Abstract
Dorsal raphe nucleus (DRN) serotonin (5-HT) neurons play an important role in feeding, mood control and stress responses. One important feature of their activity across the sleep-wake cycle is their reduced firing during rapid-eye-movement (REM) sleep which stands in stark contrast to the wake/REM-on discharge pattern of brainstem cholinergic neurons. A prominent model of REM sleep control posits a reciprocal interaction between these cell groups. 5-HT inhibits cholinergic neurons, and activation of nicotinic receptors can excite DRN 5-HT neurons but the cholinergic effect on inhibitory inputs is incompletely understood. Here, in vitro, in DRN brain slices prepared from GAD67-GFP knock-in mice, a brief (3 min) bath application of carbachol (50 μM) increased the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in GFP-negative, putative serotonin neurons but did not affect miniature (tetrodotoxin-insensitive) IPSCs. Carbachol had no direct postsynaptic effect. Thus, carbachol likely increases the activity of local GABAergic neurons which synapse on 5-HT neurons. Removal of dorsal regions of the slice including the ventrolateral periaqueductal gray (vlPAG) region where GABAergic neurons projecting to the DRN have been identified, abolished the effect of carbachol on sIPSCs whereas removal of ventral regions containing the oral region of the pontine reticular nucleus (PnO) did not. In addition, carbachol directly excited GFP-positive, GABAergic vlPAG neurons. Antagonism of both muscarinic and nicotinic receptors completely abolished the effects of carbachol. We suggest cholinergic neurons inhibit DRN 5-HT neurons when acetylcholine levels are lower i.e. during quiet wakefulness and the beginning of REM sleep periods, in part via excitation of muscarinic and nicotinic receptors located on local vlPAG and DRN GABAergic neurons. Higher firing rates or burst firing of cholinergic neurons associated with attentive wakefulness or phasic REM sleep periods leads to excitation of 5-HT neurons via activation of nicotinic receptors located postsynaptically and presynaptically on excitatory afferents.
Keywords: Patch-clamp, presynaptic modulation, sleep, GAD67-GFP knock-in mice, ventrolateral periaqueductal gray
Wakefulness and rapid-eye-movement (REM) sleep are conscious brain states which appear superficially similar, based on examination of the cortical electroencephalogram (EEG). However, brain function is dramatically different in these two states. One major neuromodulatory difference which may help explain the difference between these states is the reduced firing during REM sleep of dorsal raphe nucleus (DRN) serotonergic, and other aminergic neurons (Brown et al., 2012). The DRN contains the largest number of forebrain projecting serotonin (5-HT) neurons (Jacobs and Azmitia, 1992; Monti, 2010). Extracellular recordings showed that these neurons discharge fastest during wakefulness and discharge at a much lower rate during REM sleep (McGinty and Harper, 1976; Trulson and Jacobs, 1979), although firing may occur following phasic REM events (Sakai and Crochet, 2001).
One neurotransmitter system which may be involved in the silencing of DRN neurons during REM sleep is the brainstem cholinergic system, located in the laterodorsal and pedunculopontine tegmental nuclei (LDT/PPT), since, in contrast to aminergic neurons, these cholinergic neurons are highly active during REM sleep (Steriade et al., 1990; Williams et al., 1994; Thakkar et al., 1998; Sakai, 2012) and project to the DRN and surrounding regions (Rye et al., 1987; Woolf and Butcher, 1989). In fact, an influential theory of REM sleep control, the reciprocal interaction theory of Hobson and McCarley (Hobson et al., 1975; McCarley and Hobson, 1975), posits state-dependent interactions between cholinergic and brainstem aminergic neurons (see McCarley, 2007; Brown et al., 2012 for a critical discussion of this and other REM sleep control models). Several in vitro (Luebke et al., 1992; Leonard and Llinás, 1994) and in vivo (Thakkar et al., 1998) studies have confirmed inhibition of cholinergic LDT/PPT neurons by 5-HT. In addition, direct and indirect excitatory effects of nicotine on DRN 5-HT neurons have been shown (Li et al., 1998; Mihailescu et al., 1998; Mihailescu et al., 2001; Mihailescu et al., 2002; Galindo-Charles et al., 2008; Chang et al., 2011; Garduno et al., 2012). In some of these studies, inhibitory effects were also seen prior to excitatory effects and/or in particular subsets of neurons. Thus, inhibitory effects of cholinergic stimulation may also occur, likely in a state-dependent manner.
Recent experiments and theories of REM sleep control have emphasized a role for GABAergic neurons (Xi et al., 1999; Luppi et al., 2006; Mallick et al., 2012; Brown et al., 2012). A microdialysis study showed that GABA release in DRN is increased during REMS (Nitz and Siegel, 1997), suggesting that increased GABAergic input is important for inhibiting DRN serotonergic neurons during REM sleep. Furthermore, unit recording studies show that the discharge rate of DRN neurons loses its relationship to the vigilance state with iontophoretic application of a GABAA receptor antagonist into DRN (Gervasoni et al., 2000), supporting the hypothesis that GABAergic inputs are involved.
The DRN 5-HT neurons are surrounded by a large number of GABAergic neurons in the DRN and neighboring ventrolateral periaqueductal gray (vlPAG) (Maloney et al., 2000; Brown et al., 2008). Using retrograde-tracing, Gervasoni et al (2000) revealed that DRN receives GABAergic inputs from multiple regions, including a strong input from the vlPAG. Furthermore, retrograde tracing combined with Fos immunohistochemistry to identify recently active neurons, suggested that the vlPAG contains REM-active GABAergic neurons (Sapin et al., 2009). A unifying model to combine cholinergic and GABAergic influence of REM sleep control would suggest cholinergic excitation of GABAergic neurons which inhibit DRN neurons (McCarley, 2007; Brown et al., 2012). Thus, here, we tested the hypothesis that cholinergic neurons inhibit DRN REM-off 5-HT neurons by increasing GABAergic inputs using whole-cell recordings in coronal brain slices prepared from GAD67-GFP knock-in mice (Tamamaki et al., 2003; Brown et al., 2008). Application of the mixed cholinergic agonist, carbachol was used to stimulate both muscarinic and nicotinic receptors. Excitatory glutamatergic synaptic events were blocked so as to focus on inhibitory inputs. We then removed particular parts of the brainstem slices to determine which parts of the brainstem slice are required for carbachol effects on inhibitory inputs to DRN neurons. Lastly, we tested the effect of carbachol on one possible source of GABAergic inputs to DRN, vlPAG GABAergic neurons.
