Abstract
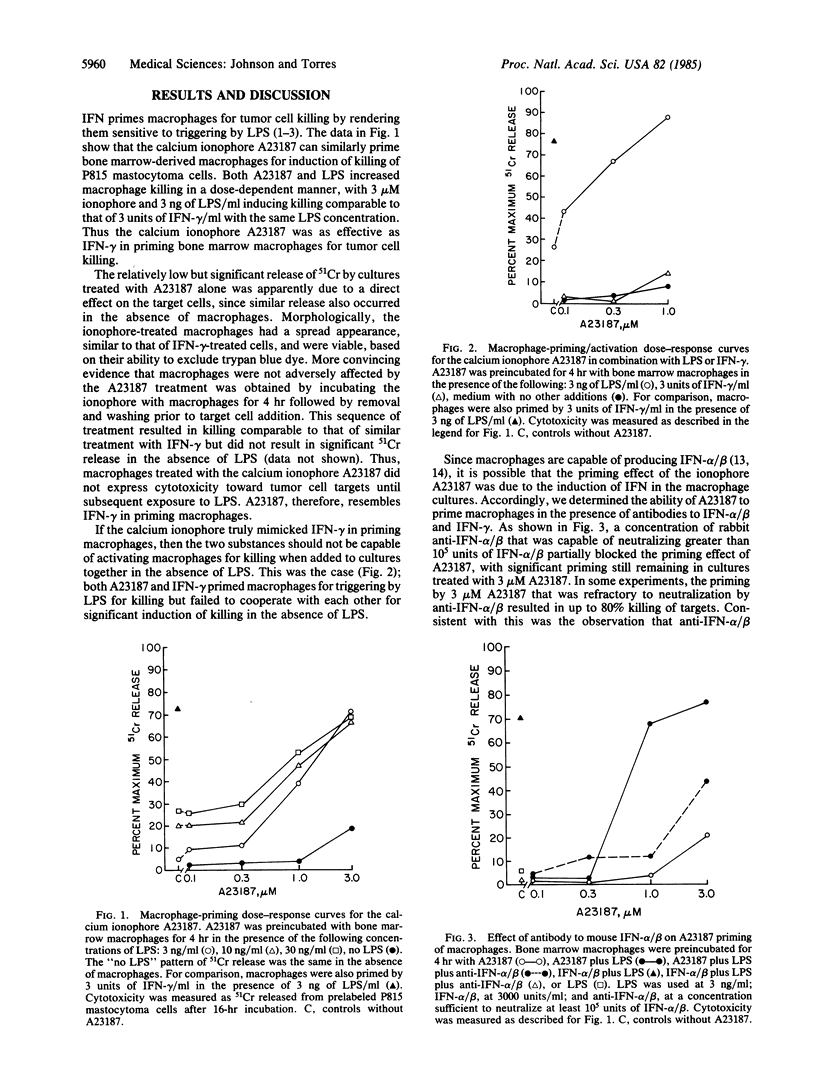
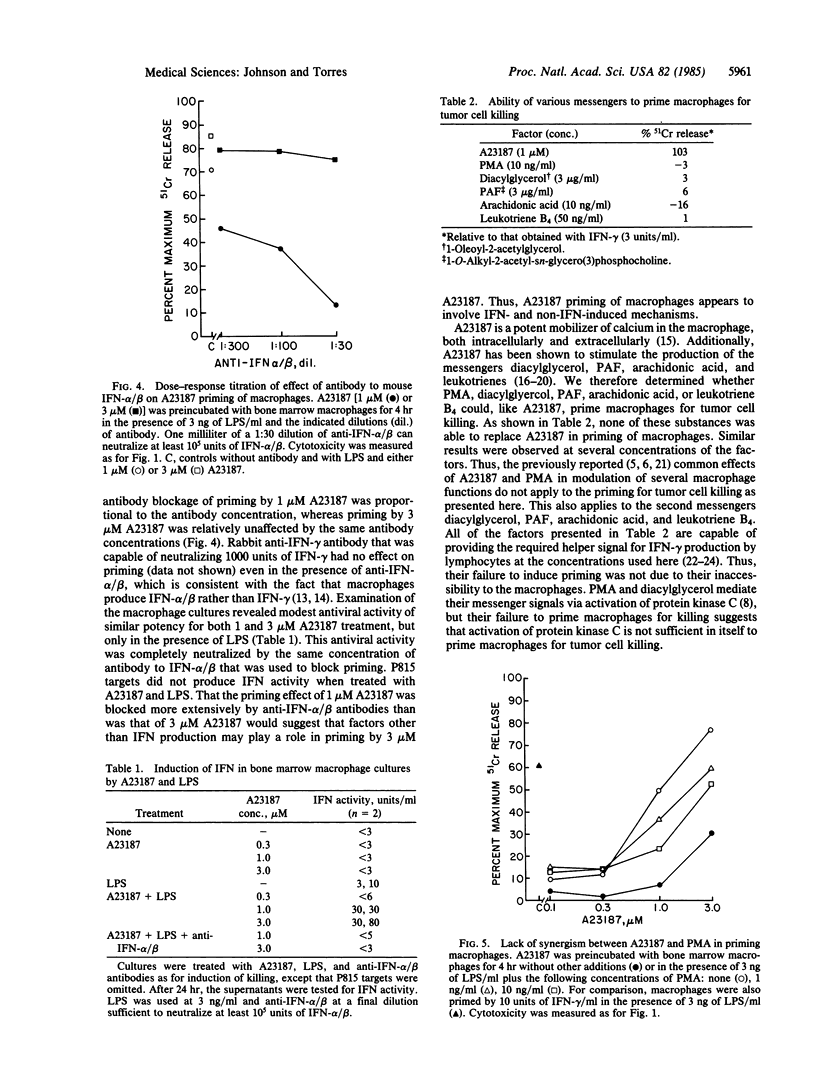
Interferon primes macrophages for tumor cell killing by rendering them sensitive to triggering agents such as lipopolysaccharide. In an attempt to determine the nature of the priming signal, we tested phorbol 12-myristate 13-acetate, diacylglycerol, platelet-activating factor, arachidonic acid, leukotriene B4, and the calcium ionophore A23187 for their ability to prime mouse bone marrow-derived macrophages for activation to kill P815 mastocytoma target cells. The ionophore A23187 was the only substance that was able to replace the interferon priming signal. A23187 priming appeared to be due in part to induction of interferon alpha/beta in the macrophage cultures, since its effect was partially but specifically blocked by antibody to interferon alpha/beta. Consistent with this was the observation that A23187 induced interferon alpha/beta production in macrophage cultures. The fact that A23187 priming was not completely reversed by antibody to interferon would suggest that factors unrelated to interferon induction also played a role in macrophage priming. The failure of phorbol myristate acetate or diacylglycerol to prime macrophages for tumor cell killing would suggest that activation of protein kinase C is not sufficient for priming. Thus, A23187 appears to provide the priming signal for macrophage killing through the combination of interferon- and non-interferon-induced mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. H., Snyder F. Biosynthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) from 1-alkyl-2-acyl-sn-glycero-3-phosphocholine by rat alveolar macrophages. Phospholipase A2 and acetyltransferase activities during phagocytosis and ionophore stimulation. J Biol Chem. 1983 Jan 10;258(1):97–102. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin H. D., Hawthorne J. N. Calcium-activated hydrolysis of phosphatidyl-myo-inositol 4-phosphate and phosphatidyl-myo-inositol 4,5-bisphosphate in guinea-pig synaptosomes. Biochem J. 1978 Nov 15;176(2):541–552. doi: 10.1042/bj1760541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A. Leukotrienes: positive signals for regulation of gamma-interferon production. J Immunol. 1984 Jan;132(1):413–416. [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A. Phorbol ester replacement of helper cell and interleukin 2 requirements in gamma interferon production. Infect Immun. 1982 Jun;36(3):911–914. doi: 10.1128/iai.36.3.911-914.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Vassallo T., Torres B. A. Interleukin 2-mediated events in gamma-interferon production are calcium dependent at more than one site. J Immunol. 1985 Feb;134(2):967–970. [PubMed] [Google Scholar]
