Abstract
The animal gut commonly contains a large reservoir of symbiotic microbes. Although these microbes have obvious functions in digestion and immune defence, gut microbes can also affect behaviour. Here, we explore whether gut microbiota has a role in kin recognition. We assessed whether relatedness, familiarity and food eaten during development altered copulation investment in three species of Drosophila with diverse ecologies. We found that a monandrous species exhibited true kin recognition, whereas familiarity determined kin recognition in a species living in dense aggregations. Finally, in a food generalist species, food eaten during development masked kin recognition. The effect of food type on copulation duration, in addition to the removal of this effect via antibiotic treatment, suggests the influence of bacteria associated with the gut. Our results provide the first evidence that varied ecologically determined mechanisms of kin recognition occur in Drosophila, and that gut bacteria are likely to have a key role in these mechanisms.
Keywords: gut microbiota, gut bacteria, copulation duration, copulation investment, kin recognition
Introduction
The animal gut contains a large and commonly biodiverse bacterial community. Although these bacteria have obvious physiological functions in digestion and immune defence, gut microbes can affect many aspects of host behaviour (Archie and Theis, 2011; Lizé et al., 2013). The gut bacteria community of any particular individual is commonly dependent on external factors, with host diet having been shown to have the closest correlation with the composition of gut microbiota across taxa (Ley et al., 2008). Recent studies highlight the importance of gut microbiota on the evolution of mate preferences (Markov et al., 2009; Sharon et al., 2010), whereas others demonstrate that social behaviours can themselves alter gut microbiota communities (Koch and Schmid-Hempel, 2011). In the 1970s it was also hypothesised that gut microbiota could alter the scent of mammals (Gorman et al., 1974; Gorman, 1976; Singh et al., 1990; Kuhn and Natsch, 2009; Archie and Theis, 2011) and could thus have a key role in kin recognition. Examination of this hypothesis has continued to focus on mammals; however, gut microbiota is likely to also be important in social insects and potentially also in all insects whose communication relies on olfaction (Lizé et al., 2013).
Many species largely rely upon olfactory communication as a cue for decisions related to reproductive fitness such as mate choice and kin recognition. In humans, and generally in mammals, skin bacteria interacts with gene products of the major histocompatibility complex to determine the scent of an individual, which in turn influences partner preferences and siblings recognition (Boyse et al., 1987; Manning et al., 1992; Wedekind et al., 1995; Setchell et al., 2011). In arthropods, cuticular hydrocarbons are also used for olfactory-based interactions such as mate (Averhoff and Richardson, 1974, 1976; Singer, 1998; Ferveur, 2005) and kin recognition (for a review see Singer, 1998). Bacteria are known to affect the smell of an organism (Natsch et al., 2006), but, although the interplay between bacteria and the major histocompatibility complex in mammals is recognised, only recently have attempts focused on the effect of gut bacteria on olfactory-determined behaviours in arthropods. Eusocial insects are known to distinguish nest mates from non-nest mate conspecifics (reviewed in Vander Meer et al., 1998), and some of these nest mate recognition systems implicate gut bacteria. For example, differences in the composition of gut microbiota are evident between termites from different species (Inoue and Ushida, 2003) and from different colonies (Matsuura, 2001; Hongoh et al., 2005), with nest mates sharing a higher proportion of gut symbionts (Minkley et al., 2006). Following antibiotic treatment the ability of termites to distinguish nest mates according to gut microbiota was eliminated. The effect of diet upon the odour of the colony supports the hypothesis that gut microbiota is implicated in the mechanism by which termites recognise their nest mates.
