Abstract
Organic matter (OM) remineralization may be considered a key function of the benthic compartment of marine ecosystems and in this study we investigated if the input of labile organic carbon alters mineralization of indigenous sediment OM (OM priming). Using 13C-enriched diatoms as labile tracer carbon, we examined shallow-water sediments (surface and subsurface layers) containing organic carbon of different reactivity under oxic versus anoxic conditions. The background OM decomposition rates of the sediment used ranged from 0.08 to 0.44 μmol C ml−1ws day−1. Algal OM additions induced enhanced levels of background remineralization (priming) up to 31% and these measured excess fluxes were similar to mineralization of the added highly degradable tracer algal carbon. This suggests that OM priming may be important in marine sediments.
Introduction
Organic matter (OM) remineralization is a key function of the benthic compartment of marine ecosystems and although extensively studied, it remains a subject of focus due to the complexity of factors and processes involved (Wakeham and Canuel 2006). It is generally thought that microbes fulfill a key role in OM recycling (e.g., Harvey 2006) but when present fauna may be responsible for a significant part of sediment community OM respiration (Heip et al. 1995, 2001). Recent short-term experimental studies utilizing isotope tracer carbon confirm the key role of bacteria, but demonstrate that fauna directly alter the fate of incoming fresh OM (Moodley et al. 2002, 2005a; Witte et al. 2003a). Fauna my out-compete bacteria and direct uptake of OM (feeding) delays short-term OM respiration (Kristensen et al. 1992; Witte et al. 2003b). Furthermore, the recent manipulation experiment studies suggest that a major impact of community structure on seafloor functioning is through bioturbation by large fauna: maintaining habitat heterogeneity (Thrush et al. 2006a) and enhancing OM recycling primarily through fauna-induced irrigation of deeper sediment layers and enhanced sediment–water solute exchange (Biles et al. 2002; Mermillod-Blondin et al. 2004; Lohrer et al. 2004; Michaud et al. 2005; Thrush et al. 2006a; Meysman et al. 2006). Simultaneously however, the same fauna-induced displacement of sediment particles (bioturbation) results in mixing and displacement of settled OM into deeper sediment layers (Levin et al. 1997); this may lead to the strong dilution of the fresh OM (Mayer et al. 2001) directly influencing biota accessibility to this OM and subsequent degradation pathways. In addition, depending on the mode of bioturbation, OM removal from contact with oxygenated waters may slow down remineralization of this fresh OM relative to rates under oxic conditions at the sediment–water interface (Sun et al. 2002a; Josefson et al. 2002).
Another potentially related but not well-documented phenomenon in marine sediments is OM “priming”; the input of relatively fresh and labile organic carbon (OC) may alter remineralization of indigenous, relatively older and less reactive OM, i.e., the remineralization of more refractory components may be linked to the decay of labile components (Canfield 1994; Aller 1994; Hee et al. 2001). This process is sometimes referred to as “cometabolism” (Wakeham and Canuel 2006). Priming has been studied intensively in soils and its effect may be positive or absent (e.g., Hamer and Marschner 2005a, b; Hoyle et al. 2008). OM priming may potentially also be an important factor in regulating the carbon balance in marine sediments, but there is very limited evidence for “priming” in marine sediments. Kristensen and Holmer (2001) added fresh diatom and hay detritus to shallow-water surface sediment and found no clear evidence for priming. Turnewitsch et al. (2007) studied the interaction between fresh and refractory organic matter for nitrogen mineralization and found non-linear effects but the results were not conclusive regarding priming. Using a pulse-tracer experiment, Hee et al. (2001) provided convincing evidence that the addition of fresh substrates stimulated the decomposition of relict, indigenous OM.
Here, we report on sediment–water slurry incubations examining OM priming in shallow-water sediments. We utilized algal carbon, pre-enriched with 13C as tracer OM addition, because its diagenetic end product it is easily isotopically distinguished from that of the indigenous OM (Blair et al. 1996). We examined continental shelf subtidal (surface and subsurface layers) and estuarine intertidal sediments that were expected to contain OM of contrasting reactivity (Dauwe and Middelburg 1998). Given that the OM degradation may be redox sensitive (e.g., Bianchi et al. 2000; Sun et al. 1993, 2002a, b; Kristensen et al. 1995; Harvey et al. 1995; Kristensen and Holmer 2001) and that oxygen exposure time may be key in determining OM preservation in marine sediments (e.g., Hartnett et al. 1998; Sun and Dai 2005; Moodley et al. 2005b), incubation of the surface and subsurface subtidal sediments were done both under oxic and anoxic conditions. In addition, we used two different concentrations of “priming” OM to explore the consequences of dilution of fresh OM that occurs due to downward bioturbation of recently settled OM.
Materials and methods
Sediment sampling
Sediment was collected from a shallow site in the Skagerrak, eastern North Sea (59-m water depth, 57°50 N, 10°01 E) using a box corer on board the RV Pelagia in April 2002. A total of six cores (14 cm i.d.; 30-cm deep) were taken from three different box cores and sediment from the surface layer (0–2 cm) and a deeper layer (18–20 cm) were pooled separately and thoroughly homogenized manually. No large macrofauna or shell debris were observed in the sediment and the sediment was therefore not sieved. Subsamples were taken from each pooled depth interval and frozen at −80°C for later analysis of sediment characteristics.
Intertidal surface sediment (upper 2 cm) was collected during low tide from a mudflat in the Scheldt estuary (Southwest Netherlands, 51°26 N, 3°57 E, April 2003 using the RV Luctor). Moist sediment from this site was first sieved (500 μm mesh opening) to remove large fauna and shell debris. Site-specific details are provided in Table 1.
Table 1.
Origin and characteristics of the sediment used for sediment–water slurry incubations
| Sampling site | Water depth (m) | Sediment sample (cm) | Median grain size (μm) | Percentage of silt (<63 μm) | TOC content (%) | δ13C-OM | δ13C-Carbonate |
|---|---|---|---|---|---|---|---|
| Skagerrak 57°50 N, 10°01 E | Subtidal 59 m | Subtidal 0–2 | 41.2 (0.7) | 69.4 (0.5) | 0.86 (0.02) | −22 (−19) | +0.5 (0.0) |
| Subtidal 18–20 | 45.8 (0.2) | 65.5 (0.1) | 0.79 (0.01) | −22 (−19) | +0.6 (0.1) | ||
| Scheldt estuary 51°26 N, 3°57 E | Intertidal low tide | Intertidal 0–2 | 109 (0.8) | 19.4 (0.1) | 0.24 (0.10) | −19 (−16) | −0.17 (0.1) |
Average median grain size (μm), average percentage of silt (fraction smaller than 63 μm), average total organic carbon content (percentage of TOC; n = 2 and standard errors in brackets)
Also given is the measured stable carbon isotope signature (‰) of bulk sediment organic matter (OM) with in brackets the value of “reactive” OM (that of bacteria PLFA, see “Materials and methods”)
In the last column, measured δ13C of sediment bulk carbonate (SE)
Experimental set-up
Sediment–water slurries were prepared basically according to Dauwe et al. (2001); volumes of 10 ml wet sediment (sampled by filling a cutoff syringe) were transferred into preweighed 80 ml glass incubation bottles (Chrompack). Sediment without any additions was used to determine background OM remineralization rates. As a source of tracer and possible priming OM, we used the cosmopolitan diatom Skeletonema costatum cultured, isotope labeled and harvested as described in Moodley et al. (2000). This produced axenic diatom carbon with ~27% 13C (δ13C = +32,000‰).
