Abstract
This study examines the composition and activity of the planktonic community during the polar night in the high Arctic Kongsfjord, Svalbard. Our results are the first published evidence of bioluminescence among zooplankton during the Arctic polar night. The observations were collected by a bathyphotometer detecting bioluminescence, integrated into an autonomous underwater vehicle, to determine the concentration and intensity of bioluminescent flashes as a function of time of day and depth. To further understand community dynamics and composition, plankton nets were used to collect organisms passing through the bathyphotometer along with traditional vertical net tows. Additionally, using a moored bathyphotometer closed to the sampling site, the bioluminescence potential itself was shown not to have a diurnal or circadian rhythm. Rather, our results provide evidence for a diel vertical migration of bioluminescent zooplankton that does not correspond to any externally detectable changes in illumination.
Introduction
Of the various behaviors and adaptations that have evolved in marine environments, bioluminescence stands out as one of the particular importance, given the fact that it has evolved independently more than 40 times (Haddock et al. 2010). While it does not necessarily give specific advantage to species in cold environments, the long periods of continuous darkness that characterize winters at high latitudes create an environment, at least with respect to light, that is similar to the deep-sea. Depending on which taxa are bioluminescent, a variety of adaptive advantages have been suggested. These include defensive functions such as the “counter-illumination,” the “burglar alarm” and offensive mechanisms such as “prey attraction” and “intraspecific communication” (Haddock et al. 2010).
Other adaptations have evolved in both phytoplankton and zooplankton to survive in these harsh conditions that relate more to metabolic rates and the actual vertical space occupied in the water column within which a species spends the winter months. One such strategy is to enter a dormant state and overwinter at depth, seen for the copepods Calanus glacialis and C. hyperboreus which are commonly reported to enter a state of diapause at depth during winter months (Fortier et al. 2001; Falk-Petersen et al. 2008). They, then, return to shallower water in the summer to take advantage of the high productivity rates (Ashjian et al. 2003). Interestingly, Berge et al. (2009) for the first time provide acoustic evidence of active vertical migrations of zooplankton throughout the polar night in the high Arctic. More recently, this phenomenon was corroborated in the Southern Hemisphere where diel vertical migration (DVM) was shown to continue through the Austral winter in the Lazarev Sea, but ceased during the Austral summer (Cisewski et al. 2010).
The goal of the current study was to characterize plankton abundance and distribution patterns during a time of year that has rarely been studied by means of vertical net tows and autonomous underwater vehicle (AUV) surveys. Fitted on the AUV were ADCPs, a CTD and a bathyphotometer designed to register bioluminescence potential in the water column.
Materials and methods
Data were collected off the coast of Ny Ålesund in Kongsfjord, Svalbard (78°57′ N, 11°56′ E) in ~120 m of water from January 19 to 22, 2010. Spatial and temporal dynamics of acoustic backscatter, salinity, temperature and bioluminescence were measured using a REMUS-100 AUV (Moline et al. 2005). The vehicle was equipped with upward and downward facing RD Instruments 1,200-kHz Workhorse navigator acoustic Doppler current profilers (ADCP), configured in this study to provide relative acoustic backscatter as an estimate of scattering volume, rather than current velocities, a Neil-Brown CTD and a bioluminescence bathyphotometer (BP; Moline et al. 2005). The BP utilizes an impeller to continuously draw a measured volume of water into a chamber through the front of the nosecone where bioluminescence is measured by a photomultiplier tube at 60 Hz [see Herren et al. (2005) for details]. For statistical purposes, we restricted our analysis to the non-zero observations.