In Drosophila sp., gut bacteria communities vary widely according to ecology, however, similarities may exist between individuals/species feeding on the same food source (Chandler et al., 2011). In Drosophila melanogaster, slight changes in the composition of cuticular hydrocarbons significantly alter mating success (Scott et al., 2011). The role of gut bacteria in mate choice was implicated by Dodd's (1989) observation that Drosophila pseudoobscura exhibited mate preference towards individuals that had developed on the same food as themselves. A recent study adapted Dodd's experiment and highlighted the importance of gut microbiota for the evolution of mate preference in D. melanogaster (Sharon et al., 2010). Adults expressed a strong homogametic preference in mates presenting similarities in their odours, and gut bacteria were directly implicated in the recognition process. In D. melanogaster, various studies have attempted to test its ability to both recognise kin and avoid inbreeding (Spiess, 1987; Mack et al., 2002; Robinson et al., 2009, 2012; Tan et al., 2012), but controversies remain. It is clear that mating with related individuals leads to inbreeding costs, but uncertainty remains on the costs/benefits associated with mating with individuals of varying degrees of relatedness. More importantly, D. melanogaster is a polyandrous species where females are known to remate 3–5 days after a mating bout (Markow, 1996, 2002). Polyandry has the potential to negate inbreeding costs and increases fitness benefits when females remate rapidly after a first mating with an inbred male, or if she can preferentially utilise more outbred sperm. This is unlikely to occur in less polyandrous species, particularly in monandrous species, as females will have few or no opportunities to remate, increasing the risk of inbreeding costs. Males can also decrease their reproductive investment in related females to limit the number of inbred offspring and/or the costs associated with inbreeding. Copulation duration is controlled by males in Drosophila (Bretman et al., 2009; Lizé et al., 2012a) and can be used as a proxy measure of male reproductive investment. The fitness benefits of longer copulation duration (greater reproductive investment) are evident for polyandrous species, increasing the success in sperm competition (Simmons et al., 1999; Wedell et al., 2002; Bretman et al., 2009, 2010, 2011; Barbosa, 2012). However, benefits and costs associated with copulation duration in monandrous species are less clear, as females will only mate once. Nevertheless, males of monandrous species alter copulation duration in response to cues of sperm competition (Lizé et al., 2012a) and could also do so when mating with a more or less related female.
We examined whether relatedness, familiarity and food eaten during development altered copulation investment (propensity to mate and copulation duration) in three species of Drosophila. We designed a three factorial experiment where first instar larvae originating from the same (sibling) or different (unrelated larvae) pairs of parents were placed in groups of 10 in the same or a different vial (familiarity) containing the same (ASG, cornmeal-based medium) or a different type of food (banana). Gut bacteria influence on reproductive investment was tested by altering the food eaten during development (for example, Sharon et al., 2010) and by adding antibiotics to the food media. Antibiotics such as streptomycin and tetracycline are known to alter gut bacteria communities (Koukou et al., 2006; Sharon et al., 2010) but the mechanism of action of these antibiotics remains unknown. For instance, they could alter the overall physiology of the fly, thereby ultimately impacting on gut bacteria. Although, potentially, the effects of antibiotics on overall fly physiology should be considered, the effects of antibiotics on D. melanogaster have been repeatedly shown not to alter mating propensity but only affect mating preferences (Koukou et al., 2006; Sharon et al., 2010). Here, we concentrated on species of Drosophila, varying both in mating system (monandrous and polyandrous) and ecology (solitary or group-living). In Drosophila subobscura, females only mate once in their lifetime (monandrous) (Maynard-Smith, 1956; Markow, 1996), and this species is therefore expected to have evolved a robust kin recognition system to avoid costs associated with mating with relatives. Drosophila bifasciata is polyandrous with 55% of females remating 9 days after their first mating (low polyandry) (Lizé et al., 2012b). D. bifasciata develops and feeds on sap fluxes, living in dense aggregations (Kimura et al., 1977). In this species, dispersal is likely to be limited or in groups (Kimura et al., 1977), which may have promoted the evolution of kin recognition (Hamilton, 1964). D. melanogaster is a generalist fruit fly species feeding on various fruit sources (Lutz, 1914; Evans, 1916; Jaenike, 1983; Lachaise et al., 1988; Miller et al., 2011), whose female are polyandrous, remating every 3–5 days (Markow, 1996, 2002). Although we expect some inbreeding costs in the monandrous species and the species living in dense aggregations, these costs are less clear and results are somewhat contradictory in D. melanogaster (Averhoff and Richardson, 1974, 1976; Tompkins and Hall, 1984; Spiess, 1987; Robinson et al., 2009, 2012; Ala-Honkola et al., 2011; Tan et al., 2012). Our study aims at testing simultaneously whether Drosophila have evolved kin recognition according to their ecologies and how this could be influenced by bacteria variations associated with food specificities.