For Skagerrak sediment, vials containing sediment were divided into three major groups: no extra additions were series to determine background OM remineralization rates and for the two experimental treatments different amounts of tracer algal carbon additions were employed. An additional experimental factor was the division into oxic and anoxic incubations (see below). Algal material was suspended in rinsing medium (artificial seawater without bicarbonate used for initial rinsing and isolation of 13C-labeled diatoms (Moodley et al. 2000), of which 1 ml was added to each vial; two concentrations were used, equivalent to an addition of 0.52 and 5.2 μmol C per ml wet sediment (ws). For the intertidal surface sediment, single additions were made equivalent to ~3.6 μmol C per ml sediment. Owing to the logistic limitations, anoxic incubations were not possible and these were incubated only under oxic conditions, but for three different durations (7, 14 and 21 days in the dark at 15°C). Skagerrak sediments were incubated for 21 days in the dark (12°C).
The final step of slurry preparation was the addition of filtered (0.2 μm) bottom water (10 ml) followed by sealing with screw caps fitted with rubber septa. After being thoroughly mixed by hand shaking, slurries (~20 ml sediment water and ~60-ml headspace) were purged for 5 min with either N2 or N2:O2 (80:20) for anoxic and oxic slurries, respectively (Dauwe et al. 2001). Oxygenation was verified using a needle-type oxygen microsensor (needle allows penetration of septum to measure oxygen without having to open the bottles, Presens, Germany) that was also used to confirm that there was oxygen remaining in the oxic incubation waters at the end. In total, 11 experimental treatments (3 replicates each) were conducted (see Table 3) that differed in sediment origin (surface versus subsurface subtidal sediment versus intertidal estuarine surface sediment), load of labile carbon addition (single versus tenfold carbon addition), the presence or absence of oxygen in incubations of subtidal sediments and three time intervals for intertidal surface sediment. These treatments will be compared with simultaneously executed background incubations without label OM additions (control-background). In addition, a set of slurries were prepared and immediately processed (see below) to establish initial conditions (used as start values of concentrations and isotope signatures of ΣCO2). Flasks with subtidal sediments were manually shaken twice a day during the first 3 days on board ship, after which they were transferred to the laboratory in a temperature-controlled vehicle and then on continuously shaken (automatically rotated). Intertidal sediment–water slurries were manually shaken once daily.
Table 3.
Control-background (C-bkgrd) remineralization rates μmol C ml−1ws day−1 and OM reactivity (k × 10−4 day−1) of the different sediment used: average under oxic (ox) and anoxic (anox) conditions
| Sediment sample (cm) | C-bkgrd OM Remineral | Bkgrd OM reactivity (k) | Percentage of tracer carbon respired | Enhanced OM decomposition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of tracer remineralization | Percentage of bkgrd remineralization | |||||||||||||||
| Ox | Anox | Ox | Anox | Ox | Anox | Ox | Anox | Ox | Anox | |||||||
| 1× | 10× | 1× | 10× | 1× | 10× | 1× | 10× | 1× | 10× | 1× | 10× | |||||
| Subtidal 0–2 | 0.26 | 0.21 | 4.4 | 3.7 | 19 | 26 | 16 | 23 | 393 | 108 | 531 | 27 | 8 | 29 | 10 | 7 |
| Subtidal 18–20 | 0.08 | 0.13 | 0.9 | 1.6 | 11 | 15 | 20 | 25 | 11 | 51 | 300 | 62 | 0.4 | 25 | 12 | 31 |
| Intertidal 0–2 | 0.44 | – | 16.3 | – | ||||||||||||
| Day 7 | 19.7 | – | 120 | – | 22 | – | ||||||||||
| Day 14 | 29.9 | – | 91 | – | 14 | – | ||||||||||
| Day 21 | 33.2 | – | 119 | – | 15 | – | ||||||||||
Also given is the percent of the tracer OM respired in the different treatments that received a single low (1×) and tenfold (10×) load of tracer amendment
In the last column, enhanced OM decomposition (priming of indigenous OM) is presented as both percentage of tracer carbon respired and as percentage of C-background OM remineralization rates, again with 1× versus 10× addition under oxic and anoxic conditions
All values are average of n = 3
Measurements and analysis
Sediment granulometry was determined on freeze-dried sediment samples with a Malvern Mastersizer 2000 and sediment porosity was calculated from water content (weight loss on freeze drying) assuming a dry density of 2.55 kg dm−3 (Dauwe et al. 2001).
In this study, we utilized the evolution of CO2 as a measure of OM remineralization because Dauwe et al. (2001) have shown that methane production can be neglected in these sediments (even for anoxic conditions). The analysis of CO2 concentrations and δ13C–CO2 was combined in one measurement, i.e., the area counts given in a standard output of an isotope ratio measurement on a mass spectrometer was used to calculate concentrations (e.g., Moodley et al. 2002). For the slurries, we first measured CO2 concentration and δ13C–CO2 in the incubation bottle headspace by sampling 1,000 μl through septum with a gas-tight syringe. Subsequently, the vials were centrifuged (3 min at 900 rpm) to obtain sediment-free water samples from which ~3 ml was transferred through a GFF filter into a helium pre-flushed vial sealed with a shrimp cap with rubber septum. Exact water volume was determined by weight. Following the acidification (10 μl 99% H3PO4 per ml sample) headspace ΣCO2 concentration and δ13C–ΣCO2 were measured using a Carlo Erba 1106 Elemental Analyser coupled online with a Finnigan Delta S isotope ratio mass spectrometer. Total ΣCO2 was the sum of that measured directly in the slurry bottle gas-phase- and liquid-phase upon acidification. Carbon isotopes are expressed in the delta notation (δ13C) relative to Vienna Pee Dee Belemnite.
Of the total ΣCO2 produced during the incubations, a part may be due to carbonate dissolution, not necessarily associated with OM remineralization (e.g., Anderson et al. 1986). Carbonate dissolution can be traced through changes in Ca+ ion concentration (e.g., Hulthe et al. 1998) or alternatively, the fraction of dissolved inorganic carbon originating from carbonate dissolution may be traced through shift in carbon isotope signatures of CO2 produced in the incubations (e.g., Ogrinc and Faganeli 2003, Ogrinc et al. 2003; Hu and Burdige 2007) and then the maximum contribution from carbonate dissolution can be estimated from a two-end member linear mixing model (e.g., Fry and Sherr 1984; Levin and Michener 2002).
We calculated the δ13C of the ΣCO2 produced during the incubations through mass balance; first, for the δ13C of the ΣCO2 at the end of the incubation in this closed system, we used the concentration-weighed average of that measured in the incubation bottle headspace and that in the water phase upon acidification after 1‰ correction for fractionation between CO2 gas in the aqueous and gaseous phase (Mook et al. 1974). Then, the δ13C of ΣCO2 produced was calculated from the difference in δ13ΣCO2 at the start and end of the incubations calculated as:
Subsequently, a two-end member linear mixing model was used to estimate maximum ΣCO2 originating from carbonate dissolution as:
where
The fraction dissolved inorganic carbon originating from carbonate dissolution (F diss), as estimated from the two-end member linear mixing model, was then subtracted from total ΣCO2 produced in the control incubations to obtain background indigenous OM remineralization. Indigenous OM decomposition in amended treatments was obtained by further subtracting CO2 originating from tracer Corg decomposition. The use of 13C-enriched tracer OM prevents direct calculation of dissolution in individual amended treatments, as described above for the different sediment types, but assuming constant dissolution dynamics for a given sediment type, we applied that determined on the corresponding background sediment (Table 2). At this point, it is important to note that the correction for carbonate dissolution is subtraction of a sediment type-specific fraction of the ΣCO2 produced and not a fixed amount of the CO2 production. Therefore, the addition of OM leading to higher CO2 production would also result in a corresponding larger ΣCO2 subtraction for dissolution correction.