Experimental Procedures
Animals
All experiments conformed to U.S. Veterans Administration, Harvard University, and U.S. National Institutes of Health guidelines and were reviewed by the institutional animal care and use committee (IACUC) of the VA Boston Healthcare System. Experimental male and female GAD67-GFP knock-in animals were obtained by crossing male heterozygous GAD67-GFP knock-in mice (Swiss-Webster background) with wild-type female Swiss-Webster mice (Charles River, Wilmington, MA). GFP-positive animals were phenotyped under a fluorescent microscope within 3 days after birth. GAD67-GFP knock-in animals have similar sleep-wake behavior and cortical rhythms as wild-type animals (Chen et al., 2010; McNally et al., 2011). The selective expression of green fluorescent protein in brainstem GABAergic neurons was validated in our previous study (Brown et al., 2008). Mice were housed under constant temperature and a 12:12 light:dark cycle (7AM:7PM), with food and water available ad libitum.
Slice preparation
Slices from young mice (9–20 d) were used for all experiments since visualization of neurons in brain slices is easier at this age and young animals have large amounts of REM sleep (Jouvet-Mounier and Astic, 1968). Mice were deeply anesthetized with isofluorane and then decapitated. Coronal brainstem slices (250 μm thickness) were cut between −4.48 mm and −4.80 mm with respect to Bregma rostrocaudally in ice-cold sucrose solution (in mM: 208.6 sucrose, 1.8 KCl, 25.6 NaHCO3, 1.2 KH2PO4, 0.6 CaCl2, 3.3 MgSO4, 10 glucose, saturated with 95% O2/5% CO2). After slicing they were placed into ACSF (in mM: 124 NaCl, 1.8 KCl, 25.6 NaHCO3, 1.2 KH2PO4, 2 CaCl2, 1.3 MgSO4 and 10 glucose, 300 mOsm, saturated with 95% O2/5% CO2) for >1 h at room temperature before being transferred to the recording chamber and superfused with warmed ACSF (32°C) at 2–3 ml/min.
Whole-cell patch-clamp recordings
For putative 5-HT neurons, electrophysiological recordings were made from somata of GFP-negative neurons in the ventral, interfascicular parts of DRN where 5-HT neurons are particularly highly concentrated (Monti, 2010). For GABAergic neurons, electrophysiological recordings were made from somata of GFP-positive neurons in lateral vlPAG (Brown et al., 2008). Neurons were photographed prior to recording using a Hamamatsu ORCA-ER CCD camera (Hamamatsu Corporation, Middlesex, NJ, USA). Fluorescent neurons were observed with Zeiss filters (GFP: filter set 38, excitation filter 470/40 and emission filter 525/50). Long-axis cell diameter was measured from these images and calibrated using a standard 25 μm grid. Intrinsic membrane properties of the neurons were tested with 1 s long current steps as previously reported (McKenna et al., 2013). The amplitude of these steps was titrated according to the input resistance of the neurons so that the maximal hyperpolarization with the largest current step was the same for each cell (McKenna et al., 2013).
In order to rapidly obtain a maximal effect, carbachol was bath applied at a concentration of 50 μM, as in other in vitro studies (Kumar, 2010; Simon et al., 2011). A brief (2–3 min) application was used in order to be able to observe a washout of the effect. Since equilibration of solutions between the bath and within the slice takes approx. 10–15 min the actual carbachol concentration in the vicinity of the recorded neurons is likely to have been considerably lower.
To test the effect of carbachol on inhibitory inputs to DRN 5-HT neurons, spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded without tetrodotoxin (TTX) (Abcam, Cambridge, MA) while miniature inhibitory postsynaptic currents (mIPSCs) were recorded in the presence of 500 nM TTX. Inhibitory postsynaptic currents (IPSCs) were recorded in the presence of 20 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX) (Abcam, Cambridge, MA) and 50 μM (2R)-amino-5-phosphonovaleric acid (AP5) (Abcam, Cambridge, MA) to block glutamatergic AMPA/Kainate and NMDA receptor mediated currents respectively. The holding potential was close to the resting membrane potential of 5-HT neurons (−65 mV). The intracellular solution contained KCl as the main constituent in order to enhance the driving force for chloride entry and thus increase the resolution of GABAA receptor mediated events (in mM: 130 KCl, 5 NaCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 Na2ATP, 0.5 NaGTP, 4 MgATP, 1 spermine, pH 7.25 with KOH, 280 mOsm) (Sigma, St. Louis, MO) . At the end of IPSC recordings, 10 μM GABAzine (Abcam, Cambridge, MA), a GABAA receptor blocker, was applied to the bath to confirm those inhibitory inputs were GABAergic. All receptor antagonists were bath applied for 5 min before the carbachol response was tested. Carbachol (Sigma, St. Louis, MO) was bath applied for ~3 min. A one minute period immediately prior to carbachol application and a one minute period after 2 min application of carbachol were used for statistical analysis with Igor software (WaveMetrics, Inc., Portland, OR, USA). Only well resolved events with amplitudes >10 pA were analyzed. The baseline current during postsynaptic current recordings was measured by lowpass filtering the trace (Bessel, 8-pole) at 10 Hz in pClamp to eliminate the synaptic currents.
To test the postsynaptic effect of carbachol on vlPAG GABAergic neurons, neurons were pre-incubated in 500 nM TTX for 5 min and then tested in response to 2 min bath application of carbachol under current clamp (Ihold = 0). Patch pipettes were filled with intracellular solution containing (in mM: 130 K-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 Na2ATP, 0.5 NaGTP, 4 MgATP, 1 spermine, pH 7.25 with KOH, 280 mOsm). Zero-Ca2+ ACSF (in mM: 124 NaCl, 1.8 KCl, 25.6 NaHCO3, 1.2 KH2PO4, 2 MgCl2, 1.3 MgSO4 and 10 glucose) was used to confirm that the effect of carbachol on vlPAG neurons was postsynaptic.
The muscarinic receptor antagonist, atropine (5 μM, Sigma, St. Louis, MO), and the nicotinic receptor antagonist, mecamylamine hydrochloride (100 μM, Sigma, St. Louis, MO), were added to the bath for 5 min before carbachol was applied in order to identify the receptor subtypes mediating the effects of carbachol.