The AUV was deployed at 10:27, 12:25 and at 13:40 (local time 19th of January) at depths of 15, 45 and 75 m, respectively. These missions were surveyed a transect of 1.5 km at each respective depth. The vehicle was also deployed at 21:30 (19th of January) along the same transect as during the “day” but ran one mission surveying the transect at 15, 45 and 75 m successively without breaks between each depth. ADCP data were processed to remove noise and calculate relative backscatter coefficient (S v) according to Deines (1999) for the data collected between 0.5 and 5.0 m away from either side of the vehicle. Following the AUV deployments, continuous BP observations were made from 15:00 on January 21, 2010 until 09:00 on January 22, 2010 at 1 m depth at the Ny Ålesund harbor about 2 km from the study site. For logistical reasons (power supply, stable holdfast for the instrument and weather conditions), it was not possible to carry out the continuous BP observations in the fjord at the same place as the AUV deployments. However, given the short distance between the two locations and the absence of any physical barriers, the two sites are regarded as comparable for the examination of circadian rhythm (see also "Discussion"). Concurrent with the REMUS deployments, vertical net hauls were conducted using a WP2 plankton net, 180-μm mesh size with a 0.25 m2 opening. In order to collect vertical net hauls from the same depths surveyed by the REMUS ADCP, two replicates from each depth were taken from 30–0 m, 60–0 m and from 90–0 m between 10:30–13:00 LT. During the nighttime AUV deployment, replicate hauls were taken between 30–0 m, but inclement weather resulted in only one sample from 60–0 m was collected and none between 90–0 m. To determine species composition and abundance for specific depth intervals of 60–30 m and 90–60 m, the results from 30–0 m were subtracted from those of 60–0 m, and results from 60–0 m from those of 90–0 m, respectively. Additionally, the REMUS BP was equipped with cylindrical plankton nets (20-μm mesh size), and these nets were fitted to each of the two exhausts of the BP (Moline et al. 2009), which hence provided two replicate samples from each of the depths (15, 45 and 75 m) sampled using the AUV. For all plankton samples, the nets were rinsed and samples collected and preserved in a 4% formaldehyde solution. Samples were later enumerated and identified to the lowest possible taxonomic unit using a Leica stereomicroscope with 6.3–40× magnification.
Results
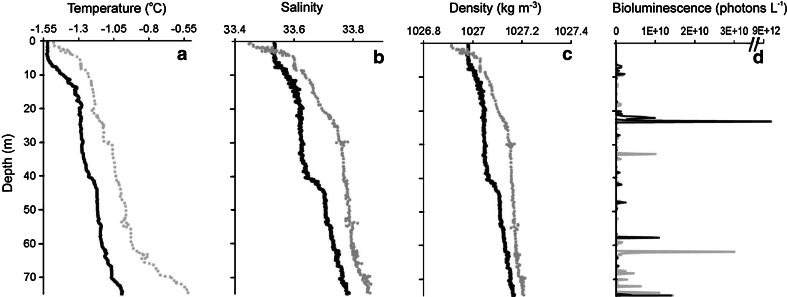
The temperature, salinity and density profiles were similar between night and day during the sample period (Fig. 1) and did hence not reveal any major advection events that would otherwise influence the measurements and results presented below. Based upon Willis et al. (2006), physical parameters indicated that the water mass in Kongsfjord was of Artic Water origin. Bioluminescence was detected throughout the water column both night and day, with higher bioluminescence at depth during the day and increased surface bioluminscence at night (Fig. 1d). Comparison of the bioluminescence at the specific depths also provided that this result with significantly greater bioluminescence intensity per flash was observed at 75 m during the day and at 15 m at night (Table 1), suggesting that the organisms with more intense flashes, i.e., larger zooplankton (see Moline et al. 2009), had migrated toward shallower depths at night.
Fig. 1.
Vertical profiles of a temperature (°C), b salinity (ppt), c density (kg m−3) and d bioluminescence (photons/l) as a function of depth (m) taken from the REMUS AUV during the final ascents from 75 m during the day and night deployments. Daytime observations represented in gray and nighttime in black. For this profile, bioluminescence for the upper water column (<45 m) was significantly less than that for the lower water column during the day (Mann–Whitney, P = 0.009, n = 102) and higher bioluminescence in the upper water column at night
Table 1.