Table 2.
Average total end ΣCO2 concentration in the different incubations (μmol C ml−1) and the amount of tracer carbon re-mineralized (μmol C ml−1ws) in the amended treatments under oxic (ox) versus anoxic (anox) conditions
| Sediment sample | End [ΣCO2] (μmol ml−1) | Tracer OM respired | δ13C of ΣCO2 produced (‰) | Maximum ΣCO2 from C-dissolution (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Ox | Anox | Ox | Anox | Ox | Anox | Ox | Anox | |
| Subtidal 0–2 | ||||||||
| C-bkgrd | 8.99 | 6.92 | −11.8 | −13.9 | 37.0 | 26.2 | ||
| +1× tracer OM | 9.29 | 7.20 | 0.11 | 0.09 | +288.2 | +332.5 | 37.0 | 26.2 |
| +10× tracer OM | 10.88 | 7.44 | 1.46 | 1.06 | +3,136.8 | +3,201.0 | 37.0 | 26.2 |
| Subtidal 18–20 | ||||||||
| C-bkgrd | 12.29 | 4.98 | −2.2 | −16.8 | 86.0 | 11.2 | ||
| +1× tracer OM | 12.09 | 5.17 | 0.06 | 0.11 | +137.8 | +824.9 | 86.0 | 11.2 |
| +10× tracer OM | 14.33 | 6.87 | 0.80 | 1.36 | +1,496.7 | +6,655.3 | 86.0 | 11.2 |
| Intertidal | ||||||||
| 7 day C-bkgrd | 9.60 | −13.4 | 16.6 | |||||
| +Tracer OM | 11.00 | 0.71 | +3,126.9 | 16.6 | ||||
| 14 day C-bkgrd | 11.74 | −14.5 | 9.8 | |||||
| +Tracer OM | 13.47 | 1.08 | +2,831.9 | 9.8 | ||||
| 21 day C-bkgrd | 13.70 | −14.5 | 9.6 | |||||
| +Tracer OM | 15.92 | 1.21 | +2,480.0 | 9.6 | ||||
The δ13C of ΣCO2 produced (‰) is shown in the fourth column followed by the maximum contribution of carbonate dissolution (%) to ΣCO2 produced
Maximum dissolution was taken to be constant per given sediment and dissolution in amended treatments therefore equal to corresponding control-background dissolution values
All values are average of n = 3
The calculations can be summarized as follows:
Total ΣCO2 (12C + 13C) originating from the strongly labeled algal OM: product of excess 13C (e.g., Middelburg et al. 2000) and end ΣCO2 content divided by the fractional abundance of 13C in the OM (0.27; Moodley et al. 2005a).
Total ΣCO2 produced in background incubations or amended treatments (i.e., sum of metabolic produced CO2 and CO2 from carbon dissolution): end ΣCO2 content − start ΣCO2 content.
Background indigenous OM remineralization: (2) − (F diss).
Indigenous OM remineralization in amended treatments: (2) − (F diss) – (1).
Priming is the difference in indigenous OM remineralization measured in amended treatments and that measured in control-background incubations, expressed as
Log-transformed data were analyzed by two-way analysis of variance (ANOVA) followed by pairwise comparisons (Tukey HSD test) using SYSTAT (Systat Inc.).
Results
Skagerrak, subtidal sediments were fine grained and organic carbon content was similar in surface and subsurface layers; it was much lower in the coarse-grained intertidal sediment (Table 1). The δ13C of ΣCO2 produced during the control-background incubations ranged from −2.2 to −16.8‰ and revealed different degrees of carbonate dissolution; 10–86% of the dissolved inorganic carbon originated from carbonate dissolution (Table 2). Subtracting the fraction originating from carbonate dissolution from total ΣCO2 produced in the control incubations revealed background OM remineralization rates in subtidal sediment ranging from 1.6 to 5.5 μmol C ml−1ws (21 days incubation; Fig. 1a) and in intertidal sediment these values ranged from 3.8 to 9.3 μmol C ml−1ws after 7–21 days of incubation (Fig. 1b). Conversion to daily OM remineralization rates and OM reactivity (k, calculated from a single pool as quotients of CO2 production rates and Corg content) show that subtidal subsurface sediment had approximately four times lower OM reactivity than its surface counterpart and was an order of magnitude less reactive than that of the intertidal sediment (Table 3).
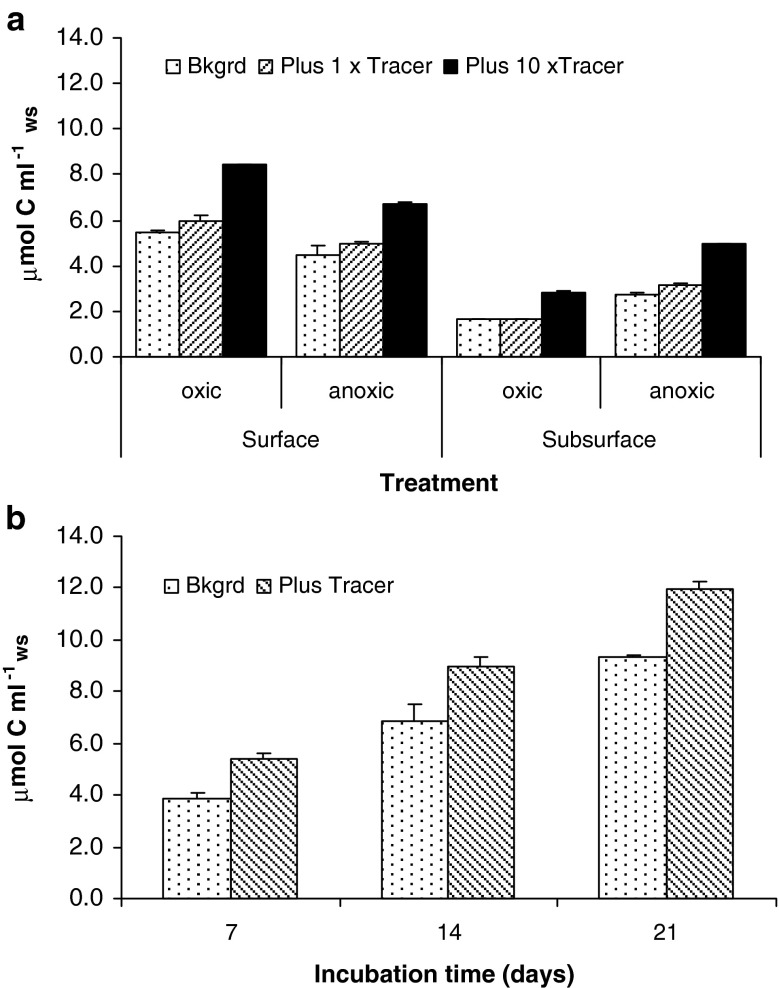
Fig. 1.