All recordings were made using a Multiclamp 700B amplifier and pClamp 9.0 software (Molecular Devices, LLC, Sunnyvale, CA, USA). Patch pipettes (3–6 MΩ) were filled with either KCl or regular intracellular solution as mentioned above. Membrane potential measurements were adjusted for liquid junction potentials between the pipette and bath solution. Bridge balance was adjusted after gaining access to the whole-cell and maintained throughout the experiment. Recordings were accepted if action potentials were overshooting and electrode resistance was less than 20 MΩ and changed by less than 10 % during the experiment. Continuous recordings of synaptic currents were made using a MiniDigi 1A system and Axoscope 9.2 software (Molecular Devices, LLC, Sunnyvale, CA, USA) with a sampling frequency of 20 kHz.
Data analysis and statistics
The morphological and intrinsic membrane properties of DRN and vlPAG neurons were analyzed using the same methods as previously reported (McKenna, et al., 2013). Data were presented as mean ± standard error of the mean (SEM). For data with normal distribution, paired t test or unpaired t test were used for statistical analysis of significance. Kolmogorov-Smirnoff two-sample test was used to test significant difference for cumulative probabilities. Statistical analysis utilized GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA, USA), and differences were considered significant when p<0.05.
Results
Recordings from DRN putative 5-HT neurons
Our previous work in GAD67-GFP knock in mice showed that there was little or no colocalization of GAD67 in tryptophan hydroxylase positive 5-HT neurons in the DRN (Brown et al., 2008). Thus, here in order to target 5-HT neurons we made whole cell recordings from GFP-negative neurons (cell size: 22.5 ± 0.5 μm, n=30) (Figure 1A) in the ventral DRN region where 5-HT neurons are highly concentrated (Brown et al., 2002). A KCl-based pipette solution was used in all recordings from DRN neurons in order to shift the equilibrium potential for chloride to a more positive potential, thereby increase the driving force for chloride entry and facilitating the detection of GABAA receptor mediated synaptic events. Under these conditions, putative 5-HT neurons exhibited large, depolarizing inhibitory postsynaptic potentials under current-clamp (Figure 1B) and inward-going currents in voltage-clamp (Figure 1C–E). These neurons did not show any depolarizing sag during hyperpolarizing current steps (Figure 1B), similar to other recordings from rodent 5-HT neurons (Brown et al., 2002; Garduño et al., 2012). Their resting membrane potential was −65.7 ± 1.0 mV and their input resistance was 368.5 ± 37.4 MΩ. Other electrophysiological properties were also consistent with those of 5-HT neurons (Table 1). In particular, they exhibited broad action potentials (half-width of 1.26 ± 0.05 ms) and large afterhyperpolarizations (−26.9 ± 0.7 mV).
Fig. 1. Carbachol increased the frequency of spontaneous inhibitory postysynaptic currents (sIPSCs) in dorsal raphe nucleus (DRN) putative serotonin (5-HT) neurons via both muscarinic and nicotinic receptors.
(A) Infrared-differential interference microscopy (IR-DIC) and black-and-white fluorescent images of a typical 5-HT neuron. Scale bar: 25 μm. Serotonergic neurons were selected according to their GFP-negative pattern under fluorescent illumination in slices prepared GAD67-GFP knock-in mice and identified by their typical intrinsic membrane properties during current pulses applied to the recording pipette (B, −350 pA to 0 pA, −70 pA interval). 5-HT neurons had a medium/large size, were normally spontaneously active, and did not exhibit any depolarizing sag during hyperpolarizing current steps. (C) A continuous recording of sIPSCs in control (preincubated in 20 μM DNQX and 50 μM AP5 for 5 min) and 50 μM carbachol (added to the bath perfusate and applied for 3 min). Holding potential was −65 mV. (D) and (E) Ten second-long representative voltage-clamp recording traces from the same neuron shown in (C) under control and carbachol. (F) and (G) Cumulative probability histograms of amplitude (left) and inter-event interval (right) of 1 min recordings from the same neuron. Distributions were compared using the Kolmogorov-Smirnoff test. (H) Summary of carbachol effect on frequency of sIPSCs in all recorded putative 5-HT neurons under different recording conditions. There was a significant increase in the frequency of sIPSCs with carbachol stimulation in control, 5 μM atropine or 100 μM mecamylamine. The combination of atropine and mecamylamine blocked the carbachol effect on sIPSC frequency. *: p<0.05, paired t test.
Table 1.
Intrinsic membrane properties of DRN putative 5-HT and identified vlPAG GABAergic neurons
| Parameter | DRN Putative 5-HT neurons (n = 30) | vlPAG GABAergic neurons (n = 25) |
|---|---|---|
| Long diameter (μm) | 22.5 ± 0.5 | 16.7 ± 0.5*** |
| Short diameter (μm) | 14.7 ± 0.4 | 10.5 ± 0.4*** |
| RMP (mV) | −65.7 ± 1.0 | −62.3 ± 1.1* |
| Rin (MΩ) | 368.5 ± 37.4 | 508.1 ± 59.4* |
| Sag (%) | -- | 16.1 ± 3.4 |
| Sag decay τ (ms) | -- | 332.5 ± 58.8 |
| Sag decay Amplitude (mV) | -- | −12.1 ± 1.4 |
| Spontaneous firing (Hz) | 5.2 ± 0.7 (n=21/30) | 5.4 ± 0.5 (n = 14/25) |
| Maximum firing (Hz) | not tested | 47.3 ± 4.0 |
| AP amplitude (mV) | 69.5 ± 1.4 | 65.7 ± 2.7 |
| AP threshold (mV) | −51.6 ± 0.6 | −41.5 ± 4.4* |
| AP half width (ms) | 1.26 ± 0.05 | 0.85 ± 0.06*** |
| AHP amplitude (mV) | −26.9 ± 0.7 | −21.8 ± 1.4** |
p<0.0001;
p<0.01;
p<0.05;
unpaired t test, comparison between DRN and vlPAG neurons.
Note: The spontaneous firing rates of DRN putative 5-HT neurons in our study were higher than those of identified serotonergic neurons in rodents reported by other studies (Garduño et al., 2012), possibly due to 1) use of young animals in current study; 2) our use of a KCl-pipette solution for recording sIPSCs. vlPAG neurons were recorded with a K-gluconate based pipette solution. Abbreviations: RMP: resting membrane potential; Rin: input resistance; AP: action potential; AHP: afterhyperpolarization.