- Kiyotaki C., Bloom B. R. Activation of murine macrophage cell lines. Possible involvement of protein kinases in stimulation of superoxide production. J Immunol. 1984 Aug;133(2):923–931. [PubMed] [Google Scholar]
- Kolesnick R. N., Gershengorn M. C. Ca2+ ionophores affect phosphoinositide metabolism differently than thyrotropin-releasing hormone in GH3 pituitary cells. J Biol Chem. 1984 Aug 10;259(15):9514–9519. [PubMed] [Google Scholar]
- Maehara N., Ho M., Armstrong J. A. Differences in mouse interferons according to cell source and mode of induction. Infect Immun. 1977 Sep;17(3):572–579. doi: 10.1128/iai.17.3.572-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Ohtsuki K., Torres B. A., Johnson H. M. Characterization of immune type interferon (IFN gamma)-induced cytoplasmic protein kinase activity in mouse L cells. Biochem Biophys Res Commun. 1982 Jan 29;104(2):422–429. doi: 10.1016/0006-291x(82)90654-4. [DOI] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W. Activation of mouse macrophages for tumor cell killing. I. Quantitative analysis of interactions between lymphokine and lipopolysaccharide. J Immunol. 1981 May;126(5):1863–1867. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Rittenhouse S. E. Activation of human platelet phospholipase C by ionophore A23187 is totally dependent upon cyclo-oxygenase products and ADP. Biochem J. 1984 Aug 15;222(1):103–110. doi: 10.1042/bj2220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Homma Y., Nagai Y. Role of Ca2+ in phosphatidylinositol response and arachidonic acid release in formylated tripeptide- or Ca2+ ionophore A23187-stimulated guinea pig neutrophils. J Immunol. 1983 Jun;130(6):2849–2855. [PubMed] [Google Scholar]
- Weiel J. E., Adams D. O., Hamilton T. A. Biochemical models of gamma-interferon action: altered expression of transferrin receptors on murine peritoneal macrophages after treatment in vitro with PMA or A23187. J Immunol. 1985 Jan;134(1):293–298. [PubMed] [Google Scholar]
- Yamamoto Y. Antigenicity of mouse interferons: two distinct molecular species common to interferons of various sources. Virology. 1981 Jun;111(2):312–319. doi: 10.1016/0042-6822(81)90335-4. [DOI] [PubMed] [Google Scholar]