a The amount of OM remineralized after 21 days of incubation of surface versus subsurface with or without oxygen in background (Bkgrd) and tracer OM amended (single and tenfold load) subtidal sediment, b the amount of OM remineralized after 7, 14, and 21 days of incubation of intertidal surface sediment under oxic conditions with or with tracer OM addition. Average μmol C ml−1 wet sediment plus standard errors (n = 3)
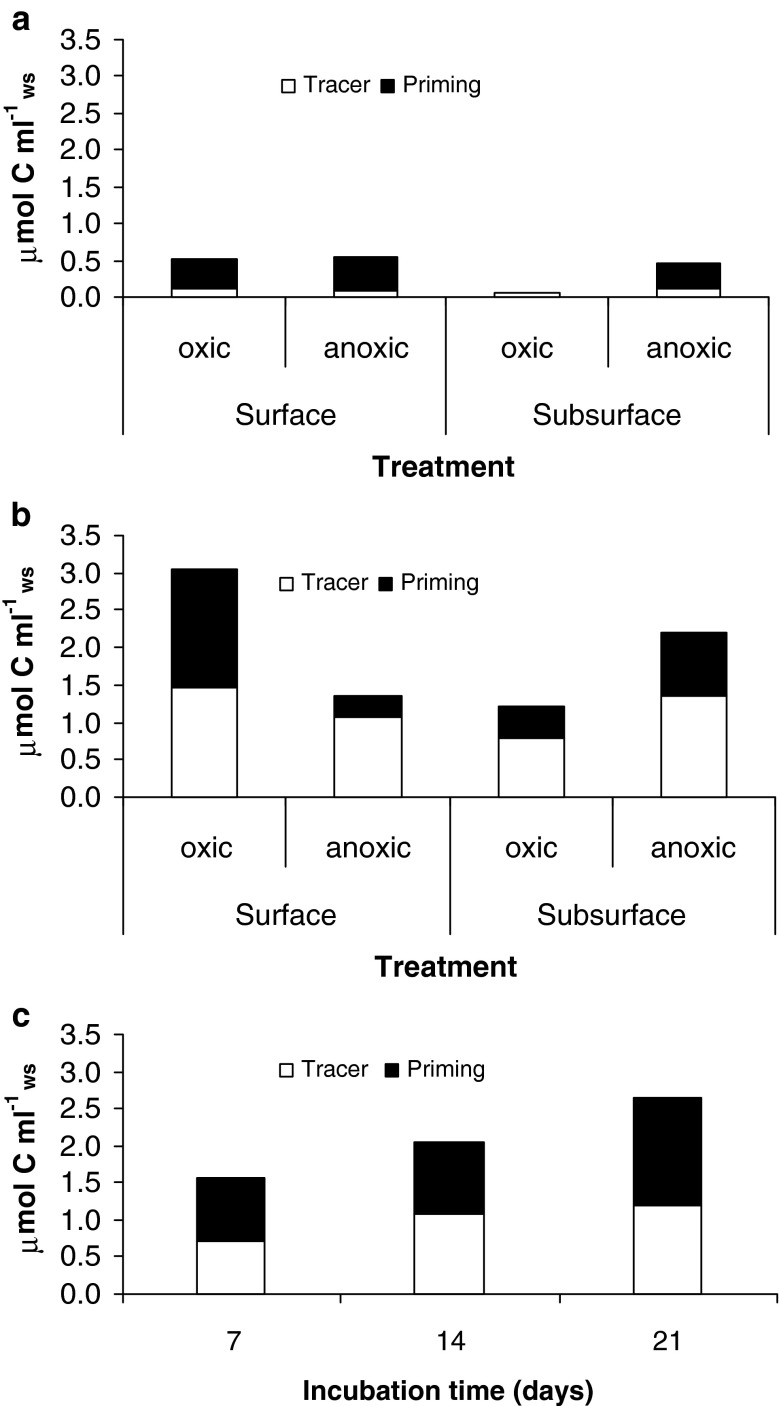
The fresh algal carbon addition significantly enhanced sediment release of ΣCO2 in surface and subsurface subtidal sediment both under oxic and anoxic conditions (Fig. 1a); treatments with amendments, especially in the higher OM addition, had higher OM remineralization rates (ANOVA, P < 0.05; Table 4). Depending on the amount of algal carbon added, sediment total OM remineralization rates were enhanced 4–80% of background values (Fig. 1a). Similarly, the addition of algal carbon significantly increased total OM remineralization in the intertidal sediments (Table 4; Fig. 1b; increasing background rates by 28–40%). However, these overall significant increases in total OM remineralization were only in part due to the respiration of the added carbon (Fig. 2). In the low load amended subtidal sediment, 16–90% (average 38 ± 35%) of excess originated from tracer respiration (Fig. 2a). For sediment with a tenfold OM addition, 48–79% (average 64 ± 13%) of excess fluxes originated from the tracer OM (Fig. 2b) and in the intertidal sediment incubations, an average of 48% of excess originated from the tracer addition (Fig. 2c). Therefore, different fractions of excess fluxes were non-tracer OM and potentially due to OM priming evident in all treatments (Table 3; Fig. 2). However, reversed redox conditions for subtidal sediments (anoxic for surface and oxic for subsurface) seemed to be relatively less facilitative for OM priming (Table 3; Fig. 2b). This is in accordance with the significant (ANOVA, P < 0.05; Table 4) impact of oxygenation on subtidal OM decomposition rates (higher rates under oxic conditions for surface sediment, but the opposite for subsurface sediment, Fig. 1a). The significant interaction term for the subsurface subtidal sediment can be attributed to the slightly different response of the 1× treatment under oxic and anoxic conditions (Fig. 1a). Although quantitatively (μmol C ml−1ws) the highest priming was found with higher OM additions, the magnitude of priming was not proportional to the amount of tracer OM added (Fig. 2): tenfold increase in OM addition did not result in tenfold higher priming. Clear cases of enhancement are seen in surface sediment under oxic conditions and subsurface sediment under anoxic conditions (compare Fig. 2a, b). For the subtidal high OM additions, priming was equivalent to 7–31% of background OM remineralization, but equivalent to 27–100% of remineralization of the highly degradable tracer added carbon (Table 3; Fig. 2b). In the low-load subtidal incubations, priming was equivalent to <1–12% of background OM remineralization and 11–500% of remineralization of the highly degradable tracer added carbon (Table 3; Fig. 2a).
Table 4.
Results of two-way ANOVA of OM remineralization rates (log-transformed
| Source | SS | df | MS | F ratio | P |
|---|---|---|---|---|---|
| Subtidal surface | |||||
| Treatment | 0.086 | 2 | 0.043 | 49.193 | 0.000 |
| Oxygenation | 0.031 | 1 | 0.031 | 34.982 | 0.000 |
| Interaction | 0.000 | 2 | 0.000 | 0.186 | 0.833 |
| Error | 0.009 | 10 | 0.001 | ||
| Subtidal subsurface | |||||
| Treatment | 0.214 | 2 | 0.107 | 1,085.722 | 0.000 |
| Oxygenation | 0.277 | 1 | 0.277 | 2,820.872 | 0.000 |
| Interaction | 0.001 | 2 | 0.001 | 10.084 | 0.003 |
| Error | 0.001 | 12 | 0.000 | ||
| Intertidal surface | |||||
| Treatment | 0.051 | 1 | 0.051 | 44.460 | 0.000 |
| Time | 0.273 | 2 | 0.136 | 118.734 | 0.000 |
| Interaction | 0.001 | 2 | 0.000 | 0.400 | 0.683 |
| Error | 0.009 | 8 | 0.001 | ||
Treatment is no amendments versus different OM additions
Fig. 2.