Carbachol increases the frequency of sIPSCs in DRN putative 5-HT neurons via both muscarinic and nicotinic receptors
To determine if cholinergic inputs modulate inhibitory synaptic inputs to DRN 5-HT neurons, we tested the effect of carbachol on spontaneous inhibitory postsynaptic currents (sIPSCs). sIPSCs were recorded at a holding potential close to the resting membrane potential (Vhold= −65 mV) in the presence of 20 μM DNQX and 50 μM AP5, which block glutamatergic AMPA/Kainate and NMDA receptors respectively, and eliminate the excitatory synaptic currents. Under these conditions bath application of 50 μM carbachol significantly increased the frequency of sIPSCs (control vs. carbachol: 3.9 ± 1.2 Hz vs 8.4 ± 2.2 Hz, p = 0.010, paired t test, n = 9), but had no significant effect on the amplitude of sIPSCs (control vs. carbachol: 42.4 ± 5.4 pA vs 42.9 ± 4.1 pA, p = 0.90, paired t test) (Figure 1). GABAzine (10 μM), a GABAA receptor blocker, applied at the end of the experiment completely blocked the events, confirming that these sIPSCs were mediated by GABAA receptors. In addition, the baseline current was significantly changed from −43.4 ± 9.1 pA to −24.1 ± 9.0 pA by carbachol (p = 0.013, paired t test, n = 5), which resulted in a outward current of 19.3 ± 4.5 pA (Figure 1C).
To determine which receptor subtypes were involved in the carbachol effect on sIPSCs of DRN neurons, we pre-incubated the neurons in DNQX+AP5 together with a muscarinic receptor antagonist, atropine (5 μM), or a nicotinic receptor antagonist, mecamylamine hydrochloride (100 μM), or a mixture of both antagonists, for 5 min before carbachol was applied to the bath. In the presence of the muscarinic antagonist, atropine, carbachol still significantly increased the frequency of sIPSCs from 4.9 ± 1.9 Hz to 9.8 ± 3.1 Hz (p = 0.026, paired t test, n = 7). In the presence of the nicotinic antagonist, mecamylamine, carbachol also significantly increased the sIPSC frequency from 5.3 ± 2.2 Hz vs. to 8.3 ± 2.7 Hz (p = 0.049, paired t test, n = 9). However, in the presence of both atropine and mecamylamine, the carbachol effect on sIPSCs was not significant (9.1 ± 2.3 Hz vs. 9.1 ± 2.4 Hz, p = 0.94, n = 8; Figure 1H). There was no significant difference among baseline frequencies of sIPSCs recorded in ACSF, atropine, mecamylamine and atropine+mecamylamine (p > 0.05, one-way ANOVA) (Figure 1H). Carbachol did not cause significant changes in the amplitude of sIPSCs under any of these recording conditions (atropine vs. atropine+carbachol: 51.2 ± 4.7 pA vs. 64.5 ± 13.5 pA, p=0.27; mecamylamine vs. mecamylamine+carbachol: 53.2 ± 7.0 pA vs. 61.2 ± 9.1 pA, p=0.061; atropine+mecamylamine vs. atropine+mecamylamine+carbachol: 66.0 ± 8.4 pA vs. 71.9 ± 7.8 pA, p=0.14).
To determine if carbachol had a direct effect on the axon terminals of GABAergic inputs to DRN neurons, we tested the effect of carbachol on miniature IPSCs (mIPSCs) in the presence of 500 nM TTX. The frequency of mIPSCs in control was 1.1 ± 0.2 Hz and in carbachol was 1.0 ± 0.1 Hz (n = 6). The amplitude of mIPSCs in control was 30.9 ± 3.9 pA and in carbachol was 32.8 ± 4.0 pA. Neither the frequency (p = 0.57, paired t test) nor the amplitude (p = 0.12, paired t test) were significantly changed by carbachol (Figure 2). The baseline current was also not significantly changed in the presence of TTX (control vs. carbachol: −46.5 ± 3.5 pA vs. −43.9 ± 6.7 pA, p = 0.47, paired t test; Figure 2A), indicating that carbachol had no direct postsynaptic effect on DRN neurons. Together, these data suggest that carbachol increased the firing of local GABAergic neurons in the slice which project to DRN 5-HT neurons, but did not directly affect the presynaptic GABAergic terminals.
Fig. 2. Carbachol had no effect on the miniature IPSCs (mIPSCs) of dorsal raphe nucleus (DRN) putative serotonin (5-HT) neurons.
(A) A continuous recording of mIPSCs from one putative DRN 5-HT neurons under control (preincubated in a mixture of 20 μ M DNQX, 50 μM AP5 and 500 nM TTX for 5 min) and 50 μM carbachol (added in the bath and applied for 3 min). Holding potential was −65 mV. (B) and (C) Ten second-long representative traces from one putative 5-HT neuron under control and carbachol recording conditions. (D) and (E) Cumulative probability histograms of amplitude (left) and inter-event interval (right) of 1 min recordings from the same neuron as in (B) and (C). Distributions were compared using the Kolmogorov-Smirnoff test. (F) and (G) Summary of carbachol effect on frequency and amplitude of mIPSCs in all recorded DRN putative 5-HT neurons. There was no significant increase in the frequency nor amplitude of mIPSCs with carbachol stimulation. Paired t-test, n = 6.
Carbachol-sensitive GABAergic inputs to DRN 5-HT neurons are located dorsal to the decussation of the superior cerebellar peduncle
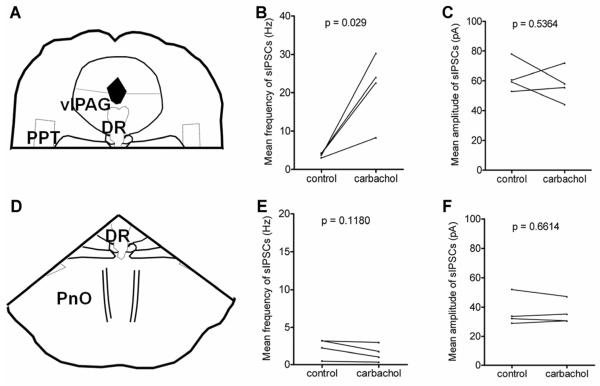
To identify the local regions sending carbachol-sensitive GABAergic inputs to DRN 5-HT neurons, we tested the effect of carbachol on sIPSCs in slices in which either the dorsal or ventral parts were transected (Figure 3A, D). Previous retrograde tracing studies in rats have shown that DRN receives GABAergic inputs from the pontine nucleus, pars oralis (PnO) and from the vlPAG (Gervasoni et al., 2000). Our previous work in the mouse brainstem showed that a subpopulation of PnO GABAergic neurons is excited by carbachol (Brown et al., 2008). Therefore, to test if this input is important in the carbachol-mediated increase of inhibitory synaptic currents, we made a horizontal cut across the decussation of the superior cerebellar peduncle (xscp) (Figure 3A), which separates the ventral part of brainstem, including PnO, from the DRN region. In these slices, carbachol still significantly increased the frequency of sIPSCs (control vs. carbachol: 3.7 ± 0.3 Hz vs. 21.3 ± 4.6 Hz, p = 0.029, paired t test, n = 4) (Figure 3B), suggesting the carbachol-sensitive GABAergic neurons that project to DRN 5-HT neurons were not located in PnO but in dorsal regions above the decussation of the cerebellar peduncle. In these slices, there was also no significant change in the amplitude of sIPSCs (control vs. carbachol: 62.6 ± 5.4 pA vs. 57.4 ± 5.7 pA, p = 0.54, paired t test) (Figure 3C).