Mean Bioluminescence intensity per flash (±SE) surveyed by the AUV at the three different depths during the daytime and nighttime deployments (LT is local time)
| Depth (m) | Mean intensity/flash (×108) daytime (10:30–14: × 25 LT) | Mean intensity/flash (×108) daytime (21:30–22:30 LT) | Mean S v difference (day–night) |
|---|---|---|---|
| 15 | 9 ± 2* | 160 ± 142† | −16 |
| 45 | 7 ± 2 | 8 ± 3 | −35 |
| 75 | 18 ± 6*‡ | 4 ± 1†,‡ | 17 |
Significant differences were found between depths using Mann–Whitney (* P = 0.016, n = 1,030; † P = 0.025, n = 286; ‡ P = 0.022, n = 126), and additionally, the difference in the mean acoustic backscatter coefficients (S v) between day and night is shown for each depth. The differences in S v between day and night were significant (Mann–Whitney, P < 0.001) at all depths
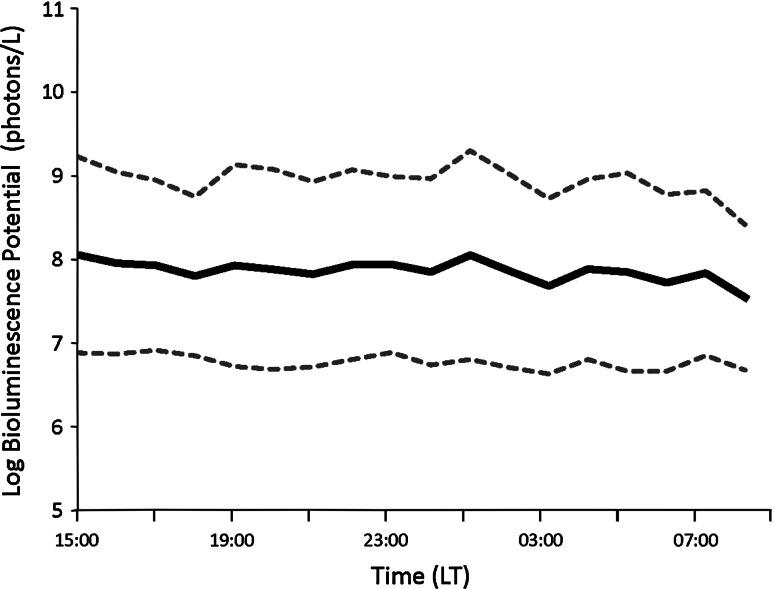
It is important when interpreting changes in bioluminescence signals that the circadian rhythms in the bioluminescence potential of planktonic organisms be taken into account (Batchelder et al. 1992). The continuous BP observations at the Ny Ålesund harbor showed no evidence of a circadian rhythm in the bioluminescence signal (Fig. 2) in a location (sheltered by the pier and with a max depth of 5 m) where vertical migration of zooplankton would be restricted. Organisms collected by net hauls next to the moored BP and by nets connected to the BP during this period using identical methods described above for the AUV showed >80% similarity to the study transects (data not shown), thus making these results applicable to the observations made by the AUV.
Fig. 2.
Hourly means of log bioluminescence potential (solid black line) with standard deviations (dotted black lines) collected at 1 m depth from 15:00 LT on January 21 to 09:00 LT on January 22, 2010, (n = 9,545, non-zero observations). Flashes per unit time were also found to be consistent throughout the time series
Estimates of relative backscatter coefficient as a relative measure of zooplankton biomass in a 10-m swath around the prescribed vehicle depths showed significantly higher intensity between 70 and 80 m during the day, and between 10 and 20 m and 40–50 m at night (Table 1). In combination with the changes in bioluminescence intensity, these data demonstrated a coordinated movement of biomass indicative of DVM.
Plankton enumerations from WP2 vertical net hauls show an increase above 60 m in the majority of the most abundant zooplankton taxa at night, including Pseudocalanus spp., Microcalanus spp., Oithona spp., Calanus spp., Metridia spp. and Thysanoessa spp. These genera have been reported to present throughout the year in this regions (Lischka and Hagen 2005). Table 2 provides specific species classification and shows that this increase above 60 m at night is also apparent in the enumerations of other less abundant taxa including Calanus finmarchicus, Acartia longiremis, Oncaea borealis and Eukrohnia hamata. Of these, Metridia lucens, Metridia longa, Oncaea borealis, Thysanoessa inermis and Thysanoessa longicaudata most likely account for the increase in high-intensity bioluminescent flashes at 15 and 45 m during the night (Table 1).