Division of excess fluxes above background rates between that from tracer OM and that of OM priming: a Twenty-one day incubations of subtidal sediment under different redox conditions amended with a single load of tracer OM. b Same as a, but with a tenfold tracer OM addition. c Intertidal sediment after 7, 14 and 21 days incubation. Average μmol C ml−1 wet sediment (n = 3)
As in the case of subtidal sediment, tracer addition to intertidal sediment clearly impacted OM remineralization rates. First, there was significantly more OM decomposition over time and OM amendment significantly increased OM decomposition rates (ANOVA, P < 0.05, Table 4; Fig. 1b). Priming was also evident in intertidal sediment already after 7 days and ranged from 91 to 120% of that derived from the tracer OM, equivalent to on an average 17.2 ± 3.5% of background OM remineralization (Fig. 2c; Table 3).
Although the sediment material used in this study differed about as much as one order of magnitude in OM reactivity, the priming effect after 21 days was rather similar for comparable treatments and the priming resulted in a 15–29% increase in background OM mineralization in Skagerrak subtidal and intertidal estuarine sediments incubated under oxic conditions with similar high OM additions (Table 3).
For subtidal sediment incubations, the amount of ΣCO2 that originated from tracer OM was proportional to the amount added (~12-fold increase in amount ΣCO2 originating from tracer OM with tenfold higher tracer addition, Fig. 2). For the high-load additions, a similar fraction of the tracer OM was re-mineralized within 21 days in subtidal sediments under different redox conditions (23–26%) except lowered value of ~15% for subsurface sediment under oxic conditions (Table 3). A similar trend among the treatments was also evident in the low-load OM amendments; albeit with a slightly lower percent of tracer carbon respired after 21 days (16–20%; Table 3). In the case of intertidal sediment approximately 33% of the added tracer was respired within 21 days (Table 3).
Discussion
The concept and consequences of priming have been discussed in the marine literature (e.g., Graf 1992; Canfield 1994; Aller 1994; Gribsholt and Kristensen 2002), but few conclusive results have been reported because of experimental difficulties. It is not only necessary to detect the small enhancement of total OM mineralization due to labile carbon addition, but it is also needed to partition this excess mineralization between tracer and enhanced indigenous carbon mineralization. Moreover, carbonate dissolution may complicate interpretation of dissolved inorganic production rates as proxy for mineralization. Closed, small volume sediment–water incubations as used in this study may exhibit elevated sensitivity to carbonate dissolution due to the possible exhaustion of alkalinity or buffer capacity. This is particularly evident in the subtidal subsurface sediment under oxic conditions where the high incubation end ΣCO2 concentration was primarily due to carbonate dissolution. Oxidation of compounds, such as ammonium that often accumulate in deeper sediment layers not exposed to bioturbation (e.g., Nielsen et al. 2004; Mermillod-Blondin et al. 2005) can lead to extensive loss of alkalinity (Wilson and Thomson 1998; Soetaert et al. 2007). This is supported by more limited dissolution under anoxic conditions (Table 2).
It is clearly evident that sediments may differ strongly in their dissolution dynamics (Table 2) depending on origin and redox conditions and highlights the importance of individual estimates per sediment type and treatment as conducted in this study (Table 2). At the same time, this draws attention to the application of dissolution dynamics determined for background to amended treatments, but we estimate that this has not led to gross overestimation of priming because of underestimating dissolution. First, dissolution dynamics are expected to be similar because OM additions were by no means unusually high. In addressing OM priming, the amount of matter added should preferably be close to in situ OM fluxes. In this study, using the CO2 production rates, we estimate OM input for the subtidal surface sediment at 2.6 mmol C m−2 day−1 and for the intertidal sediment at 4.4 mmol C m−2 day−1. This means that the additions used for the subtidal sediment equal approximately 10–100% of in situ input for that experiment period of 21 days. For the intertidal sediments, this is estimated at being 40% of a 3-week input. Therefore, the overall amounts employed here are within ranges expected to prevail in the field. The amount that can be mixed deep down in the subtidal sediment used in this study is unknown, but it is expected to represent a small fraction of that settling on the surface (that may vary depending on the mode of bioturbation) and the 10% of estimated incoming fluxes employed is not unrealistically high.
Secondly, OM amendment treatments were otherwise identical to background incubations and as used in this study, carbonate dissolution correction (calculated as percentage of CO2 production) is proportional to the amount of CO2 produced thereby accounting for potential increase in the dissolution coupled to enhanced OM decomposition (tracer and indigenous OM). In addition, the two end mixing model method represents a maximum percentage contribution of dissolution, so that priming could in fact be larger. Therefore, isotope insensitive complementary tools, such as changes in Ca+ ion concentration (e.g., Hulthe et al. 1998) may constitute an important refinement in this method of addressing the subject OM priming in marine sediments.
Another potential complication of our incubation technique relates to rapid changes in redox conditions as imposed in this study, which may destroy a portion of the microbes with consequences for the net efficiency of remineralization (Aller 1994). However, overall, a substantial fraction of fresh tracer OM was readily and rapidly processed and respired by the sediment microbiota, consistent with recent isotope tracer studies (e.g., Blair et al. 1996; Moodley et al. 2005a, Buhring et al. 2006). For tenfold tracer OM addition in the subtidal sediment, 23–26% was respired within 21 days except for a low 15% in subsurface sediment exposed to oxic conditions (Table 3). In contrast to surface layers, deeper subsurface layers are not naturally exposed to changing redox conditions and this lowered activity may reflect reduced flexibility of deep subsurface microbes. After 21 days of incubation, 33 ± 3% of the added carbon was respired in intertidal sediments (Table 3) indicating that coarse permeable sediments may indeed be favorable for microbial OM remineralization despite an expected relatively low-microbial standing stock (Dauwe et al. 2001; Buhring et al. 2006).
The results of this study clearly revealed that input of fresh OM can potentially enhance remineralization rates of native OM both under oxic and anoxic conditions. There are very few data to compare with our results. Kristensen and Holmer (2001) incubated thin layers of homogenized surface sediment in a continuous flow system and found no significant effects that could be attributed to priming. Turnewitsch et al. (2007) focused on the interaction of labile and refractory organic matter on nitrogen cycling and reported a transient positive effect on nitrogen mineralization, but no significant effect on particulate nitrogen pools. Hee et al. (2001) incubated coastal deep subsurface sediments retrieved from below the active remineralization zone and demonstrated priming under anoxic conditions using both dissolved inorganic carbon and carbon isotope data as we did.
In contrast to marine sediments, OM priming in soils has been the target of numerous studies and it has been established that it is strongly influenced by the diversity and concentration of substrates employed. Moreover, priming may be evident, but substrate addition may also inhibit OM degradation and its magnitude can vary strongly and depends on the concentration of the substrate added (e.g., Fontaine et al. 2003; Kuzyakov et al. 2000; Hamer and Marschner 2005a, b; Waldrop and Firestone 2004; Brant et al. 2006). These studies also revealed that priming in soils can exceed background remineralization rates, while it represented only a small fraction of background OM remineralization in our study (up to 31%, Table 3). However, the rates of these enhanced fluxes were in the range of remineralization rates measured for the highly reactive and degradable amended OM (Fig. 2). Given that OM lability is a prime factor governing OM remineralization (Wakeham and Canuel 2006 and references therein), this magnitude of priming, therefore, albeit a small percentage of background OM remineralization may be considered highly relevant.