Materials and methods
Fly stocks
Multi-female lines of D. subobscura and D. bifasciata were collected in 2008 from Vancouver Island (British Columbia, Canada) and in 2003 from the campus of Hokkaido University (Sapporo, Japan), respectively. Wild-type D. melanogaster flies were from an outbred population collected in Dahomey (Benin) in 1970 and have been maintained in the laboratory since that time. Flies were reared at 18 °C for D. subobscura, 22 °C for D. bifasciata and 25 °C for D. melanogaster in a 12/12 h light/dark cycle in standard plastic vials (23 mm by 73 mm) containing 15 ml of standard ASG food (for 1 l of water: 85 g of sugar, 60 g of corn, 20 g of yeast, 10 g of agar and 25 ml of nipagin (100 g l−1)).
Larval development and production of families
Singly mated females were placed in small cages upon banana medium, supplemented with Marmite and a yeast water mixture and allowed to oviposit. Larvae were collected from the banana plates and placed in family groups of 10 individuals in standard plastic vials containing 15 ml of either ASG food (as above) or banana medium (for 1 l of water: 137.5 g of banana, 47.5 g of sugar syrup, 30 g of malt, 27.5 g of yeast, 6.5 g of agar and 2 g of mould inhibitor). To create vials of unrelated individuals, a single larva from 10 different families was placed together in a vial. Maternal influence was controlled in these experiments as all larvae were isolated and transferred to a new vial for development without their mothers. Mothers could therefore not inoculate the medium with their own gut microbiota.
Removal of gut microbiota
Gut microbiota is expected to be similar in composition between individuals having developed in the same environment and/or on the same type of food (but in different environments). To test whether gut microbiota suppression altered copulation investment, fly media was supplemented with streptomycin (4 ml of 10 g streptomycin/100 ml ethanol solution per litre melted into the growth medium), an antibiotic known to alter gut bacteria communities (for example, Sharon et al., 2010). This procedure was followed when food and/or familiarity altered copulation investment, which was the case for D. melanogaster and D. bifasciata.
Mating trials
At eclosion, flies were collected and virgin individuals isolated using CO2 anaesthesia. Emergents were isolated twice a day and placed individually in vials containing the same food as the larval growth medium.
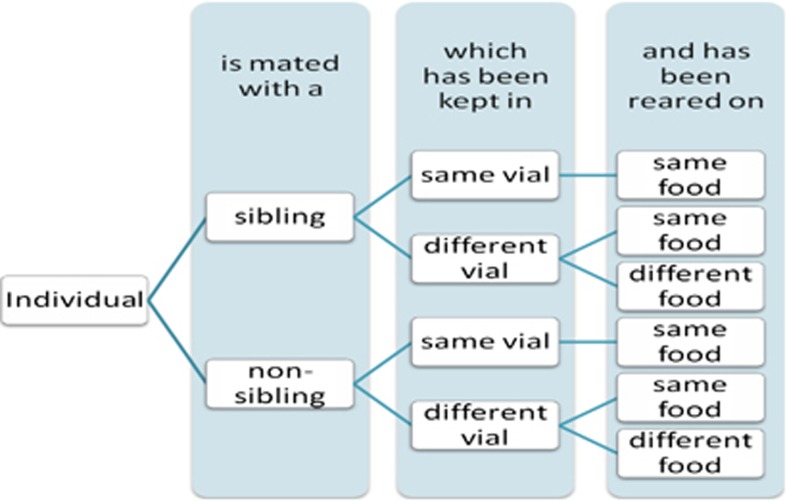
At sexual maturity (4 days after emergence for D. melanogaster and 7 days for D. subobscura and D. bifasciata), a male and female were placed by aspiration together into a vial containing 10 ml of a neutral medium (composed of sugar, agar and yeast). Mating pairs were assigned according to a three factorial design (Figure 1), where the sexual partner was either related or not, developed in the same or a different vial (environment) and reared on the same type of food or a different type of food (ASG and banana-based media), making six possible treatments (Figure 1).
Figure 1.
