Abstract
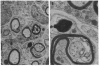
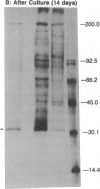
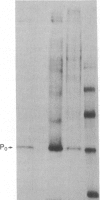
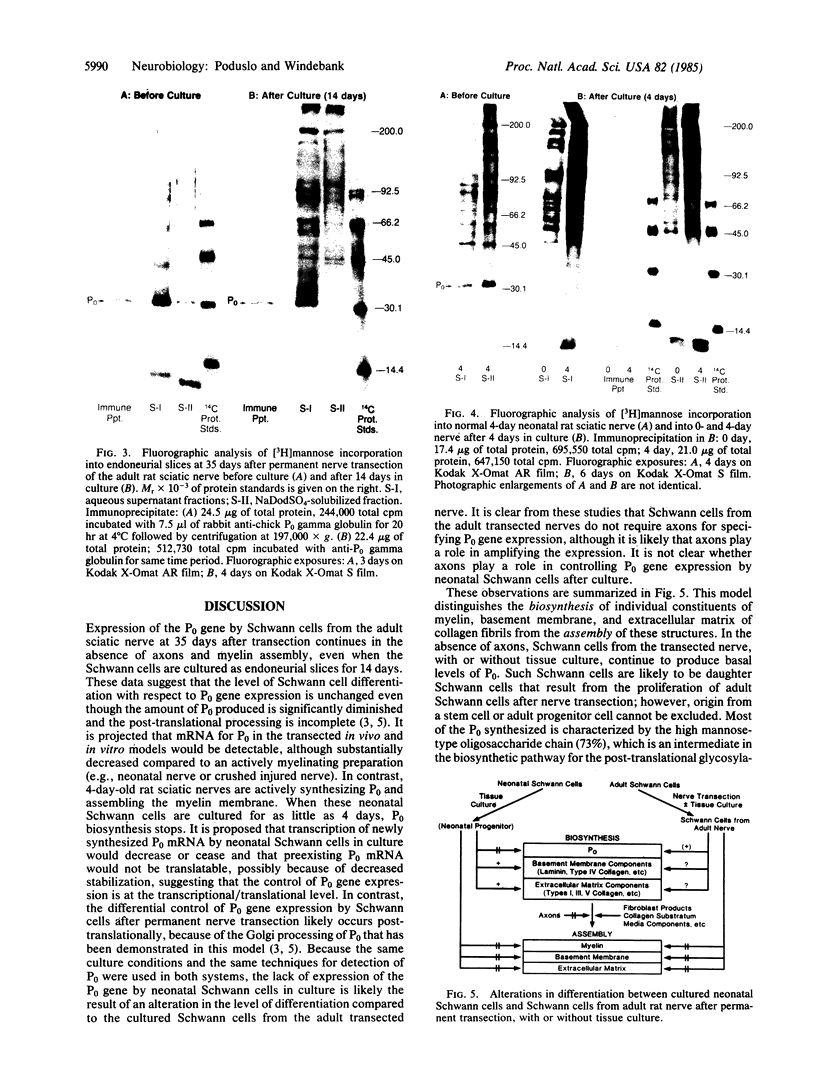
Previous experiments demonstrated that Schwann cells from permanently transected sciatic nerves of adult rats synthesize basal levels of the major myelin glycoprotein (P0). This denervated preparation at 35 days after transection was characterized by the absence of both axons and myelin assembly. The present investigation demonstrates that production of P0 continues after culture of the Schwann cells as endoneurial slices for 14 days. Thus, the level of differentiation is unchanged in culture even though only basal levels of P0 are produced and post-translational processing is incomplete. In contrast, Schwann cells from 4-day-old rat sciatic nerves actively synthesized P0 and assembled myelin membrane; however, after only 4 days in culture biosynthesis of P0 ceased. Because the same culture conditions and precursor incorporation procedures were used for both neonatal and transected nerves, it is proposed that neonatal Schwann cells in culture return to a progenitor state that is not capable of P0 gene expression. This comparison, both before and after culture, of neonatal Schwann cells that are programmed to myelinate and Schwann cells from the adult transected nerve that were in a myelin-maintaining mode provides a useful model for investigating the mechanisms by which differential gene expression is controlled. These results confirm that axons are not necessary for specifying P0 gene expression by Schwann cells from the adult transected nerves. The role that axons play in controlling P0 gene expression by neonatal Schwann cells in culture, however, has yet to be determined. It is concluded that the differentiation and maturation of Schwann cells is multistage process that allows the sequential production of specific gene products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Charron L., Bray G. M. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976 Oct;5(8):565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- Aguayo A. J., Epps J., Charron L., Bray G. M. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976 Mar 5;104(1):1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- Billings-Gagliardi S., Webster H. F., O'Connell M. F. In vivo and electron microscopic observations on Schwann cells in developing tadpole nerve fibers. Am J Anat. 1974 Nov;141(3):375–391. doi: 10.1002/aja.1001410308. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fryxell K. J., Lemke G. E. Studies on cultured Schwann cells: the induction of myelin synthesis, and the control of their proliferation by a new growth factor. J Exp Biol. 1981 Dec;95:215–230. doi: 10.1242/jeb.95.1.215. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Raff M. C., Nishiguchi D. J., Winter J. Studies on cultured rat Schwann cells. III. Assays for peripheral myelin proteins. J Neurocytol. 1980 Feb;9(1):67–77. doi: 10.1007/BF01205227. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Williams A. K., Wood P. M. Neuron-Schwann cell interaction in basal lamina formation. Dev Biol. 1982 Aug;92(2):449–460. doi: 10.1016/0012-1606(82)90190-7. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Williams A. K., Wood P. M., Uitto J., Jeffrey J. J. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980 Jan;84(1):184–202. doi: 10.1083/jcb.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge R. P., Bunge M. B. Evidence that contact with connective tissue matrix is required for normal interaction between Schwann cells and nerve fibers. J Cell Biol. 1978 Sep;78(3):943–950. doi: 10.1083/jcb.78.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge R. P. Recent observations on the control of Schwann cell functions. Anat Rec Suppl. 