Our experiment revealed that even a very small addition of fresh OM induced OM priming (Fig. 2). Consistently, Mondini et al. (2006) showed that the addition of trace amounts of different simple and complex substrates can induce immediate, significant activation of soil microbes. However, with a high load, there seems to be preferential uptake of fresh OM resulting in relatively decreased priming (compare Fig. 2a, b). Although this study demonstrates that OM priming may also be prevalent in marine sediments and, therefore, potentially an important determinant of the final carbon balance, clearly more experimental work is required. We expected that input of fresh OM would have the strongest stimulation on microbial activity under anoxic conditions (remineralization is generally more complete in presence of oxygen) and in sediments with relatively less reactive native OM. This does seem to be the case: priming as percentage of control-background remineralization was indeed highest in subtidal subsurface sediment under anoxic incubations (31%, Table 3). However, priming was similarly high in oxic subtidal surface sediment incubations (29%) and intertidal sediment (15%) with relatively high OM reactivity (Table 3). In addition, the highest absolute amount primed (μmol C ml−1ws) was 1.43 in the intertidal sediment after 21 days and 1.58 in the subtidal sediments (oxic surface with tenfold OM additions, Fig. 2). Moreover, the actual mechanisms involved or driving priming are still unclear (e.g., Hee et al. 2001; Hamer and Marschner 2005b; Fontaine et al. 2003). Since the initial proposal of OM priming (Löhnis 1926), it has been a subject of debate whether or not priming was an artifact: priming may not reflect increase in native soil organic matter remineralization, but remineralization of bacterial biomass replaced by new tracer substrate derived biomass. However, studies including soil microbial biomass dynamics confirm that true priming does occur (e.g., Fontaine et al. 2004a and references therein; Kuzyakov et al. 2007) and recently further insight into microbial dynamics involved in soil OM priming have been gained through the application of compound-specific isotope analysis (bacteria-specific fatty acid biomarkers, e.g., Waldrop and Firestone 2004; Brant et al. 2006). Overall for soils, it seems that energy-rich substrate additions rapidly induce shifts in microbial community structure and activity. Depending not only on the reactivity and standing stock of native OM, but also on the substrate added (composition and quantity); priming may be absent, neutral or positive (Fontaine et al. 2004b and references therein). Clearly more experiments with marine sediments are required and although small volume sediment–slurry incubations may be convenient, intact sediment incubated in flow through systems including microbial dynamics and diversity analysis will provide a more complete assessment of the ecological significance of OM priming. Finally, although experimental mineralization rates may deviate from field values, it does not invalidate the study of OM priming which is a comparison-based approach employing same physical conditions for all treatments. Irrespective, this study shows that organic matter priming may indeed occur in marine sediments. This highlights a potential impact of bioturbation (mixing and displacement of sediment by macrofauna) on sediment OM mineralization that is rarely considered. Bioturbation by large organisms has been repeatedly observed to enhance sediment OM remineralization primarily through oxygenation of subsurface sediments (e.g., Kristensen 2000; Meysman et al. 2006). This can now be extended with OM priming driven by the same down-core mixing of incoming fresh OM. This link between OM priming and fauna activity is further supports the hypothesis that basic seafloor ecological processes are strongly linked to the structure of seafloor communities (Waldbusser et al. 2004; Lohrer et al. 2004; Thrush et al. 2006b; Mermillod-Blondin and Rosenberg 2006; Raffaelli 2006) and is further substantiates that bioturbation by large organisms may strongly govern the carbon flow pathways and the organic carbon balance in marine sediments (e.g., Bianchi et al. 2000; Moodley et al. 2005a; Thrush et al. 2006a; Meysman et al. 2006.
Acknowledgments
Special thanks to Lennart van Ijzerloo for technical assistance and the captain and crew of the R.V. Pelagia and the R.V. Luctor for excellent services. Peter van Breugel and Marco Houtekamer are acknowledged for carrying out different laboratory analysis. Special thanks to Suzan Verdegaal and Hubert Vonhof (Faculty of Earth and Life Sciences, Vrije University, Amsterdam, The Netherlands) for sediment bulk carbonate carbon isotope measurements. Pim van Avesaath and Nicole Dijkman are acknowledged for valuable discussions. Detailed and highly constructive reviews by two anonymous reviewers greatly improved the manuscript. This research was supported by the Netherlands Organization of Scientific Research (PIONIER 833.02.2006). This is publication number 4581 of the Netherlands Institute of Ecology (NIOO-KNAW), Yerseke.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aller RC. Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol. 1994;114:331–345. doi: 10.1016/0009-2541(94)90062-0. [DOI] [Google Scholar]
- Anderson LG, Hall PO, Iverfeldt A, van der Rutgers Loeff MM, Sundby B, Westerland SFG. Benthic respiration measured by total carbonate production. Limnol Oceanogr. 1986;31:319–329. [Google Scholar]
- Bianchi TS, Johansson B, Elmgren R. Breakdown of phytoplankton pigments in Baltic sediments: effects of anoxia and loss of deposit-feeding macrofauna. J Exp Mar Biol Ecol. 2000;251:161–183. doi: 10.1016/S0022-0981(00)00212-4. [DOI] [PubMed] [Google Scholar]
- Biles CL, Paterson DM, Ford RB, Solan M, Raffaelli DG. Bioturbation, ecosystem functioning and community structure. Hydrol Earth Sys Sci. 2002;6:999–1005. [Google Scholar]
- Blair NE, Levin LA, De Master DJ, Plaia G. The short-term fate of fresh algal carbon in continental slope sediments. Limnol Oceanogr. 1996;41:1208–1219. [Google Scholar]
- Boschker HTS, Brouwer JFC, Cappenberg TE. The contribution of macrophyte-derived organic matter to microbial biomass in salt marsh sediments: stable carbon isotope analysis of microbial biomarkers. Limnol Oceanogr. 1999;44:309–319. [Google Scholar]
- Brant JB, Sulzman EW, Myrold DD. Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem. 2006;38:2219–2232. doi: 10.1016/j.soilbio.2006.01.022. [DOI] [Google Scholar]
- Buhring SI, Ehrenhauss S, Kamp A, Moodley L, Witte U. Enhanced benthic activity in sandy sublittoral sediments: evidence from C-13 tracer experiments. Mar Biol Res. 2006;2:120–129. doi: 10.1080/17451000600678773. [DOI] [Google Scholar]