Schematic representation of fully factorial design showing the six treatments across which mating propensity and copulation investment was measured in the three Drosophila species.
Mating trials were conducted during 3 h in the morning as this is when Drosophila are most active in the wild (Hardeland, 1972). We recorded proportions of mating, latencies and copulation duration. Although copulation latencies did not vary in the experiment, copulation duration did and was used as a proxy for reproductive investment (Bretman et al., 2009). Sample sizes for each pairwise comparison for the three factors (relatedness, environment and food type) are reported in Table 1.
Table 1. Sample size for each pairwise comparison of the three factors (relatedness, environment and food type) for D. subobscura, D. bifasciata and D. melanogaster antibiotic treated flies (Streptomycin) or not (−).
| D. subobscura |
D. bifasciata |
D. melanogaster |
|||
|---|---|---|---|---|---|
| — | — | Streptomycin | — | Streptomycin | |
| Related | 61 | 157 | 23 | 89 | 68 |
| Unrelated | 65 | 106 | 14 | 69 | 67 |
| Same environment (vial) | 52 | 86 | 11 | 48 | 34 |
| Different environment | 74 | 177 | 26 | 110 | 101 |
| Same food | 93 | 200 | 25 | 101 | 83 |
| Different food | 33 | 63 | 12 | 57 | 52 |
Statistical analysis
All statistical analyses were performed using R 2.15.0 (Ihaka and Gentleman, 1996). All data that were not normally distributed (Shapiro tests) were square root transformed. Variances were homogenous (Bartlett test: P<0.05). Data were analysed using a generalised linear model procedure assuming (i) a binomial error distribution with a logit link function for differences in propensity to mate and (ii) a Gaussian distribution with an identity link function for copulation duration differences (McCullagh and Nelder, 1989). The maximal model including all relevant factors was created in each case and reduced by stepwise elimination of non-significant factors to the minimal adequate model using analysis of deviance. Throughout this paper, we report effect sizes (‘lsr' package from R) for copulation investment data using partial eta squared (ηρ2) (Nakagawa and Cuthill, 2007). Partial eta squared was calculated on the analysis of variance derived from the maximal model before being reduced by stepwise elimination to take into account all factors. Effect size, when measured as partial eta squared, is considered as small when around 0.01, medium around 0.06 and large around 0.14.
Results
Propensity to mate
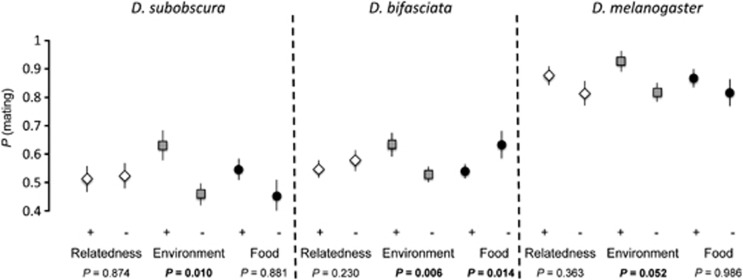
All three species were more prone to mate with a partner from the same environment (vial) (D. subobscura: χ2 test: LRT (likelihood ratio test) =6.54, 243 df (degrees of freedom), P=0.010, D. bifasciata: χ2 test: LRT=7.36, 462 df, P=0.006, D. melanogaster: χ2 test: LRT=3.77, 185 df, P=0.052) (Figure 2). The proportion of mating observed in D. subobscura and D. bifasciata was higher when the sexual partner came from the same vial environment, where they had fed on the same substrate and encountered each other previously. However, D. bifasciata sexual partners coming from different vials mated more easily with a sexual partner that had fed and developed on a different type of food (χ2 test: LRT=5.92, 328 df, P=0.014) (Figure 2). Relatedness and food eaten during development had no effect on propensity to mate across all three species (all P>0.05).
Figure 2.
Mating propensity of the three species of Drosophila in no choice three factorial design experiment, where relatedness, environment (familiarity) and food used during development varied. Error bars: s.e.