Table 2.
Concentrations of the plankton captured by the 180 μm WP2 plankton net during the day and at night for depth intervals 30–0, 60–30 and 90–60 m
| Taxa | Depth (m) | ind./m3 (day) | ind./m3 (night) | Taxa | Depth (m) | ind./m3 (day) | ind./m3 (night) |
|---|---|---|---|---|---|---|---|
| Calanus finmarchicus | 0−30 | 50 | 82 | Heterorhabdus norvegicus* | 0–30 | <1 | <1 |
| 30–60 | 41 | 148 | 30−60 | <1 | 6 | ||
| 60–90 | 89 | – | 60–90 | 4 | – | ||
| Calanus glacialis | 0–30 | 4 | 10 | Harpacticus chelifer | 0–30 | 9 | 50 |
| 30–60 | 31 | 31 | 30–60 | 54 | 30 | ||
| 60–90 | 147 | – | 60–90 | <1 | – | ||
| Calanus hyperboreus | 0–30 | <1 | 1 | Harpacticoida spp. | 0–30 | 2 | <1 |
| 30–60 | <1 | <1 | 30–60 | 11 | <1 | ||
| 60–90 | 4 | – | 60–90 | <1 | |||
| Pseudocalanus spp. | 0–30 | 3,100 | 10,300 | Oithona atlantica | 0–30 | 388 | 725 |
| 30–60 | <1 | 16,820 | 30–60 | <1 | 555 | ||
| 60–90 | 775 | – | 60–90 | <1 | – | ||
| Microcalanus spp. | 0–30 | 563 | 2,300 | Oithona similis | 0–30 | 400 | 825 |
| 30–60 | 163 | 1,900 | 30–60 | <1 | 1,335 | ||
| 60–90 | 63 | – | 60–90 | 131 | – | ||
| Metridia lucens* | 0–30 | 3 | 17 | Appendicularia* | 0–30 | 17 | 15 |
| 30–60 | 4 | 18 | 30–60 | 3 | 3 | ||
| 60–90 | 6 | – | 60–90 | 96 | – | ||
| Metridia longa* | 0–30 | <1 | <1 | Limacina helicina | 0–30 | 2 | <1 |
| 30–60 | <1 | 3 | 30–60 | <1 | 1 | ||
| 60–90 | 11 | – | 60–90 | 12 | – | ||
| Acartia longiremis | 0–30 | 175 | 375 | Sagitta elegans | 0–30 | <1 | 2 |
| 30–60 | <1 | 385 | 30–60 | 5 | 11 | ||
| 60–90 | <1 | – | 60–90 | 12 | – | ||
| Paraeuchaeta norvegica | 0–30 | <1 | <1 | Eukrohnia hamata | 0–30 | 4 | 30 |
| 30–60 | 1 | <1 | 30–60 | 2 | 21 | ||
| 60–90 | <1 | – | 60–90 | 11 | – | ||
| Diastylis lucifera* | 0–30 | <1 | <1 | Thysanoessa longicaudata* | 0–30 | <1 | 2 |
| 30–60 | 1 | <1 | 30–60 | 3 | 7 | ||
| 60–90 | <1 | – | 60–90 | 2 | – | ||
| Bradyidius similis | 0–30 | <1 | 1 | Thysanoessa inermis* | 0–30 | <1 | <1 |
| 30–60 | <1 | 3 | 30–60 | <1 | 22 | ||
| 60–90 | 6 | – | 60–90 | <1 | – | ||
| Oncaea borealis* | 0–30 | 12 | <1 | ||||
| 30–60 | 51 | 200 | |||||
| 60–90 | <1 | – |
Asterisks indicate organisms known to be bioluminescent
Plankton enumerated from the >20 μm net collection of the BP exhaust suggests that during the day, the greatest biomass occurred at 45 m and was dominated by copepod nauplii, copepod eggs and the Tintinnid Acantostomella norvegica (Table 3). The same three groups of organisms dominated the biomass at 15 and 75 m. Other major contributors at each of these three depths were Ceratium and Protoperidinium spp., Salpingella acuminata Pseudocalanus spp., Microcalanus spp., Oithona similis and Oithona atlantica (Table 3), consistent with the WP2 nets samples (Table 2).
