Abstract
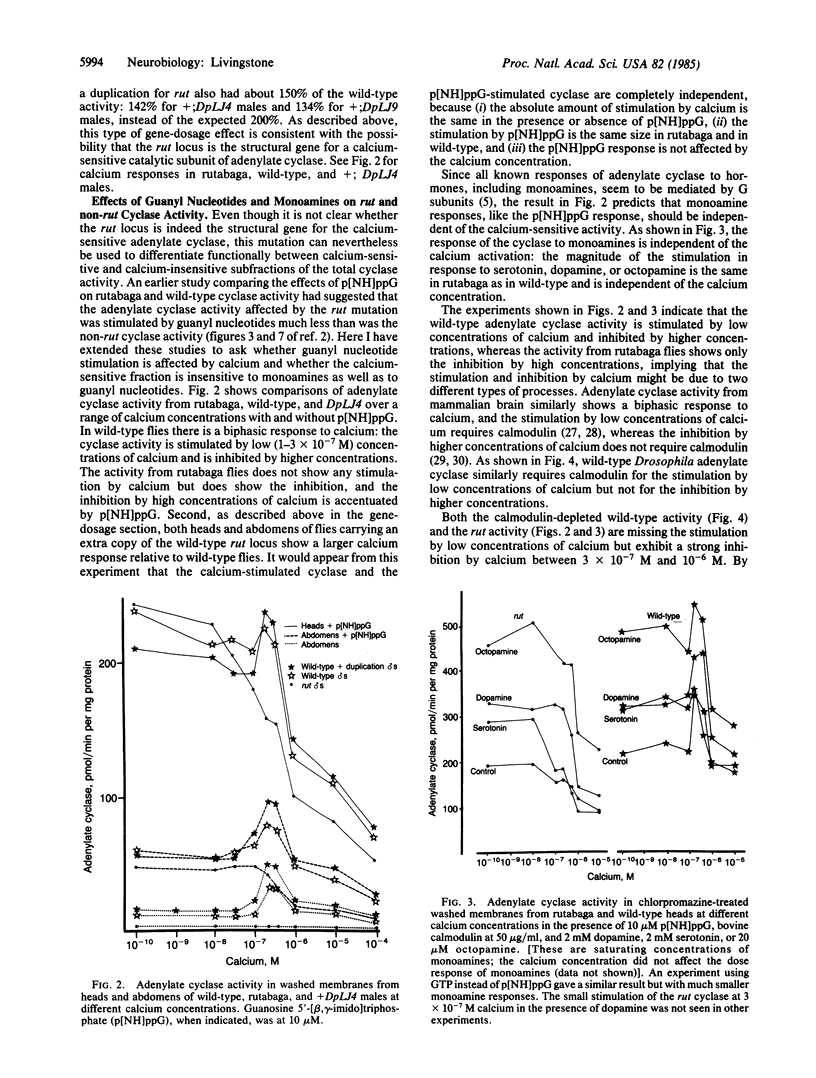
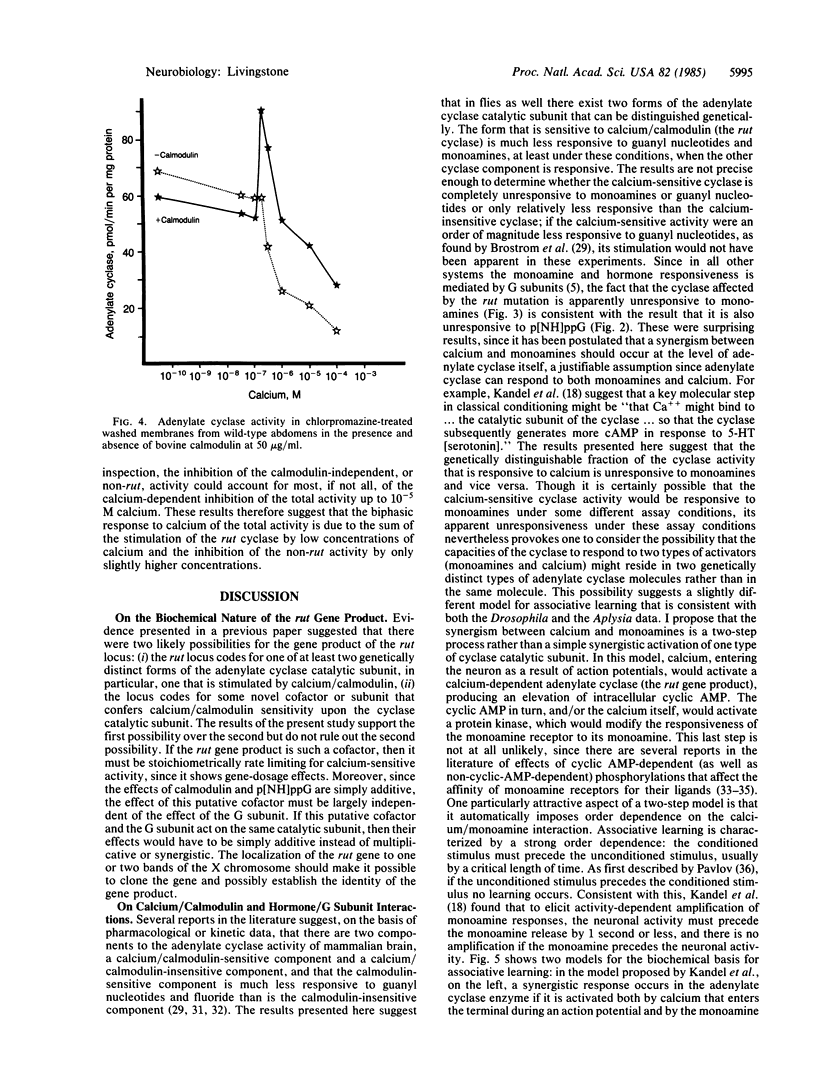
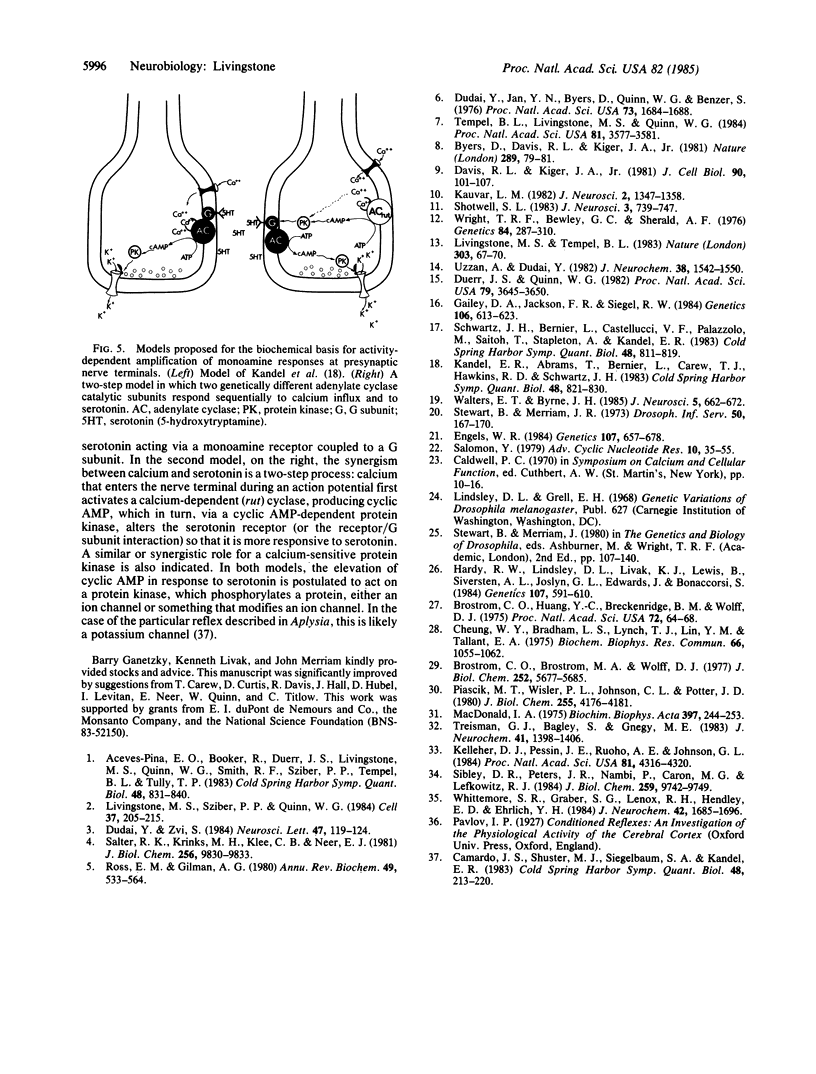
The Drosophila learning mutant rutabaga is missing calcium/calmodulin activation of adenylate cyclase (EC4.6.1.1). The mutation was mapped at a finer resolution to X chromosome bands 12F5-7. By comparing wild-type and mutant cyclase activities, the relative responsiveness of the calcium-sensitive and calcium-insensitive components to different ligands could be determined; the calcium-sensitive fraction of the total cyclase activity was significantly less responsive to guanyl nucleotides and monoamines. The results suggest that the component of cyclase activity that is stimulated by calcium/calmodulin, possibly a genetically distinct catalytic subunit, is not coupled to the G subunit or the G subunit/monoamine receptor complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves-Piña E. O., Booker R., Duerr J. S., Livingstone M. S., Quinn W. G., Smith R. F., Sziber P. P., Tempel B. L., Tully T. P. Learning and memory in Drosophila, studied with mutants. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):831–840. doi: 10.1101/sqb.1983.048.01.086. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A., Wolff D. J. Calcium-dependent adenylate cyclase from rat cerebral cortex. Reversible activation by sodium fluoride. J Biol Chem. 1977 Aug 25;252(16):5677–5685. [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D., Davis R. L., Kiger J. A., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981 Jan 1;289(5793):79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Camardo J. S., Shuster M. J., Siegelbaum S. A., Kandel E. R. Modulation of a specific potassium channel in sensory neurons of Aplysia by serotonin and cAMP-dependent protein phosphorylation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):213–220. doi: 10.1101/sqb.1983.048.01.024. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Kiger J. A., Jr Dunce mutants of Drosophila melanogaster: mutants defective in the cyclic AMP phosphodiesterase enzyme system. J Cell Biol. 1981 Jul;90(1):101–107. doi: 10.1083/jcb.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Jan Y. N., Byers D., Quinn W. G., Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976 May;73(5):1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Zvi S. Adenylate cyclase in the Drosophila memory mutant rutabaga displays an altered Ca2+ sensitivity. Neurosci Lett. 1984 Jun 15;47(2):119–124. doi: 10.1016/0304-3940(84)90416-6. [DOI] [PubMed] [Google Scholar]
- Duerr J. S., Quinn W. G. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., Preston C. R. Formation of chromosome rearrangements by P factors in Drosophila. Genetics. 1984 Aug;107(4):657–678. doi: 10.1093/genetics/107.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailey D. A., Jackson F. R., Siegel R. W. Conditioning Mutations in DROSOPHILA MELANOGASTER Affect an Experience-Dependent Behavioral Modification in Courting Males. Genetics. 1984 Apr;106(4):613–623. doi: 10.1093/genetics/106.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Lindsley D. L., Livak K. J., Lewis B., Siversten A. L., Joslyn G. L., Edwards J., Bonaccorsi S. Cytogenetic analysis of a segment of the Y chromosome of Drosophila melanogaster. Genetics. 1984 Aug;107(4):591–610. doi: 10.1093/genetics/107.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Abrams T., Bernier L., Carew T. J., Hawkins R. D., Schwartz J. H. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- Kauvar L. M. Defective cyclic adenosine 3':5'-monophosphate phosphodiesterase in the Drosophila memory mutant dunce. J Neurosci. 