- Canfield DE. Factors influencing organic carbon preservation in marine sediments. Chem Geol. 1994;114:315–329. doi: 10.1016/0009-2541(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Dauwe B, Middelburg JJ. Amino acids and hexosamines as indicators of organic matter degradation state in North Sea sediments. Limnol Oceanogr. 1998;43:782–798. [Google Scholar]
- Dauwe B, Middelburg JJ, Herman PMJ. Effect of oxygen on the degradability of organic matter in subtidal and intertidal sediments of the North Sea area. Mar Ecol Prog Ser. 2001;215:13–22. doi: 10.3354/meps215013. [DOI] [Google Scholar]
- Fontaine S, Mariotti A, Abbadie L. The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem. 2003;35:837–843. doi: 10.1016/S0038-0717(03)00123-8. [DOI] [Google Scholar]
- Fontaine S, Bardoux G, Benest D, Verdier B, Mariotti A, Abbadie L. Mechanisms of priming effect in a Savannah soil amended with cellulose. Soil Sci Soc Am J. 2004;68:125–131. [Google Scholar]
- Fontaine S, Bardoux G, Abbadie L, Mariotti A. Carbon input to soil decrease soil carbon content. Ecol Lett. 2004;7:314–320. doi: 10.1111/j.1461-0248.2004.00579.x. [DOI] [Google Scholar]
- Fry B, Sherr EB. δ13C measurements as indicators of carbon flow in marine and fresh water systems. Contrib Mar Sci. 1984;27:13–46. [Google Scholar]
- Graf G. Benthic–pelagic coupling: a benthic view. Oceanogr Mar Biol Ann Rev. 1992;30:149–190. [Google Scholar]
- Gribsholt B, Kristensen E. Effects of bioturbation and plant roots on salt marsh biogeochemistry: a mesocosm study. Mar Ecol Prog Ser. 2002;241:71–87. doi: 10.3354/meps241071. [DOI] [Google Scholar]
- Hamer U, Marschner B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol Biochem. 2005;37:445–454. doi: 10.1016/j.soilbio.2004.07.037. [DOI] [Google Scholar]
- Hamer U, Marschner B. Priming effects in soils after combined and repeated substrate additions. Geoderma. 2005;128:38–51. doi: 10.1016/j.geoderma.2004.12.014. [DOI] [Google Scholar]
- Hartnett HE, Keil RG, Hedges JI, Devol AH. Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature. 1998;391:572–574. doi: 10.1038/35351. [DOI] [Google Scholar]
- Harvey HR (2006) Sources and cycling of organic matter in the marine water column. In: Volkman JK (ed) Marine organic matter: the handbook of environmental chemistry, vol 2. Part N. Springer, Berlin, pp 1–25
- Harvey HR, Tuttle JH, Bell JT. Kinetics of phytoplankton decay during simulated sedimentation—changes in biochemical composition and microbial activity under oxic and anoxic conditions. Geochim Cosmochim Acta. 1995;59:3367–3377. doi: 10.1016/0016-7037(95)00217-N. [DOI] [Google Scholar]
- Hee CA, Pease TK, Alperin MJ, Martens CS. Dissolved organic carbon production and consumption in anoxic marine sediments: a pulsed-tracer experiment. Limnol Oceanogr. 2001;46:1908–1920. [Google Scholar]
- Heip CHR, Goosen NK, Herman PMJ, Kromkamp J, Middelburg JJ, Soetaert K. Production and consumption of biological particles in temperate tidal estuaries. Oceanogr Mar Biol Annu Rev. 1995;33:1–149. [Google Scholar]
- Heip CHR, Duineveld GCA, Flach E, Graf G, Helder W, Herman PMJ, Lavaleye M, Middelburg JJ, Pfannkuche O, Soetaert K, Soltwedel T, de Stigter H, Thomsen L, Van Averbeke J, de Wilde P. The role of the benthic biota in sedimentary metabolism and sediment–water exchange processes in the Goban Spur area (NE Atlantic) Deep Sea Res Part II. 2001;48:3223–3243. doi: 10.1016/S0967-0645(01)00038-8. [DOI] [Google Scholar]
- Hoyle FC, Murphy DV, Brookes PC. Microbial response to the addition of glucose in low-fertility soils. Biol Fertil Soils. 2008;44:571–579. doi: 10.1007/s00374-007-0237-3. [DOI] [Google Scholar]
- Hu XP, Burdige DJ. Enriched stable carbon isotopes in the pore waters of carbonate sediments dominated by seagrasses: evidence for coupled carbonate dissolution and reprecipitation. Geochim Cosmochim Acta. 2007;71:129–144. doi: 10.1016/j.gca.2006.08.043. [DOI] [Google Scholar]
- Hulthe G, Hulth S, Hall POJ. Effect of oxygen on degradation rate of refractory and labile organic matter in continental margin sediments. Geochim Cosmochim Acta. 1998;62:1319–1328. doi: 10.1016/S0016-7037(98)00044-1. [DOI] [Google Scholar]
- Josefson AB, Forbes TL, Rosenberg R. Fate of phytodetritus in marine sediments: functional importance of macrofaunal community. Mar Ecol Prog Ser. 2002;230:71–85. doi: 10.3354/meps230071. [DOI] [Google Scholar]
- Kristensen E. Organic matter diagenisis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia. 2000;426:1–24. doi: 10.1023/A:1003980226194. [DOI] [Google Scholar]
- Kristensen E, Holmer M. Decomposition of plant materials in marine sediment exposed to different electron acceptors (O2, NO3−, and SO42−), with emphasis on substrate origin, degradation kinetics, and the role of bioturbation. Geochim Cosmochim Acta. 2001;65:419–433. doi: 10.1016/S0016-7037(00)00532-9. [DOI] [Google Scholar]
- Kristensen E, Andersen FO, Blackburn TH. Effects of benthic macrofauna and temperature on degradation of macroalgal detritus: the fate of organic carbon. Limnol Oceanogr. 1992;37:1404–1419. [Google Scholar]
- Kristensen E, Ahmed SI, Devol AH. Aerobic and anaerobic decomposition of organic matter in marine sediment: which is fastest? Limnol Oceanogr. 1995;40:1430–1437. [Google Scholar]
- Kuzyakov Y, Friedel JK, Stahr K. review of mechanisms and quantification of priming effects. Soil Biol Biochem. 2000;32:1485–1498. doi: 10.1016/S0038-0717(00)00084-5. [DOI] [Google Scholar]
- Kuzyakov Y, Hill PW, Jones DL. Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil. 2007;290:305. doi: 10.1007/s11104-006-9162-8. [DOI] [Google Scholar]
- Levin LA, Michener RH. Isotopic evidence for chemosynthesis-bases nutrition of macrobenthos: the lightness of being at Pacific methane seeps. Limnol Oceanogr. 2002;47:1336–1345. [Google Scholar]
- Levin L, Blair N, DeMaster D, Plaia G, Fornes W, Martin C, Thomas C. Rapid subduction of organic matter by maldanid polychaetes on the North Carolina slope. J Mar Res. 1997;55:595–611. doi: 10.1357/0022240973224337. [DOI] [Google Scholar]
- Löhnis F. Nitrogen availability in green manures. Soil Sci. 1926;22:253–290. doi: 10.1097/00010694-192610000-00001. [DOI] [Google Scholar]
- Lohrer AM, Thrush SF, Gibbs MM. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature. 2004;431:1092–1095. doi: 10.1038/nature03042. [DOI] [PubMed] [Google Scholar]
- Mayer LM, Jumars PA, Bock MJ, Vetter YA, Schmidt JL. Two roads to sparagmos: extracellular digestion of sedimentary food by bacterial inoculation versus deposit-feeding. In: Aller JY, Woodin SA, Aller RC, editors. Organism–sediment interactions. Columbia: University of South Carolina Press; 2001. pp. 335–347. [Google Scholar]
- Mermillod-Blondin F, Rosenberg R. Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat Sci. 2006;68:434–442. doi: 10.1007/s00027-006-0858-x. [DOI] [Google Scholar]