Fig. 3. Carbachol sensitive GABAergic inputs to dorsal raphe nucleus (DRN) putative serotonin (5-HT) neurons originated from dorsal regions of the slice.
(A) and (D) Schematic (adapted from mouse atlas of Franklin and Paxinos, 2008; AP −4.72 mm) showing the brainstem recording slices with either ventral part (A) or dorsal part (D) removed. (B) and (C) Summary of carbachol effect on frequency and amplitude of sIPSCs in all recorded DRN putative 5-HT neurons with the ventral part from DRN removed. The carbachol effect on frequency of sIPSCs persisted. Paired t-test, n=4. (E) and (F) Summary of carbachol effect on frequency and amplitude of sIPSCs in all recorded DRN putative 5-HT neurons with the dorsal part removed. The carbachol effect was eliminated. Paired t-test, n=4. DR: dorsal raphe; vlPAG: ventrolateral periaqueductal gray; PPT: pedunculopontine tegmental nuclei; PnO: pontine reticular nucleus, oral part.
In the second set of experiments, we separated the dorsal part of the slices, including vlPAG, from the DRN region (Figure 3D). Carbachol had no significant effect on either the frequency or amplitude of sIPSCs in DRN putative 5-HT neurons (control vs. carbachol: 2.3 ± 0.6 Hz vs. 1.5 ± 0.6 Hz, p = 0.12, paired t test; 36.8 ± 5.2 pA vs. 36.1 ± 3.9 pA, p = 0.66, paired t test, n = 4) (Figure 3E, F), suggesting that the carbachol-sensitive GABAergic inputs to DRN originate from dorsal regions, including vlPAG.
vlPAG GABAergic neurons are directly excited by carbachol
To directly test if carbachol excites vlPAG GABAergic neurons, we made whole-cell recordings from GFP-positive (i.e. GABAergic) neurons in vlPAG (Figure 4A, B). vlPAG GABAergic neurons exhibited a small depolarizing sag (16.1 ± 3.4%, n = 25) during hyperpolarizing current steps (Figure 4C). About half of them were spontaneously active with a firing frequency of 5.4 ± 0.5 Hz (n = 14/25) (Table 1). In contrast to putative 5-HT neurons, they had significantly narrower action potentials (half width 0.85 ± 0.06 ms) and smaller afterhyperpolarizations (−21.8 ± 1.4 mV) (Table 1). Carbachol responses were recorded under current-clamp (Ihold = 0) in the presence of 500 nM TTX (Figure 4D) to eliminate action potential-dependent presynaptic effects. In the majority of vlPAG GABAergic neurons, carbachol induced a depolarization (4.9 ± 0.6 mV, n = 7/8) (Figure 4E). The input resistance was significantly decreased from 205.6 ± 22.0 MΩ to 182.7 ± 18.9 MΩ (p = 0.0095, n = 5), indicating that carbachol caused opening of ion channels on vlPAG GABA neurons. One vlPAG GABAergic neuron did not show any response to carbachol. Although these responses were recorded in TTX, this does not completely eliminate the possibility of a direct action of carbachol on presynaptic terminals. Thus, to further test the postsynaptic nature of this response we recorded the response to carbachol in zero-Ca2+ ACSF solution containing TTX, thus eliminating calcium-dependent neurotransmitter release. Carbachol also induced depolarization under these recording conditions (11.3 ± 5.8 mV, n = 3). These data suggested that carbachol directly excites vlPAG GABAergic neurons.
Fig. 4. Carbachol postsynaptically excited vlPAG GABAergic neurons.
(A) Schematic (adapted from mouse atlas of Franklin and Paxinos, 2008; AP −4.72 mm) showing the location of the brainstem recording sites in the ventrolateral periaqueductal gray (vlPAG). (B) Infrared-differential interference microscopy (IR-DIC) and black-and-white fluorescent images of a GABAergic neuron in vlPAG. Scale bar: 25 μm. GABAergic neurons were selected by their GFP-positive pattern under fluorescent illumination in slices prepared from GAD67-GFP knock-in mice. (C) Intrinsic membrane properties of the same neurons in (B) during current pulses (−300 pA to 60 pA, −60 pA interval). vlPAG GABAergic neurons were either silent or spontaneously active with low firing rate and had a small depolarizing sag during hyperpolarizing current steps. (D) Preincubation in 500 nM TTX blocked the action potential firing in the same neuron in C with the same current steps. (E) In the presence of TTX, 50 μM carbachol caused a depolarization. Neurons were recorded continuously under current clamp (Ihold = 0) with a hyperpolarizing current injected every 15 s (−50 pA, 500 ms duration). The recorded neuron was the same as that in B–D. DC: period of hyperpolarizing current injection to check input resistance changes.
To determine the receptors involved in the postsynaptic response induced by carbachol on vlPAG GABAergic neurons, we applied atropine, mecamylamine or both into the bath together with TTX and tested the carbachol-induced response. In the presence of atropine, carbachol still induced a depolarization (4.5 ± 0.5 mV, n = 4). Similarly, in the presence of mecamylamine, carbachol also induced a depolarization (3.8 ± 1.6 mV, n = 5). The amplitude of the response in atropine or mecamylamine was not significantly different from that in regular ACSF (p > 0.05, one-way ANOVA). However, in the presence of both atropine and mecamylamine, the carbachol-induced depolarization was completely blocked (changes in voltage: −0.096 ± 0.19 mV, n = 4; p < 0.05, compared to the response in ACSF, atropine and mecamylamine, one-way ANOVA).
