Abstract
Background:
Along with conventional electrodiagnostic studies, several other indexes including residual latency (RL) were introduced in patients with different types of peripheral neuropathies. RL is the time difference between measured and predicted distal conduction times. This study was performed to determine the values of the median nerve RL and to investigate its sensitivity and specificity in the diagnosis of carpal tunnel syndrome (CTS).
Materials and Methods:
The study was carried out among 100 hands of 75 healthy volunteers and 64 patients who had a positive history of pain or paresthesia in upper extremities and 2 of 3 signs suggesting CTS. Information including age, gender and results of sensory and motor nerve conduction velocity, compound motor action potential of proximal and distal stimulation and RL were recorded for analysis.
Results:
Normal range of the median nerve RL was found to be 1.03-2.65 (mean = 1.84 ± 0.41). The cut-off point of median RL was 2.37 for CTS diagnosis with sensitivity of 85.9% (95% of confidence interval [CI]: 84.4-87.5%) and specificity of 91.1% (95% CI: 87.8-92.2%).
Conclusion:
In mild cases of CTS, which conventional nerve conduction studies (NCSs) shows abnormalities only in sensory studies, RL may better demonstrate the effect on the median nerve motor fibers. We conclude that RL measurement of the median nerve may raise the sensitivity of NCSs for the diagnosis of CTS.
Keywords: Carpal tunnel syndrome, median nerve, reference values, residual latency
INTRODUCTION
Electrodiagnostic studies are the most common methods for evaluating and confirming peripheral neuropathies. Beside the routine nerve conduction studies (NCSs), several indexes were introduced in patients with different types of peripheral neuropathies. Among these, residual latency (RL) referred to the time difference between the measured distal conduction time and the predicted time based upon the application of proximal nerve conduction velocity (NCV) to the distal distance.[1,2] It may be more sensitive than a routine nerve conduction study for early diagnosis of distal peripheral neuropathies.[2,3]
RL determinations may provide a smaller standard deviation (SD) and tighter normal range than distal latencies for both motor and sensory NCSs.[1,2] It's proposed diagnostic utility is to provide additional information about distal nerve segments without requiring additional electrical stimulation and it may help to early diagnosis of peripheral neuropathies. As a study mentioned that RL measurements were as effective and as accurate as terminal latency measurements in determining the presence of a neuropathy distal to the wrist.[4] However, another recent study also found that the sensitivity and specificity of median RL and distal motor latency (DML) are similar but it showed that it is not superior to traditional NCS.[5]
As the normal values of median RL and its clinical utility in the diagnosis of peripheral neuropathies has been examined in a limited number of studies,[1,2,3,4,5,6,7,8] This study was performed to determine the cut-off values of the median nerve RL; and its sensitivity and specificity in the diagnosis of carpal tunnel syndrome (CTS) and compare with other electrodiagnostic parameters.
MATERIALS AND METHODS
This is a cross-sectional study, which was carried out from June 2012 to March 2013 among 100 hands of 75 healthy volunteers and 64 hands of 44 patients. It was performed at electrodiagnosis center of Alzahra Hospital, located at Department of Physical Medicine and Rehabilitation, Isfahan, Iran, after explaining the procedure and taking written consent. Control group were persons who had neither signs nor symptoms of neurologic abnormalities of upper extremities in their history and physical examination. Patients who had a positive history of pain or paresthesia in upper extremities and 2 of 3 signs suggesting CTS (Tinel's sign-paresthesias radiating in a median nerve distribution with tapping on the wrist or over the median nerve, median compression test-pressure over the proximal edge of the carpal ligament [proximal wrist crease] with thumbs causes paresthesia to develop or increase in the median nerve distribution and Phalen's sign-paresthesias radiating in a median nerve distribution within 60 s of sustained flexion of the wrist) were included as CTS group. Patients who had any history of hereditary polyneuropathies (e.g., Charcot-Marie-Tooth), acquired polyneuropathies (e.g., diabetic polyneuropathy), who underwent surgery or local steroid injections for CTS and patients who had any scar formation or history of fracture at the sites of stimulation or recording were excluded from the study.[5]
A normal room temperature (mean: 25°C) and a skin temperature of over 31°C (32-34°C) were maintained and the study was performed with surface stimulation electrode using constant current and surface bar recording electrodes.[9]
For obtaining the median nerve compound motor action potential (CMAP), while the active electrode (E-I) was located on the abductor pollicis brevis motor point with the reference electrode (E-2) placed distally, stimulation has been made 8 cm proximally at wrist. A second stimulus was applied to the median nerve at the antecubital fossa. Using a supramaximal impulse for both stimulation sites, the CMAP recorded and forearm NCV was obtained.[9]
For anti-dromic median nerve sensory conduction studies, the E-I recording electrode was located on the second or third digit just distal to the metacarpophalangeal joint region and the E-2 electrode placed at least 4 cm more distal on the respective digit. The median nerve is excited 7 cm[5] and 14 cm proximal to E-I at wrist again with a supramaximal current intensity.[10] With similar techniques, ulnar nerve CMAP from abductor digiti minimi motor point and ulnar nerve sensory nerve action potential (SNAP) from fifth digit were recorded.