1983;1:3–25. doi: 10.1016/0732-118x(83)90024-7. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Eldridge C. F., Cornbrooks C. J., Timpl R., Bunge R. P. Biosynthesis of type IV collagen by cultured rat Schwann cells. J Cell Biol. 1983 Aug;97(2):473–479. doi: 10.1083/jcb.97.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Winter J., Abney E. R., Pruss R. M., Gavrilovic J., Raff M. C. Myelin-specific proteins and glycolipids in rat Schwann cells and oligodendrocytes in culture. J Cell Biol. 1980 Mar;84(3):483–494. doi: 10.1083/jcb.84.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya F., Bunge M. B., Bunge R. P. Schwann cells proliferate but fail to differentiate in defined medium. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6902–6906. doi: 10.1073/pnas.77.11.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. J., Mezei C. Solid-phase immunoassay of PO glycoprotein of peripheral nerve myelin. J Neurochem. 1984 Jan;42(1):158–165. doi: 10.1111/j.1471-4159.1984.tb09712.x. [DOI] [PubMed] [Google Scholar]
- Payer A. F. An ultrastructural study of Schwann cell response to axonal degeneration. J Comp Neurol. 1979 Jan 15;183(2):365–383. doi: 10.1002/cne.901830209. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Berg C. T., Dyck P. J. Schwann cell expression of a major myelin glycoprotein in the absence of myelin assembly. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1864–1866. doi: 10.1073/pnas.81.6.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F., Dyck P. J., Berg C. T. Regulation of myelination: Schwann cell transition from a myelin-maintaining state to a quiescent state after permanent nerve transection. J Neurochem. 1985 Feb;44(2):388–400. doi: 10.1111/j.1471-4159.1985.tb05428.x. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F. Posttranslational protein modification: biosynthetic control mechanisms in the glycosylation of the major myelin glycoprotein by Schwann cells. J Neurochem. 1985 Apr;44(4):1194–1206. doi: 10.1111/j.1471-4159.1985.tb08743.x. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F. Regulation of myelination: biosynthesis of the major myelin glycoprotein by Schwann cells in the presence and absence of myelin assembly. J Neurochem. 1984 Feb;42(2):493–503. doi: 10.1111/j.1471-4159.1984.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Yao J. K. Association and release of the major intrinsic membrane glycoprotein from peripheral nerve myelin. Biochem J. 1985 May 15;228(1):43–54. doi: 10.1042/bj2280043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Booth G. M. Improved methods for the fluorographic detection of weak beta-emitting radioisotopes in Agarose and acrylamide gel electrophoresis media. J Biochem Biophys Methods. 1981 Jun;4(5-6):339–346. doi: 10.1016/0165-022x(81)90074-9. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. Studies on the control of myelinogenesis. I. Myelination of regenerating axons after entry into a foreign unmyelinated nerve. J Neurocytol. 1975 Aug;4(4):395–418. doi: 10.1007/BF01261372. [DOI] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. Studies on the control of myelinogenesis. II. Evidence for neuronal regulation of myelin production. Brain Res. 1976 Aug 27;113(2):363–378. doi: 10.1016/0006-8993(76)90947-1. [DOI] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. The fate of Schwann cells isolated from axonal contact. J Neurocytol. 1978 Oct;7(5):555–569. doi: 10.1007/BF01260889. [DOI] [PubMed] [Google Scholar]
- Windebank A. J., Wood P., Bunge R. P., Dyck P. J. Myelination determines the caliber of dorsal root ganglion neurons in culture. J Neurosci. 1985 Jun;5(6):1563–1569. doi: 10.1523/JNEUROSCI.05-06-01563.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]