Copulation investment
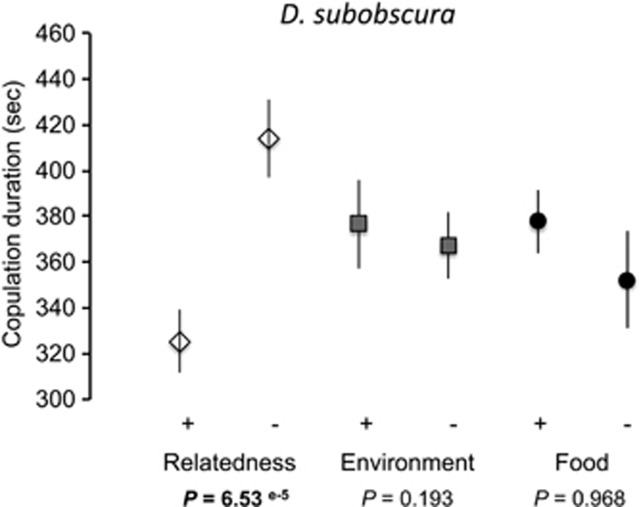
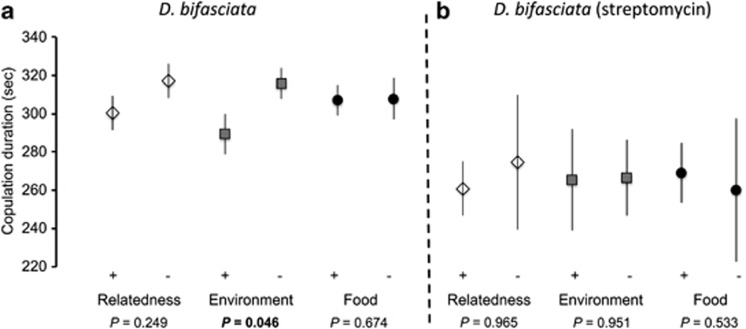
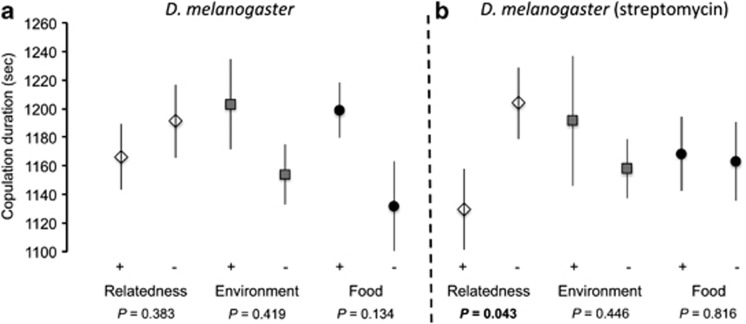
Despite the fact that all three species were more prone to mate with individuals coming from the same environment, investment in copulation differed between species. We found a significant decrease in copulation duration when partners were related in the monandrous species D. subobscura (F-test: F1,124 =17.08, P=8e-5, ηρ2=0.121), whereas there was no effect of environment of origin (familiarity and food type, all P>0.05) (Figure 3). In contrast, the two other Drosophila species which are polyandrous did not respond primarily to relatedness. D. bifasciata invested less in copulation when mating with a partner coming from the same environment (F-test: F1,261 =3.99, P=0.046, ηρ2=0.015) (Figure 4a). In D. melanogaster, food type significantly antagonistically interacted with relatedness to alter copulation duration (F-test: F3,154 =4.13, P=0.043, ηρ2=0.026) (Figure 5a).
Figure 3.
Copulation investment measured as average copulation duration in D. subobscura when sexual partners varied according to relatedness, environment they grew in (familiarity) and food they used to eat during their development. Error bars: s.e.
Figure 4.
Copulation investment measured as average copulation duration in D. bifasciata (a) when sexual partners varied according to relatedness, environment they grew in (familiarity) and food they used to eat during their development. The vial was also treated with streptomycin (b) to remove gut bacteria effects. Error bars: s.e.
Figure 5.
Copulation investment measured as average copulation duration in D. melanogaster (a) when sexual partners varied according to relatedness, environment they grew in (familiarity) and food they used to eat during their development. The vial was also treated with streptomycin (b) to remove gut bacteria effects. Error bars: s.e.