Discussion
Our data presented here show: 1) The cholinergic receptor agonist, carbachol, increased the frequency of spontaneous inhibitory GABAergic synaptic currents onto DRN putative 5-HT neurons; 2) Carbachol had no direct postsynaptic effect on DRN 5-HT neurons. 3) Carbachol-mediated increases in the frequency of sIPSCs in 5-HT neurons were abolished by removal of the dorsal region of the slice including vlPAG; 4) Carbachol postsynaptically excited vlPAG GABAergic neurons; and 5) The effects of carbachol involved both muscarinic and nicotinic receptors. Several previous studies have investigated the effects of activation of nicotinic receptors on dorsal raphe neurons (Li et al., 1998; Mihailescu et al., 1998; Mihailescu et al., 2001; Mihailescu et al., 2002; Chang et al., 2011; Garduno et al., 2012). However, to the best of our knowledge, this is the first study to investigate the effects of carbachol, a mixed muscarinic/nicotinic cholinergic agonist, on inhibitory inputs to DRN neurons. Furthermore, no previous study has identified the intrinsic membrane properties or carbachol modulation of identified vlPAG GABAergic neurons. Thus, our results advance our knowledge of the interactions between these two important neurotransmitter systems which play an important role in the control of behavioral state.
Mechanisms involved in the reduced firing of DRN 5-HT neurons during sleep
Both withdrawal of excitatory inputs (Baraban and Aghajanian, 1980; Sakai and Crochet, 2000; Brown et al., 2002) and an increase in inhibitory tone (Nitz and Siegel, 1997; Gervasoni et al., 2000) likely contribute to the reduced firing of DRN 5-HT neurons during sleep (McGinty and Harper, 1976; Trulson and Jacobs, 1979). During wakefulness, DRN neurons are strongly excited by noradrenaline, histamine and orexins via activation of a mixed cation current (Baraban and Aghajanian 1980; Sakai and Crochet 2000; Brown et al., 2002; Liu et al., 2002). Like 5-HT neurons, noradrenaline, histamine, and orexin neurons reduce their firing during non-REM (NREM) sleep and are almost silent during REM sleep (reviewed in Brown et al., 2002). Thus, the excitatory effects of these neuromodulators will be absent during REM sleep. Inhibitory inputs are also likely to be important since microdialysis in the cat revealed an increased release of GABA in the DRN during REM sleep (Nitz and Siegel, 1997). Furthermore, Gervasoni et al., (2000) showed that local application of a GABAA receptor antagonist abolished the silence of DRN neurons during REM. However, the mechanisms underlying this putative increase in GABAergic inhibition are not well understood.
Our in vitro work on brainstem slices reported here indicates that increased GABAergic inhibition of DRN neurons during REM sleep could occur as a result of cholinergic excitation of a subpopulation of GABAergic inputs via muscarinic and nicotinic receptors. The cholinergic agonist carbachol increased the frequency of inhibitory GABAA-receptor mediated synaptic currents in DRN putative 5-HT neurons. Antagonism of both muscarinic and nicotinic receptors with atropine and mecamylamine, respectively, was necessary to completely block this effect. An inhibitory action mediated by muscarinic receptors is consistent with the ability of systemic atropine to enhance DRN 5-HT release (Kumari et al., 2007). Carbachol did not affect miniature synaptic currents, suggesting that it was not acting directly at GABAergic presynaptic terminals but rather by increasing the excitability of local GABAergic neurons in the slice. Carbachol also caused an outward current which was not observed in the presence of TTX. Thus, we believe it was due to increased GABA release due to carbachol excitation of local GABAergic neurons, which in addition to causing fast GABAA receptor-mediated IPSCs, acts on GABAB receptors on DRN neurons to cause a slow outward current (Kohlmeier et al., 2013).
Although some previous studies in the rat have demonstrated a direct postsynaptic nicotinic excitation of DRN serotonin neurons (Galindo-Charles et al., 2008; Chang et al., 2011), not all studies have observed this effect (Li et al., 1998; Garduno et al., 2012) and we did not observe a direct presynaptic effect here in the mouse. One explanation for the difference between our study and previous studies that observed postsynaptic effects could be that we used bath application of carbachol rather than pressure application of acetylcholine or electrical stimulation of the PPT. Thus, with this slow application method, desensitization of the nicotinic receptors on DRN serotonin neurons may have occurred. However, we did observe nicotinic effects on GABAergic neurons suggesting that if this explanation is correct then the subunit combination present on GABAergic neurons may be less sensitive to desensitization. Other possibilities are that postsynaptic effects of nicotine are only present on a subset of DRN 5-HT neurons (e.g. those projecting to the nucleus accumbens; Chang et al., 2011) and/or a species difference, since our work was performed in mouse and the previous work was performed in rat.
Sources of GABAergic input to DRN 5-HT neurons
DRN receives inputs from many brain regions, including several sites involved in sleep-wake control (Jacobs and Azmitia, 1992; Peyron et al., 1998). Gervasoni et al (2000) used retrograde tracing and immunohistochemical verification to identify GABAergic inputs to DRN and demonstrated inputs from neurons in hypothalamus, basal forebrain and brainstem. Among those regions, GABAergic neurons in brainstem may be responsible for silencing DRN 5-HT neurons during REM sleep, since REM-like episodes are still associated with a decreased activity of DRN 5-HT neurons in decerebrate animals (Hoshino and Pompeiano, 1976). In particular, the vlPAG region may be important since retrogradely labeled GABAergic neurons were observed in this region in the rat following injections targeting the DRN (Gervasoni et al., 2000) and Sapin and colleagues (2009) demonstrated REM-active GABAergic neurons in this region using Fos immunohistochemistry. However, GABAergic neurons are also present in other brainstem regions surrounding the DRN 5-HT neurons, including lateral regions of the DRN itself (Stamp and Semba, 1995; Brown et al., 2008). Our experiments cannot rule out that excitation of these neurons also contributes to the effect of carbachol and in fact direct nicotinic excitation of DRN non 5-HT, possibly GABAergic neurons, has been previously demonstrated (Galindo-Charles et al., 2008). Here we focused on the vlPAG GABA neurons since our recent retrograde tracing work in the same GAD67-GFP knock-in mice that we used for our in vitro experiments, confirmed previous findings of brainstem GABAergic inputs from vlPAG as well as from PnO (McKenna et al., 2010). In our in vitro electrophysiology experiments, the carbachol effect on sIPSCs of DRN neurons was eliminated when the dorsal part (including vlPAG) from DRN was removed from the slices. In contrast, removal of ventral regions containing the PnO did not abolish the carbachol-mediated increase in sIPSCs. Furthermore, identified vlPAG GABAergic neurons were depolarized by carbachol. Together, these findings suggest that carbachol increases inhibitory inputs to DRN 5-HT neurons at least in part by increasing the excitability of vlPAG GABAergic afferents.
