000385951. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G. The regulation of cell proliferation: advances in the biology and mechanism of action of epidermal growth factor. J Invest Dermatol. 1978 Nov;71(5):283–288. doi: 10.1111/1523-1747.ep12529177. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J., Auersperg N. Epidermal growth factors enhances viral transformation of granulosa cells. Science. 1981 Jul 10;213(4504):218–219. doi: 10.1126/science.6264597. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Twardzik D. R., De Larco J. E., Stephenson J. R., Todaro G. J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: amino acid sequence homology with epidermal growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4684–4688. doi: 10.1073/pnas.80.15.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Oka T. Evidence for physiological function of epidermal growth factor: pregestational sialoadenectomy of mice decreases milk production and increases offspring mortality during lactation period. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6059–6063. doi: 10.1073/pnas.81.19.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Stahn R., Passovoy D. S., Herschman H. Epidermal growth factor enhancement of skin tumor induction in mice. Experientia. 1976;32(7):913–915. doi: 10.1007/BF02003764. [DOI] [PubMed] [Google Scholar]
- Taketani Y., Oka T. Biological action of epidermal growth factor and its functional receptors in normal mammary epithelial cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2647–2650. doi: 10.1073/pnas.80.9.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani Y., Oka T. Epidermal growth factor stimulates cell proliferation and inhibits functional differentiation of mouse mammary epithelial cells in culture. Endocrinology. 1983 Sep;113(3):871–877. doi: 10.1210/endo-113-3-871. [DOI] [PubMed] [Google Scholar]
- Taketani Y., Oka T. Tumor promoter 12-O-tetradecanoylphorbol 13-acetate, like epidermal growth factor, stimulates cell proliferation and inhibits differentiation of mouse mammary epithelial cells in culture. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1646–1649. doi: 10.1073/pnas.80.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P., Marquardt H., Rosberger D. F., Wong T. W., Todaro G. J. Synthesis of biologically active rat transforming growth factor I. Nature. 1984 May 24;309(5966):376–378. doi: 10.1038/309376a0. [DOI] [PubMed] [Google Scholar]
- Tonelli Q. J., Sorof S. Epidermal growth factor requirement for development of cultured mammary gland. Nature. 1980 May 22;285(5762):250–252. doi: 10.1038/285250a0. [DOI] [PubMed] [Google Scholar]
- Turkington R. W. Stimulation of mammary carcinoma cell proliferation by epithelial growth factor in vitro. Cancer Res. 1969 Jul;29(7):1457–1458. [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Imagawa W., McCormick K., Nandi S. Growth factor- and cyclic nucleotide-induced proliferation of normal and malignant mammary epithelial cells in primary culture. Endocrinology. 1980 Jul;107(1):35–41. doi: 10.1210/endo-107-1-35. [DOI] [PubMed] [Google Scholar]