1982 Oct;2(10):1347–1358. doi: 10.1523/JNEUROSCI.02-10-01347.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher D. J., Pessin J. E., Ruoho A. E., Johnson G. L. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P., Quinn W. G. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984 May;37(1):205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Tempel B. L. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983 May 5;303(5912):67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- MacDonald I. A. Differentiation of fluorides-stimulated and non-fluoride-stimulated components of beef brain cortex adenylate cyclase cy calcium ions, ethyleneglycol-bis-(beta-aminoethyl ether) N,N'-tetraacetic acid and Triton X-100. Biochim Biophys Acta. 1975 Jul 27;397(1):244–253. doi: 10.1016/0005-2744(75)90197-7. [DOI] [PubMed] [Google Scholar]
- Piascik M. T., Wisler P. L., Johnson C. L., Potter J. D. Ca2+-dependent regulation of guinea pig brain adenylate cyclase. J Biol Chem. 1980 May 10;255(9):4176–4181. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Salter R. S., Krinks M. H., Klee C. B., Neer E. J. Calmodulin activates the isolated catalytic unit of brain adenylate cyclase. J Biol Chem. 1981 Oct 10;256(19):9830–9833. [PubMed] [Google Scholar]
- Schwartz J. H., Bernier L., Castellucci V. F., Palazzolo M., Saitoh T., Stapleton A., Kandel E. R. What molecular steps determine the time course of the memory for short-term sensitization in Aplysia? Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):811–819. doi: 10.1101/sqb.1983.048.01.084. [DOI] [PubMed] [Google Scholar]