Table 3.
Concentrations of the plankton captured by the 20 μm plankton nets covering the REMUS BP exhaust for daytime deployments at 15, 45 and 75 m
| Taxa | Depth (m) | ind/m3 | Taxa | Depth (m) | ind/m3 |
|---|---|---|---|---|---|
| Ceratium arcticum | 15 | 229 | Copepod nauplii* | 15 | 1,409 |
| 45 | 352 | 45 | 2,726 | ||
| 75 | 246 | 75 | 1,403 | ||
| Ceratium fusus* | 15 | 9.6 | Copepod eggs | 15 | 903 |
| 45 | 91.3 | 45 | 1,631 | ||
| 75 | 80.3 | 75 | 1,372 | ||
| Ceratium furca | 15 | <1 | Oncaea borealis* | 15 | 5 |
| 45 | 13 | 45 | 52 | ||
| 75 | 4 | 75 | 4 | ||
| Protoperidinium spp.* | 15 | 211 |
Harpacticoida spp. Microsetella norvegica |
15 | 7 |
| 45 | 391 | 45 | 7 | ||
| 75 | 387 | 75 | 4 | ||
| Diatom spp. | 15 | 10 | 15 | 5 | |
| 45 | 59 | 45 | 7 | ||
| 75 | 80 | 75 | 4 | ||
| Acantostomella norvegica | 15 | 1,252 | Oithona atlantica | 15 | 67 |
| 45 | 2,023 | 45 | 117 | ||
| 75 | 827 | 75 | 59 | ||
| Salpingella acuminata | 15 | 241 | Oithona similis | 15 | 72 |
| 45 | 163 | 45 | 111 | ||
| 75 | 122 | 75 | 108 | ||
| Heliocostomella subulata | 15 | 2 | Eukrohnia hamata | 15 | <1 |
| 45 | 13 | 45 | 1 | ||
| 75 | <1 | 75 | <1 | ||
| Parafavella denticulata | 15 | 7 | Gastropoda larvae | 15 | 23 |
| 45 | 33 | 45 | 33 | ||
| 75 | 45 | 75 | 11 | ||
| Calanus finmarchicus | 15 | 8 | Appendicularia* | 15 | 11 |
| 45 | 8 | 45 | 39 | ||
| 75 | 11 | 75 | 21 | ||
| Paraeuchaeta norvegica CIII | 15 | <1 | Membranipora larvae | 15 | 19 |
| 45 | 1 | 45 | 20 | ||
| 75 | <1 | 75 | 21 | ||
| Acartia longiremis | 15 | 29 | Bivalve larvae | 15 | <1 |
| 45 | 7 | 45 | 7 | ||
| 75 | 18 | 75 | <1 | ||
| Pseudocalanus spp. | 15 | 205 | Limacina helicina | 15 | <1 |
| 45 | 215 | 45 | <1 | ||
| 75 | 154 | 75 | 4 | ||
| Microcalanus spp. | 15 | 137 | Thysanoessa longicaudata* | 15 | <1 |
| 45 | 437 | 45 | <1 | ||
| 75 | 140 | 75 | 1 |
Asterisks indicate organisms known to be bioluminescent
Evident from organisms collected from both net sampling approaches is that the day/night changes in the vertical distributions of bioluminescence and acoustic scattering resulted from the larger zooplankton. Zooplankton taxa > 2.3 mm (Calanus spp., Metridia spp., Thysanoessa spp. and Appendicularia) collected by the WP2 vertical net tows showed decreased abundance at the surface (upper two depth layers) during the day than during the night and the highest abundance of this size class was found at depth during the day (Table 2). The smaller size class (Pseudocalanus spp, Microcalanus spp., Oithona spp.) did not show this trend. Histograms of the bioluminescence intensity (data not shown) in conjunction with plankton enumerations from each depth suggest that an underlying low-to-intermediate-intensity bioluminescence was consistent between day and night and likely attributed to the dinoflagellates, from Protoperidinium that occurred throughout the water column and to a lesser degree, Ceratium furca and C. fusus that were present in significantly lower abundances (Table 3).