The instrument settings for median CMAP assessment were an amplifier sensitivity of 1,000 μV/div, a sweep of 2 or 5 ms/div and it was changed to an amplifier sensitivity of 10-20 μV/div and a sweep of I or 2 ms/div for SNAP recordings. RL was calculated by the formula “median RL = median DML (ms) – (distal distance [mm]/median motor nerve conduction velocity [MNCV] [m/s])”[1,2]
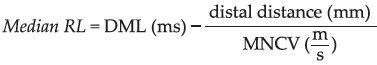
CTS was accepted as sensory distal latency (SDL) ≥3.6 ms, sensory nerve conduction velocity (SNCV) ≤40 m/s or DML ≥4.2 ms.[11]
All tests were performed or directly supervised by a physiatrist attending. Cadwell EMG machine was used for the study. Information including age, gender and results of (SNCV and MNCV), latency, CMAP of proximal and distal stimulation was recorded for analysis.
Statistical analysis
The statistical package for social sciences (SPSS) 20 (Chicago, IL, USA) was used to calculate the average values and SD. Independent Student t-test was used for comparison of mean values among study groups. Comparison between the averages NCS values of the CTS and control groups also were performed by independent Student's t-test. Chi-square test was used for the comparison of nominal data. Sensitivity and specificity of variables were based on receiver operating characteristic (ROC) curve analysis and sensitivity was calculated as (true positive/[true positive + false negative]), specificity as (true negative/[true negative + false positive]), positive predictive value was determined as (true positive/[true positive + false positive]) and negative predictive value as (true negative/[true negative + false negative]). P < 0.05 accepted as statistically significant.
RESULTS
A total number of 64 hands with CTS (54 women, 10 men) and 100 healthy controls (75 women, 25 men) were investigated after taking anamnesis and performing neurological and electrophysiological examinations. 20 CTS cases (62.5%) were diagnosed as bilateral CTS, 13 (20.3%) in the right hand and 11 (17.1%) in the left hand.
No significant difference was found between the mean ages of the CTS (47.12 ± 11.01 years) and the control groups (44.27 ± 12.24 years) (P > 0.05) [Table 1].
Table 1.
Comparison of mean RL of median nerve among males and females of CTS and normal groups
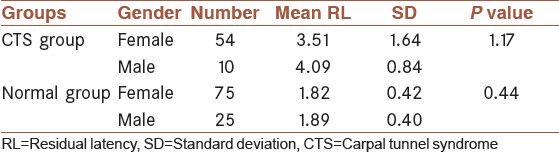
Normal values were found from the average values of these parameters recorded from the controls ± 2 SD. Mean (SD) of median nerve RL was found to be 1.84 ± 0.41 (range: 1.03-2.65 ms). It was found that mean RL of right and left hands had no significant difference (P = 0.84).
Table 2 shows the results of the electrophysiological examination of 64 upper extremities from the CTS group and 100 upper extremities from the control group.
Table 2.