- Shotwell S. L. Cyclic adenosine 3':5'-monophosphate phosphodiesterase and its role in learning in Drosophila. J Neurosci. 1983 Apr;3(4):739–747. doi: 10.1523/JNEUROSCI.03-04-00739.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Peters J. R., Nambi P., Caron M. G., Lefkowitz R. J. Desensitization of turkey erythrocyte adenylate cyclase. Beta-adrenergic receptor phosphorylation is correlated with attenuation of adenylate cyclase activity. J Biol Chem. 1984 Aug 10;259(15):9742–9749. [PubMed] [Google Scholar]
- Tempel B. L., Livingstone M. S., Quinn W. G. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman G. J., Bagley S., Gnegy M. E. Calmodulin-sensitive and calmodulin-insensitive components of adenylate cyclase activity in rat striatum have differential responsiveness to guanyl nucleotides. J Neurochem. 1983 Nov;41(5):1398–1406. doi: 10.1111/j.1471-4159.1983.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Uzzan A., Dudai Y. Aminergic receptors in Drosophila melanogaster: responsiveness of adenylate cyclase to putative neurotransmitters. J Neurochem. 1982 Jun;38(6):1542–1550. doi: 10.1111/j.1471-4159.1982.tb06631.x. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci. 1985 Mar;5(3):662–672. doi: 10.1523/JNEUROSCI.05-03-00662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore S. R., Graber S. G., Lenox R. H., Hendley E. D., Ehrlich Y. H. Activation of adenylyl cyclase by preincubation of rat cerebral-cortical membranes under phosphorylating conditions: role of ATP, GTP, and divalent cations. J Neurochem. 1984 Jun;42(6):1685–1696. doi: 10.1111/j.1471-4159.1984.tb12760.x. [DOI] [PubMed] [Google Scholar]
- Wright T. R., Bewley G. C., Sherald A. F. The genetics of dopa decarboxylase in Drosophila melanogaster. II. Isolation and characterization of dopa-decarboxylase-deficient mutants and their relationship to the alpha-methyl-dopa-hypersensitive mutants. Genetics. 1976 Oct;84(2):287–310. doi: 10.1093/genetics/84.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]