Comparison of vlPAG GABAergic neurons with other brainstem GABAergic neurons
Previous work in our laboratory has verified the GABA expression in GFP-positive brainstem neurons from GAD67-GFP knock in mice, allowing characterization of the properties of identified GABAergic neurons (Brown et al., 2008). Here, for the first time, we reported the intrinsic membrane properties of vlPAG GABAergic neurons. Similar to those of subcoeruleus (SubC) and PnO GABAergic neurons, vlPAG GABAergic neurons were small (10–15 μm) and medium (15–25 μm)-sized and had a medium sized depolarizing sag (~16%) during hyperpolarizing current pulses. In contrast, 5-HT neurons lack this sag, which is due to a hyperpolarization-activated cation current (Brown et al., 2008). Their action potential duration (half width) and afterhyperpolarization amplitude were also similar to those of SubC/PnO GABAergic neurons, but were significantly smaller than those of DRN serotonergic neurons (Table 1). Despite the similarities in morphology and action potential parameters, vlPAG GABAergic neurons showed some differences in resting membrane potential and spontaneous firing from SubC/PnO neurons. In the presence of TTX, vlPAG GABAergic neurons had a resting membrane potential of −66.2 ± 1.6 mV (n = 18), which was more hyperpolarized than that of SubC/PnO neurons recorded under the same conditions (−59.9 ± 1.0 mV. Brown et al., 2008). Accordingly, vlPAG neurons were less likely to be spontaneously active than SubC/PnO GABAergic neurons, most of which were spontaneously active and fired at 7.0 ± 0.4 Hz (Brown et al., 2008). We found that only about half of the vlPAG GABAergic neurons were spontaneously active and the ones which were spontaneously active fired at a slightly lower rate (5.4 ± 0.5 Hz) than SubC/PnO neurons (Table 1). In terms of response to carbachol, vlPAG GABAergic neurons were similar to SubC GABAergic neurons. The majority of vlPAG GABAergic neurons (7/8) were excited by carbachol, suggesting they represent REM-on or wake/REM-on neurons. Fos immunohistochemistry experiments in rats which showed an increase in the number of Fos-positive/GAD67-positive neurons in vlPAG during REM-rebound following deprivation, support this view (Sapin et al., 2009). Similar to the effect of carbachol on sIPSCs, both muscarinic and nicotinic receptors were involved in this effect. The depolarization caused by carbachol was not blocked by tetrodotoxin or under nominally calcium-free conditions suggesting a postsynaptic effect.
Functional implications
The precise role of brainstem cholinergic, aminergic and vlPAG GABAergic neurons in REM sleep control is controversial (reviewed in Brown et al., 2012), particularly in rodents. Our in vitro study focused narrowly on answering the following question: can cholinergic stimulation increase inhibitory inputs to DRN 5-HT neurons, as predicted by the modified reciprocal interaction theory of REM-sleep control (McCarley, 2007). Our results confirmed this prediction and identified vlPAG GABAergic neurons as one possible source of this increased inhibition. Thus, cholinergic neurons may play a role in silencing 5-HT neurons during REM sleep.
As discussed earlier, several previous studies have demonstrated either direct or indirect excitatory effects of nicotine on DRN serotonin neurons. How are these results to be reconciled with our results here? The overall functional effect (excitation or inhibition) likely depends on the firing rate and pattern of cholinergic neurons, differences in the affinity of nicotinic and muscarinic receptors for the endogeneous ligand, acetylcholine, the density of the receptors and the location of receptors in relation to cholinergic release sites. Thus, high-affinity/high density muscarinic and nicotinic receptors on vlPAG and DRN GABAergic neurons may be activated by low levels of acetylcholine release caused by tonic firing cholinergic neurons during non-phasic REM periods or quiet wakefulness (el Mansari et al., 1989), leading to inhibition, whereas during presentation of arousing/rewarding stimuli or phasic REM periods (Heym et al., 1982; Koyama et al., 1994), burst firing of cholinergic neurons will lead to higher concentrations of acetylcholine which activate lower affinity/lower density nicotinic receptors on DRN serotonin neurons themselves and on their excitatory inputs, leading to excitation. Two lines of evidence support this model: 1) raphe neurons in cats with large pontine lesions including areas containing cholinergic neurons show increased firing during REM sleep (Trulson et al., 1981), suggesting disinhibition due to withdrawal of cholinergic inhibition of serotonin neurons; and 2) Serotonergic DRN neurons show a reciprocal relationship with Ponto-geniculo-occipital (PGO) waves (Lydic et al., 1983), their firing decreases prior to PGO waves but increases when PGO waves occur (Sakai and Crochet, 2001, their figure 2). A related interpretation, in line with the reciprocal interaction model (McCarley et al., 2007) is that cholinergic inhibition of DRN 5-HT neurons (via enhanced GABAergic inputs) predominates at the beginning of a REM cycle but reverses to excitation at the end of the cycle due to enhanced cholinergic firing rate, thereby terminating the state. In the future, this model could be tested in vivo by combining cell-specific stimulation or inhibition of cholinergic neurons with unit recordings from raphe neurons.
Conclusion
We conclude that cholinergic neurons can inhibit DRN 5-HT neurons during active wakefulness and REM sleep via excitation of local vlPAG GABAergic neurons.
Highlights
The cholinergic agonist, carbachol, strengthens inhibition of dorsal raphe neurons
Removal of dorsal but not ventral regions of the slice abolish this effect
Carbachol depolarizes GABAergic neurons in the ventrolateral periaqueductal gray
Both nicotinic and muscarinic receptors are involved in the effect of carbachol
ACKNOWLEDGEMENTS
This work was supported by a Department of Veterans Affairs Medical Research Service Awards to R. W. McCarley, by NIMH R01 MH039683 (to R.W. McCarley) and by NIMH R21 MH094803 (to R.E. Brown).
Abbreviations
- DRN
dorsal raphe nucleus
- 5-HT
serotonin
- REM
rapid eye movement
- sIPSCs
spontaneous inhibitory postsynaptic currents
- vlPAG
ventrolateral periaqueductal gray
- EEG
electroencephalogram
- LDT
laterodorsal tegmentum
- PPT
pedunculopontine tegmentum
- TTX
tetrodotoxin
- mIPSCs
miniature inhibitory postsynaptic currents
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- AP5
(2R)-amino-5-phosphonovaleric acid
- PnO
pontine nucleus, pars oralis
- xscp
decussation of the superior cerebellar peduncle
- NREM
non-REM
- SubC
subcoeruleus
- RMP
resting membrane potential
- Rin
input resistance
- AP
action potential
- AHP
afterhyperpolarization
- PGO
Ponto-geniculo-occipital
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT The authors are not aware of any conflicts of interest, financial commercial, or otherwise.