- Mermillod-Blondin F, Rosenberg R, Francois-Carcaillet F, Norling K, Mauclaire L. Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquat Microb Ecol. 2004;36:271–284. doi: 10.3354/ame036271. [DOI] [Google Scholar]
- Mermillod-Blondin F, Francois-Carcaillet F, Rosenberg R. Biodiversity of benthic invertebrates and organic matter processing in shallow marine sediments: an experimental study. J Exp Mar Biol Ecol. 2005;315:187–209. doi: 10.1016/j.jembe.2004.09.013. [DOI] [Google Scholar]
- Meysman FJR, Middelburg JJ, Heip CHR. Bioturbation: a fresh look at Darwin’s last idea. Trends Ecol Evol. 2006;21:688–695. doi: 10.1016/j.tree.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Michaud E, Desrosiers G, Mermillod-Blondin F, Sundby B, Stora G. The functional group approach to bioturbation: the effects of biodiffusers and gallery-diffusers of the Macoma baltic community on sediment oxygen uptake. J Exp Mar Biol Ecol. 2005;326:77–88. doi: 10.1016/j.jembe.2005.05.016. [DOI] [Google Scholar]
- Middelburg JJ, Barranguet C, Boschker HTS, Herman PMJ, Moens T, Heip CHR. The fate of intertidal microphytobentos: an in situ 13C labeling study. Limnol Oceanogr. 2000;45:1224–1234. [Google Scholar]
- Mondini C, Cayuela ML, Sanchez-Monedero MA, Roig Brookes PC. Soil microbial biomass activation by trace amounts of readily available substrate. Biol Fertil Soils. 2006;42:542–549. doi: 10.1007/s00374-005-0049-2. [DOI] [Google Scholar]
- Moodley L, Boschker HTS, Middelburg JJ, Pel R, Herman PMJ, de Deckere EMGT, Heip CHR. Ecological significance of benthic foraminifera: C-13 labelling experiments. Mar Ecol Prog Ser. 2000;202:29–289. doi: 10.3354/meps202289. [DOI] [Google Scholar]
- Moodley L, Middelburg JJ, Boschker HTS, Duineveld GCA, Pel R, Herman PMJ, Heip CHR. Bacteria and Foraminifera: key players in a short-term deep-sea benthic response to phytodetritus. Mar Ecol Prog Ser. 2002;236:23–29. doi: 10.3354/meps236023. [DOI] [Google Scholar]
- Moodley L, Middelburg JJ, Soetaert K, Boschker HTS, Herman PMJ, Heip CHR. Similar rapid response to phytodetritus deposition in shallow and deep-sea sediments. J Mar Res. 2005;63:457–469. doi: 10.1357/0022240053693662. [DOI] [Google Scholar]
- Moodley L, Middelburg JJ, Herman PMJ, Soetaert K, de Lange GJ. Oxygenation and organic-matter preservation in marine sediments: direct experimental evidence from ancient organic carbon-rich deposits. Geology. 2005;33:889–892. doi: 10.1130/G21731.1. [DOI] [Google Scholar]
- Mook WG, Bommerson JC, Staverman WH. Carbon isotope fractionation between dissolved and gaseous carbon dioxide. Earth Planet Sci Lett. 1974;22:169–176. doi: 10.1016/0012-821X(74)90078-8. [DOI] [Google Scholar]
- Nielsen OL, Gribsholt B, Kreistensen E, Revsbech NP. Microscale distribution of oxygen and nitrate in sediment inhabited by Nereis diversicolor: spatial patterns and estimated reaction rates. Aquat Microb Ecol. 2004;34:23–32. doi: 10.3354/ame034023. [DOI] [Google Scholar]
- Ogrinc N, Faganeli J. Stable carbon isotopes in pore waters of coastal marine sediments (the Gulf of Trieste, N Adriatic) Acta Chim Slovenica. 2003;50:645–662. [Google Scholar]
- Ogrinc N, Faganeli J, Pezdic J. Determination of organic carbon remineralization in near-shore marine sediments (Gulf of Trieste, Northern Adriatic) using stable carbon isotopes. Org Geochem. 2003;34:681–692. doi: 10.1016/S0146-6380(03)00023-8. [DOI] [Google Scholar]
- Raffaelli D. Biodiversity and ecosystem functioning: issues of scale and trophic complexity. Mar Ecol Prog Ser. 2006;311:285–294. doi: 10.3354/meps311285. [DOI] [Google Scholar]
- Soetaert K, Hofmann AF, Middelburg JJ, Meysman FJR. The effect of biogeochemical processes on pH. Mar Chem. 2007;105:30–51. doi: 10.1016/j.marchem.2006.12.012. [DOI] [Google Scholar]
- Sun MY, Dai JH. Relative influences of bioturbation and physical mixing on degradation of bloom-derived particulate organic matter: clue from microcosm experiments. Mar Chem. 2005;96:201–218. doi: 10.1016/j.marchem.2004.11.003. [DOI] [Google Scholar]
- Sun M-Y, Aller RC, Lee C. Laboratory studies of oxic and anoxic degradation of chlorophyll a in Long Island sound sediments. Geochim Cosmochim Acta. 1993;57:147–157. doi: 10.1016/0016-7037(93)90475-C. [DOI] [Google Scholar]
- Sun MY, Aller RC, Lee C, Wakeham SG. Effects of oxygen and redox oscillation on degradation of cell-associated lipids in surficial marine sediments. Geochim Cosmochim Acta. 2002;66:2003–2012. doi: 10.1016/S0016-7037(02)00830-X. [DOI] [Google Scholar]
- Sun MY, Cai WJ, Joye SB, Ding HB, Dai JH, Hollibaugh JT. Degradation of algal lipids in microcosm sediments with different mixing regimes. Org Geochem. 2002;33:445–459. doi: 10.1016/S0146-6380(02)00005-0. [DOI] [Google Scholar]
- Thrush SF, Hewitt JE, Gibbs M, Lundquist C, Norkko A. Functional role of large organisms in intertidal communities: community effects and ecosystem function. Ecosystems. 2006;9:1029–1040. doi: 10.1007/s10021-005-0068-8. [DOI] [Google Scholar]
- Thrush SF, Gray JS, Hewitt JE, Ugland KI. Predicting the effects of habitat homogenization on marine biodiversity. Ecol Appl. 2006;16:1636–1642. doi: 10.1890/1051-0761(2006)016[1636:PTEOHH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Turnewitsch R, Domeyer B, Graf G. Experimental evidence for an effect of early-diagenetic interaction between labile and refractory marine sedimentary organic matter on nitrogen dynamics. J Sea Res. 2007;57:270–280. doi: 10.1016/j.seares.2006.08.001. [DOI] [Google Scholar]
- Wakeham SG, Canuel EA (2006) Degradation and preservation of organic matter in marine sediments. In: Volkman JK (ed) Marine organic matter: the handbook of environmental chemistry, vol 2. Part N. Springer, Berlin, pp 295–321
- Waldbusser GG, Marinelli RL, Whitlatch RB, Visscher PT. The effects of infaunal biodiversity on biogeochemistry of coastal marine sediments. Limnol Oceanogr. 2004;49:1482–1492. [Google Scholar]
- Waldrop MP, Firestone MK. Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak–woodland plant communities. Oecologia. 2004;138:275–284. doi: 10.1007/s00442-003-1419-9. [DOI] [PubMed] [Google Scholar]
- Wilson TRS, Thomson J. Calcite dissolution accompanying early diagenesis in turbiditic deep ocean sediments. Geochim Cosmochim Acta. 1998;62:2087–2096. doi: 10.1016/S0016-7037(98)00129-X. [DOI] [Google Scholar]
- Witte U, Aberle N, Sand M, Wenzhofer F. Rapid response of a deep-sea benthic community to POM enrichment: an in situ experimental study. Mar Ecol Prog Ser. 2003;251:27–36. doi: 10.3354/meps251027. [DOI] [Google Scholar]
- Witte U, Wenzhofer F, Sommer S, Boetius A, Heinz P, Aberle N, Sand M, Cremer A, Abraham WR, Jorgensen BB, Pfannkuche O. In situ experimental evidence of the fate of a phytodetritus pulse at the abyssal sea floor. Nature. 2003;424:763–766. doi: 10.1038/nature01799. [DOI] [PubMed] [Google Scholar]