Values of the various electrophysiological diagnostic tests of median nerve in CTS and normal groups
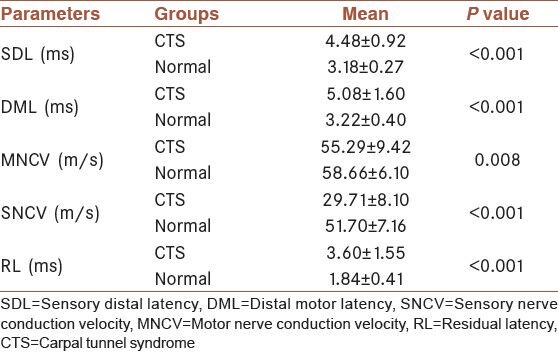
In the CTS group, DML, SDL and RL were found to be significantly higher compared with the control group (P < 0.001 for each parameter) and mean MNCV and SNCV were significantly lower than the control group [Table 2].
Table 3 shows the sensitivity, specificity, of electrophysiological diagnostic tests for the CTS patients.
Table 3.
Sensitivity, specificity, and AUC of the various electrophysiological diagnostic tests of median nerve in CTS patients
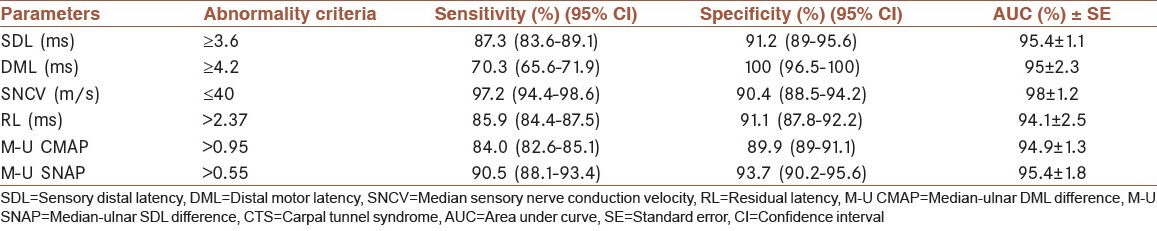
By using ROC curve, the cut-off point of RL was 2.37 for CTS diagnosis with sensitivity of 85.9% and specificity of 91.1%. As we compared this data with control group, 87.7% of them had RLs smaller than 2.37 [Figure 1].
Figure 1.
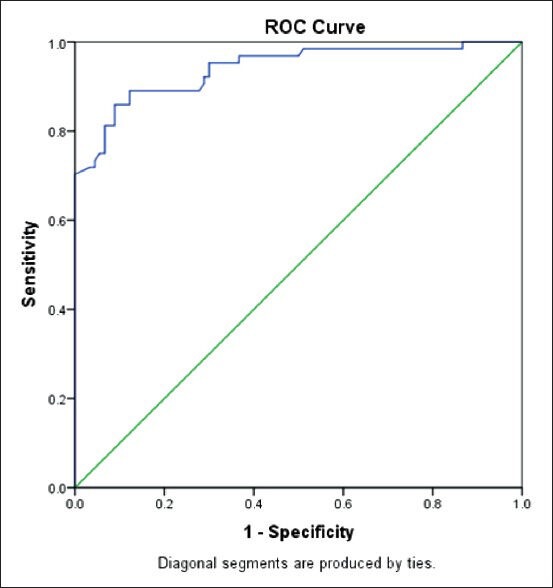
Graph showing receiver operating characteristic curve for the residual latency of the median nerve
Median – Ulnar CMAP (M-U CMAP) and Median – Ulnar SNAP (M-U SNAP) differences showed a cut-off point of 0.95 ms and 0.55 ms, respectively.
The most sensitive electrophysiological findings in CTS patients, were SNCV (97.2%), M-U SNAP (90.5%), DSL (87.3%), RL (85.9%), M-U CMAP (84.0%), DML (70.3%). In order of specificity, the most specific electrophysiological findings in CTS patients were DML (100%), M-U SNAP (93.7%), DSL (91.2%), RL (91.1%) and SNCV (90.4%). M-U CMAP (89.9%) [Table 3] positive predictive value and negative predictive value of median nerve RL were 87.3% and 90%, respectively.