REFERENCES
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, McCarley RW. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci. 2008;27:352–363. doi: 10.1111/j.1460-9568.2008.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Daniele CA, Gallagher K, Madonia M, Mitchum RD, Barrett L, Vezina P, McGehee DS. Nicotinic excitation of serotonergic projections from dorsal raphe to the nucleus accumbens. J Neurophysiol. 2011;106:801–808. doi: 10.1152/jn.00575.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd Edition Academic Press; New York: 2008. [Google Scholar]
- Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduno J, Frias-Dominguez C, Drucker-Colin R, Mihailescu S. Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse. 2008;62:601–615. doi: 10.1002/syn.20526. [DOI] [PubMed] [Google Scholar]
- Garduño J, Galindo-Charles L, Jiménez-Rodríguez J, Galarraga E, Tapia D, Mihailescu S, Hernandez-Lopez S. Presynaptic α4β2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. J Neurosci. 2012;32:15148–15157. doi: 10.1523/JNEUROSCI.0941-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervations of dorsal raphe serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heym J, Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: effects of phasic auditory and visual stimuli. Brain Res. 1982;232:29–39. doi: 10.1016/0006-8993(82)90608-4. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Pompeiano O. Tonic inhibition of dorsal pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat. Arch Ital Biol. 1976;114:310–334. [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L. Study of the course of sleep in the young rat during the 1st postnatal month. C R Seances Soc Biol Fil. 1968;162:119–123. [PubMed] [Google Scholar]
- Kohlmeier KA, Vardar B, Christensen MH. γ-Hydroxybutyric acid induces actions via the GABAB receptor in arousal and motor control-related nuclei: Implications for therapeutic actions in behavioral state disorders. Neuroscience. 2013;248C:261–277. doi: 10.1016/j.neuroscience.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Jodo E, Kayama Y. Sensory responsiveness of “broad-spike” neurons in the laterodorsal tegmental nucleus, locus coeruleus and dorsal raphe of awake rats: implications for cholinergic and monoaminergic neuron-specific responses. Neuroscience. 1994;63:1021–1031. doi: 10.1016/0306-4522(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kumar A. Carbachol-induced long-term synaptic depression is enhanced during senescence at hippocampal CA3–CA1 synapses. J Neurophysiol. 2010;104:607–616. doi: 10.1152/jn.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Sreetama S, Mohanakumar KP. Atropine, a muscarinic cholinergic receptor antagonist increases serotonin, but not dopamine levels in discrete brain regions of mice. Neurosci Lett. 2007;423:100–103. doi: 10.1016/j.neulet.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Llinás R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci. 1998;18:1904–1912. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Greene RW, Semba K, Kamondi A, McCarley RW, Reiner PB. Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc Natl Acad Sci U S A. 1992;89:743–747. doi: 10.1073/pnas.89.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. The time-course of dorsal raphe discharge, PGO waves, and muscle tone averaged across multiple sleep cycles. Brain Res. 1983;274:365–370. doi: 10.1016/0006-8993(83)90720-5. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Singh A, Khanday MA. Activation of inactivation process initiates rapid eye movement sleep. Prog Neurobiol. 2012;97:259–276. doi: 10.1016/j.pneurobio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189:58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Albu SM, Winston S, Yanagawa Y, McCarley RW, Brown RE. Investigation of GABAergic brainstem projections to the dorsal raphe nucleus in GAD67-GFP knock-in mice: possible implications for REM sleep regulation. Associated Professional Sleep Societies 24th Annual Meeting; 2010. Abs:0136. [Google Scholar]
- McKenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, Yanagawa Y, McCarley RW, Brown RE. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J Comp Neurol. 2013;521:1225–1250. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailescu S, Guzmán-Marín R, Domínguez Mdel C, Drucker-Colín R. Mechanisms of nicotine actions on dorsal raphe serotoninergic neurons. Eur J Pharmacol. 2002;452(1):77–82. doi: 10.1016/s0014-2999(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Guzmán-Marín R, Drucker-Colín R. Nicotine stimulation of dorsal raphe neurons: effects on laterodorsal and pedunculopontine neurons. Eur Neuropsychopharmacol. 2001;11:359–66. doi: 10.1016/s0924-977x(01)00104-3. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Palomero-Rivero M, Meade-Huerta P, Maza-Flores A, Drucker-Colín R. Effects of nicotine and mecamylamine on rat dorsal raphe neurons. Eur J Pharmacol. 1998;360:31–36. doi: 10.1016/s0014-2999(98)00658-x. [DOI] [PubMed] [Google Scholar]
- Monti JM. The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep Med Rev. 2010;14:307–317. doi: 10.1016/j.smrv.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Nitz DA, Siegel JM. Inhibitory amino acid neurotransmission in the dorsal raphe nucleus during sleep/wake states. Am J Physiol. 1997;273:R451–R454. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Sakai K. Discharge properties of presumed cholinergic and noncholinergic laterodorsal tegmental neurons related to cortical activation in non-anesthetized mice. Neuroscience. 2012;224:172–190. doi: 10.1016/j.neuroscience.2012.08.032. [DOI] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Serotonergic dorsal raphe neurons cease firing by disfacilitation during paradoxical sleep. Neuroreport. 2000;11:3237–3241. doi: 10.1097/00001756-200009280-00037. [DOI] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience. 2001;104:1141–1155. doi: 10.1016/s0306-4522(01)00103-8. [DOI] [PubMed] [Google Scholar]
- Sapin E, Lapray D, Bérod A, Goutagny R, Léger L, Ravassard P, Clément O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009:4–e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Williams DK, Urbano FJ, Garcia-Rill E. Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons. Am J Physiol Cell Physiol. 2011;301:C327–335. doi: 10.1152/ajpcell.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Paré D, Oakson G, Curró Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Thakkar M, Portas C, McCarley RW. Chronic low-amplitude electrical stimulation of the laterodorsal tegmental nucleus of freely moving cats increases REM sleep. Brain Res. 1996;723:223–227. doi: 10.1016/0006-8993(96)00256-9. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL, Morrison AR. Raphe unit activity during REM sleep in normal cats and in pontine lesioned cats displaying REM sleep without atonia. Brain Res. 1981;226:75–91. doi: 10.1016/0006-8993(81)91084-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J Neurosci. 1994;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull. 1989;23:519–540. doi: 10.1016/0361-9230(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–2019. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]