Discussion
Though ubiquitous in the world’s oceans and important from an ecological and evolutionary perspective (for review see Haddock et al. 2010), few studies have described bioluminescent communities and their distributions in the Arctic, particularly during the winter darkness. Buskey (1992), and Lapota et al. (1989, 1992) examined bioluminescence distributions and community structure with the goal of developing methodology to use bioluminescence as a way to measure total biomass and light budgets of a given water mass during the spring in the Greenland Sea, during the fall in the Beaufort Sea and in summer in a Norwegian Fjord, respectively. In contrast, this study quantified the bioluminescent community during the polar night and demonstrated the absence of circadian rhythm in bioluminescence.
Furthermore, both the diurnal distribution of bioluminescence intensity and concurrent changes in acoustic backscattering provide independent evidence for an active DVM of the larger bioluminescent zooplankton (and likely non-bioluminescent zooplankton) within the upper 75 m of the water column. Histograms of intensities showed the major differences between day and night occurring at the highest intensities, which is consistent with larger zooplankton (Lapota et al. 1992; Moline et al. 2009) and with the enumerations in this study. Bioluminscence and acoustic backscatting may in fact not be directly linked, but the circumstantial evidence provided herein suggests that the diurnal signal in bioluminescence is in fact caused by vertically migrating organisms. Numerous studies have examined proximal triggers for DVM (Forward 1988; Ringelberg 1995; Ringelberg and Van Gool 2003; Benoit-Bird et al. 2009), and many others have looked at triggers for the inhibition of bioluminescence as related to its circadian rhythm (Batchelder et al. 1992; Kelly and Katona 1966; Raymond and DeVries 1976; Swift et al. 1995). Interestingly all studies have in one way or another implicated a relative or absolute change in irradiance intensity, angle or daylength as a means of regulation for both DVM and the circadian rhythm of bioluminescence. Although changes in light during the time of this study are not visible to the human eye, it is possible that they were sufficient to initiate DVM in the organisms present at the time of the study as was suggested in the study by Berge et al. (2009). However, while external light cues may play a role in regulating DVM, it might not be the only factor relevant to consider for understanding this behavior. It has been well established for photosynthetic dinoflagellates and for heterotrophic dinoflagellates of the Protoperidinium genus that the bioluminescent inhibition occurs when light intensities are greater than the intensity of bioluminescence of the organisms themselves (Sweeney et al. 1959; Buskey et al. 1992). This phenomenon could explain why no circadian rhythm existed in bioluminescence during December and January in Antarctica (Raymond and DeVries 1976) and may also be related to the absence of a circadian rhythm in bioluminescence in the current study. Previous studies have found that Protoperidinium spp contributed between 20 and 90% of the total light budget from the surface to a depth of 100 m in the Beaufort Sea (Lapota et al. 1992) and that dinoflagellates were estimated to account for 96% of the total light budget in Vestfjord, Norway (Lapota et al. 1989), so it is reasonable to assume that they were significant contributors to the overall light budget during this study. Wherein the lack of sunlight facilitated an environment where the intensity of bioluminescence was not inhibited by light greater than the bioluminescence of the organisms themselves. While this regulatory factor has been established for dinoflagellates, it is possible that it plays a role in other bioluminescent taxa as well, such as copepods, appendicularians and Arctic krill as in the case of this study.