According to statistical analysis, of all studied subjects, 44 had both RL >2.37 and SDL ≥3.6, which 97.7% of these were patients and among 79 subjects who had both RL and SDL below these values 94.9% were normal controls. 67.2% of the case group had RL >2.37 and SDL ≥3.6 and 82.4% of the control group had values below these.
Combination of both RL >2.37 and DML ≥4.2, was found among 63 subjects. From these 63 subjects, 87.3% were patients and from 53 subjects who had both RL and DMLs below these values 96.2% were normal subjects.85.9% of the case group had values above and 56% of the control group had values below both the mentioned parameters.
DISCUSSION
There are several proposed factors for the concept of RL: utilization time; neuromuscular junction (NMJ) transmission delay; gradual slowing of conduction due to reduction of temperature distally; distal tapering of axons and slowed conduction across the distal unmyelinated segments.[1] A study that was performed regarding the RL of sensory fibers, mentioned that NMJ transmission delay has much less effect on this parameter.[4] Thus it concluded that the most consistent etiology which may describe the concept of RL is the tapering of the nerve distal to the wrist.
Several studies were carried out using RL as an electrodiagnostic parameter for the early diagnosis of distal peripheral neuropathies. Conflicting results have been obtained from RL in terms of sensitivity in the diagnosis of these diseases.
Kraft and Halvorson have revealed that RL determinations may be especially useful in confirming early or mild CTS and should be calculated in patients with suspected symptoms.[1] Kaplan indicated that RL measurements were as effective and as accurate as terminal latency measurements in determining the presence of a neuropathy distal to the wrist.[4] A study that was performed in 1978, concluded that with a recent onset of CTS, prolonged residual latencies may be the only abnormality.[2]
Lee et al. had performed a study and assessed the median nerve RL in normal subjects and patients with diabetic polyneuropathy. They concluded that RL will provide a narrower normal range in diagnosis of diabetic polyneuropathy irrespective of age or duration of diabetes mellitus.[12] Another study which evaluated the RL of four nerves (median, ulnar, deep peroneal and posterior tibial nerves) also confirmed this data.[13]
Karata et al. performed a study, which proposed RL was a sensitive indicator of the compression of motor nerves.[6] However, Kuntzer mentioned that although it had high sensitivity, but its specificity was low.[7]
A study also found that RL do not enhance the sensitivity of median DML in the diagnosis of CTS.[14] Another recent study found that the sensitivity and specificity of RL and DML are similar and it is not superior to traditional NCS.[5] In present study, temperatures were all standardized. We found a normal range of RL 1.84 ± 0.41. In a study with 40 control subjects the normal value of median RL was 2.39 ± 0.33[14] while another found 2.06 ± 0.45 as the normal ranges.[5] Other studies also demonstrated values, which were more similar to our results.[1,13] This variability probably is because of effect of temperature, variable methods and number of subjects.
Based on our findings, the sensitivity and specificity of RL and SDL are similar. SNCV has greater sensitivity but its specificity is lower. Area under curve of RL is approximately similar to SDL and DML. Indeed, a practitioner may benefit from RL by eliminating additional electrical stimulations for obtaining SDLs and SNCVs. Although the sensitivity for CTS diagnosis of RL was less than that of SDL and SNCV, it was more sensitive than DML. The results showed that among subjects who had both RL >2.37 and SDL ≥3.6, 97.7% were patients with CTS diagnosis. It may contribute to this fact that combination the results of RL and SDL can provide an excellent predictor of the diagnosis of CTS.
It was claimed that more accuracy will find by comparing the median nerve responses with another nerve, which does not travel through the carpal tunnel, as opposed to using “normal” values of the amplitude and latency of them individually.[15] Joshi et al. performed a study among 125 clinically diagnosed patients with CTS and found increased differences between SDL and DML of median and ulnar nerves among them.[16] our findings demonstrated comparable sensitivity and specificity of RL with either M-U CMAP or M-U SNAP. Further studies may explain the significance of these comparisons in CTS diagnosis as well.
CONCLUSION
It seems that, in mild cases of CTS which traditional NCS shows abnormalities only in sensory studies, RL may better demonstrate the effect on median nerve motor fibers. Indeed, in some cases (5 patients), although sensory nerve conduction study of the median nerve were normal, RL were abnormal, which may provide a guide in the diagnosis of CTS. In present study, we did not evaluate RL of sensory nerves which may need to be examined in future studies.
ACKNOWLEDGMENT
This study was supported by the Isfahan University of Medical Sciences (Research project Number 392222).
Footnotes
Source of Support: This study was supported by the Isfahan University of Medical Sciences (Research project Number 392222).
Conflict of Interest: None declared.
REFERENCES
- 1.Kraft GH, Halvorson GA. Median nerve residual latency: Normal value and use in diagnosis of carpal tunnel syndrome. Arch Phys Med Rehabil. 1983;64:221–6. [PubMed] [Google Scholar]
- 2.Kaplan P, Sahgal V. Residual latency: New applications of an old technique. Arch Phys Med Rehabil. 1978;59:24–7. [PubMed] [Google Scholar]
- 3.Radziwill AJ, Steck AJ, Renaud S, Fuhr P. Distal motor latency and residual latency as sensitive markers of anti-MAG polyneuropathy. J Neurol. 2003;250:962–6. doi: 10.1007/s00415-003-1128-7. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan PE. Sensory and motor residual latency measurements in helathy patients and patients with neuropathy-part 1. J Neurol Neurosurg Psychiatry. 1976;39:338–40. doi: 10.1136/jnnp.39.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uzar E, Tamam Y, Acar A, Yucel Y, Palanci Y, Cansever S, et al. Sensitivity and specificity of terminal latency index and residual latency in the diagnosis of carpal tunnel syndrome. Eur Rev Med Pharmacol Sci. 2011;15:1078–84. [PubMed] [Google Scholar]
- 6.Karata M, Sozay S, Bayramo LU. Terminal latency index and residual latency in Carpal Tunnel Syndrome. Romatizma. 2000;15:105–11. [Google Scholar]
- 7.Kuntzer T. Carpal tunnel syndrome in 100 patients: Sensitivity, specificity of multi-neurophysiological procedures and estimation of axonal loss of motor, sensory and sympathetic median nerve fibers. J Neurol Sci. 1994;127:221–9. doi: 10.1016/0022-510x(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 8.Simovic D, Weinberg DH. The median nerve terminal latency index in carpal tunnel syndrome: A clinical case selection study. Muscle Nerve. 1999;22:573–7. doi: 10.1002/(sici)1097-4598(199905)22:5<573::aid-mus4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Dumitru D, Amato AA, Zwartz MJ. Nerve conduction studies. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2002. p. 195. [Google Scholar]
- 10.Dumitru D, Amato AA, Zwartz MJ. Nerve conduction studies. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2002. p. 201. [Google Scholar]
- 11.Dumitru D, Zwartz MJ. Focal peripheral neuropathies. In: Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic Medicine. 2nd ed. Philadelphia: Hanley & Belfus; 2002. p. 1062. [Google Scholar]
- 12.Lee SY, Kim TH, Choung SY, Chung JS. Median nerve residual latency in normal controls and patients with diabetes mellitus. J Korean Acad Rehabil Med. 1997;21:703–8. [Google Scholar]
- 13.Suh J, Park JH, Jung KH, Chang JY, Choi JH, Kim YS. The clinical significance of residual latency in diagnosis of diabetic neuropathy. J Korean Acad Rehabil Med. 1998;22:1254–62. [Google Scholar]
- 14.Goh KJ, Tan CB, Yeow YK, Tjia H. Electrodiagnosis of carpal tunnel syndrome - A comparison of the sensitivities of the various nerve conduction tests. Neurol J Southeast Asia. 1999;4:37–43. [Google Scholar]
- 15.Ibrahim I, Khan WS, Goddard N, Smitham P. Carpal tunnel syndrome: A review of the recent literature. Open Orthop J. 2012;6:69–76. doi: 10.2174/1874325001206010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi AG, Gargate AR, Patil SN. Electrophysiological assessment of clinically diagnosed patients of carpal tunnel syndrome in Western Maharashtra. Indian J Physiother Occup Ther. 2013;7:29–33. [Google Scholar]