Conclusions and perspectives
The most notable finding in this study is the detection of bioluminescent activity among zooplankton during the polar night, which may be an important ecological feature. While the ultimate and proximate explanations for both the bioluminescence and the DVM behavior detected during the campaign fall outside the data collected during this study, these results provide evidence for both endogenous and exogenous control of poorly understood or previously unknown processes. Also, during this expedition, which took place during the darkest period of the polar night, we observed five different species of seabirds actively foraging at sea; little auk (Alle alle), black-legged kittiwake (Rissa tridactyla), northern fulmar (Fulmarus glacialis), black guillemot (Cepphus grylle) and brünnich’s guillemot (Uria lomvia). These seabirds have, to the best of our knowledge, not been reported to overwinter at these latitudes. Whether bioluminescence and/or DVM are playing roles in the foraging behavior of these visual predators is an exciting possibility, although still an open question. Despite the limited scope of this study, results open new lines of enquiry regarding the function and process during a time of year when classical paradigms of Arctic ecosystems postulate that organisms are predominately in a state of hibernation (see Berge et al. 2009). Ultimately, these questions also have implications for human activities (i.e., oil exploration) in the high Arctic, which up until to now has been considered “without life” during the polar night.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Ashjian CJ, Campbell RG, Welch HE, Butler M, Van Keuren D. Annual cycle in abundance, distribution, and size in relation to hydrography of important copepod species in the western Arctic Ocean. Deep-Sea Res I. 2003;50:1235–1261. doi: 10.1016/S0967-0637(03)00129-8. [DOI] [Google Scholar]
- Batchelder HP, Swift E, Van Keuren R. Diel patterns of plankton bioluminescence in the northern Sargasso Sea. Mar Biol. 1992;113:329–339. [Google Scholar]
- Benoit-Bird K, Au WWL, Wisdon DW. Nocturnal light and lunar cycle effects on diel migration of micronekton. Limnol Oceanogr. 2009;54:1789–1800. doi: 10.4319/lo.2009.54.5.1789. [DOI] [Google Scholar]
- Berge J, Cottier F, Last KS, Varpe Ø, Leu E, Søreide J, Eiane K, Falk-Petersen S, Willis K, Nygård H, Vogedes D, Griffiths C, Johnsen G, Lorentzen D, Brierley AS. Diel vertical migration of Arctic zooplankton during the polar night. Biol Lett. 2009;5:69–72. doi: 10.1098/rsbl.2008.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskey EJ. Epipelagic bioluminescence in the marginal ice zone of the Greenland Sea. Mar Biol. 1992;113:689–698. doi: 10.1007/BF00349712. [DOI] [Google Scholar]
- Buskey EJ, Strom SL, Coulter CJ. Bioluminescence of heterotrophic dinoflagellates from Texas coastal waters. J Exp Mar Biol Ecol. 1992;159:37–49. doi: 10.1016/0022-0981(92)90256-A. [DOI] [Google Scholar]
- Cisewski B, Strass VH, Rhein M, Kragefsky S. Seasonal variation of diel vertical migration of zooplankton from ADCP backscatter time series data in the Lazarev Sea, Antarctica. Deep-Sea Res I. 2010;57:78–94. doi: 10.1016/j.dsr.2009.10.005. [DOI] [Google Scholar]
- Deines KL (1999) Backscatter estimation using broadband acoustic Doppler current profilers. In: Proceedings of IEEE 6th conference on current measurement. 249–253
- Falk-Petersen S, Leu E, Berge J, Kwasniewski S, Nygard H, Rostad A, Keskinen E, Thormar J, von Quillfeldt C, Wold A, Gulliksen B. Vertical migration in high Arctic waters during autumn 2004. Deep-Sea Res II. 2008;55:2275–2284. doi: 10.1016/j.dsr2.2008.05.010. [DOI] [Google Scholar]
- Fortier M, Fortier L, Hattori H, Saito H, Legendre L. Visual predators and the diel vertical migration of copepods under Arctic sea ice during the midnight sun. J Plankton Res. 2001;23:1263–1278. doi: 10.1093/plankt/23.11.1263. [DOI] [Google Scholar]
- Forward RB. Diel vertical migration: zooplankton photobiology and behavior. Oceangr Mar Bio Ann Rev. 1988;26:361–393. [Google Scholar]
- Haddock SHD, Moline MA, Case JF. Bioluminescence in the Sea. Annu Rev Mar Sci. 2010;2:443–493. doi: 10.1146/annurev-marine-120308-081028. [DOI] [PubMed] [Google Scholar]
- Herren CM, Haddock SHD, Johnson C, Moline MA, Case JF. A multi-platform bathyphotometer for fine-scale, coastal bioluminescence research. Limnol Oceanogr Methods. 2005;3:247–262. doi: 10.4319/lom.2005.3.247. [DOI] [Google Scholar]
- Kelly MG, Katona S. An endogenous diurnal rhythm of bioluminescence in a natural population of dinoflagellates. Biol Bull. 1966;131:115–126. doi: 10.2307/1539652. [DOI] [Google Scholar]
- Lapota D, Geiger ML, Stiffey AV, Rosenberger DE, Young DK. Correlations of planktonic bioluminescence with other oceanographic parameters from a Norwegian fjord. Mar Ecol Prog Ser. 1989;55:217–227. doi: 10.3354/meps055217. [DOI] [Google Scholar]
- Lapota D, Rosenberger DE, Liebermann SH. Planktonic bioluminescence in the pack ice and the marginal ice zone of the Beaufort Sea. Mar Biol. 1992;112:665–675. doi: 10.1007/BF00346185. [DOI] [Google Scholar]
- Lischka S, Hagen W. Life histories of the copepods Psuedocalanus minutus, P. acuspes (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard) Polar Biol. 2005;28:910–921. doi: 10.1007/s00300-005-0017-1. [DOI] [Google Scholar]
- Moline MA, Blackwell SM, von Alt C, Allen B, Austin T, Case J, Forrester N, Goldsborough R, Purcell M, Stokey R. Remote environmental monitoring units: an autonomous vehicle for characterizing coastal environments. J Atm Ocean Tech. 2005;22:1797–1808. doi: 10.1175/JTECH1809.1. [DOI] [Google Scholar]
- Moline MA, Blackwell SM, Case JF, Haddock SHD, Herren CM, Orrico CM, Terrill E. Bioluminescence to reveal structure and interaction of coastal planktonic communities. Deep-Sea Res II. 2009;56:232–245. doi: 10.1016/j.dsr2.2008.08.002. [DOI] [Google Scholar]
- Raymond JA, DeVries AL. Bioluminescence in McMurdo Sound, Antarctica. Limnol Oceanogr. 1976;21:599–602. doi: 10.4319/lo.1976.21.4.0599. [DOI] [Google Scholar]
- Ringelberg J. Changes in light intensity and diel vertical migration: a comparison of marine and freshwater environments. J Mar Biol Assoc UK. 1995;28:99–113. [Google Scholar]
- Ringelberg J, Van Gool E. On the combined analysis of proximate and ultimate aspects in diel vertical migration (DVM) research. Hydrobiologia. 2003;491:85–90. doi: 10.1023/A:1024407021957. [DOI] [Google Scholar]
- Sweeney BM, Haxo FT, Hasting JW. Action spectra for two effects of light on luminescence in Gonyaulax polyedra. J Gen Physiol. 1959;43:285–299. doi: 10.1085/jgp.43.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift E, Sullivan JM, Batchelder HP, Van Keuren J, Vaillancourt RD, Bidigare RR. Bioluminescent organisms and bioluminescence measurements in the North Atlantic Ocean near latitude 59.5 N, longitude 21 W. J Geophys Res. 1995;100:6527–6547. doi: 10.1029/94JC01870. [DOI] [Google Scholar]
- Willis K, Cottier F, Kwasniewski S, Falk-Petersen S. The influence of advection on zooplankton community composition in an Arctic fjord (Kongsfjorden, Svalbard) J Mar Syst. 2006;61:39–54. doi: 10.1016/j.jmarsys.2005.11.013. [DOI] [Google Scholar]