Gut bacteria effect on copulation investment
In an attempt to evaluate the effect of gut bacteria on copulation investment in Drosophila, food eaten during development and familiarity (potential exchange of gut bacteria between individuals) was also evaluated. Copulation investment was indeed altered when partners came from the same environment for D. bifasciata (familiarity) and D. melanogaster (through an interaction between food type and familiarity). To evaluate the implication of gut bacteria in the process, we removed their potential influence in these two species by adding antibiotic (streptomycin) to the medium in which the larvae developed.
Removal of gut bacteria cancelled the effect of familiarity in D. bifasciata (F-test: F5,31 =0.05, P=0.51, ηρ2=1.19e-4) (Figure 4b) and highlighted the significant effect of relatedness in D. melanogaster (F-test: F1,133 =3.85, P=0.043, ηρ2=0.030) (Figure 5b). In D. melanogaster, the food interaction with relatedness was removed as a result of streptomycin treatment (F-test: F5,129 =0.05, P=0.518, ηρ2=0.003) (Figure 5b).
Discussion
Our results provide the first evidence that varied ecologically determined mechanisms of kin recognition occur in Drosophila, and suggest that gut bacteria may have strong impacts on kin recognition systems. Despite the fact that all three species were more prone to mate with individuals coming from the same environment, investment in copulation differed across species. Copulation duration was altered by relatedness in the monandrous D. subobscura, familiarity in the group-living D. bifasciata and food eaten during development in the generalist species D. melanogaster. Both the familiarity effect in D. bifasciata and the food effect in D. melanogaster were removed through antibiotic treatment suggesting for the first time that gut bacteria can alter copulation investment in Drosophila.
In our no-choice mating trials, all three species were more prone to mate with a familiar partner, having developed in the same environment. Studies utilising choice tests both in the laboratory (Sharon et al., 2010) and in the field (Robinsons et al., 2009) have demonstrated that D. melanogaster prefers to mate with an individual coming from the same environment. In contrast, other studies have demonstrated female's preference to mate with unfamiliar males (Ödeen and Moray, 2008), or no effect of social familiarity on female mate choice (Tan et al., 2012). This could explain why in our study D. melanogaster propensity to mate with a familiar individual (coming from the same environment) is at the limit of the significance level. Further experiments are needed to understand the scenario of mate choice/preferences in D. melanogaster.
Our hypothesis that individuals should invest less in related sexual partners in monandrous species is supported here; D. subobscura reduced copulation duration when mating with a sibling. Monandrous species are expected to suffer higher costs of inbreeding and should thus be able to mitigate these costs. However, only males may benefit from this reduced investment in related monandrous females as these females will not remate. Males may thus save mating resources for future mating opportunities with unrelated males. In contrast, polyandry has been demonstrated to facilitate the avoidance of inbreeding costs in both vertebrates (for example, in house mice: Firman and Simmons, 2008) and invertebrates (for example in red flour beetles: Michalczyk et al., 2011). The observed reduction in investment in copulation duration between related partners in D. subobscura is to our knowledge the first empirical evidence of kin recognition based on relatedness in a Drosophila species. Although the functional significance of decreasing copulation duration in this species is unknown and has not been measured here, the specific response of males to related females at the point of copulation is evidence of kin recognition whether it is biologically adaptive or not. Kin recognition in D. subobscura is not based on familiarity as common environment, and thus repeated interactions between familiar larvae had no effect on copulation duration. Consequently, kin recognition in this species is likely based on phenotype matching, a process allowing the recognition of unfamiliar but related individuals (Gadagkar, 1985; Waldman, 1987; Waldman et al., 1988; Hepper, 1991; Holmes, 2004).
Investment in copulation was affected by familiarity in the group-living D. bifasciata. Familiarity refers to interactions between individuals regardless of genetic relatedness; any individual will become familiar if it is encountered often, whether or not it is a relative. Surprisingly, this effect was removed when larvae developed on a food medium containing the antibiotic streptomycin. This suggests that in D. bifasciata, sexual partners developing as larvae in the same environment recognise each other via a cue, likely odour, determined by gut flora. Gut bacteria used in this recognition process are not altered by variation of food resources eaten during the development; thus, bacteria involved are not subject to food alterations. However, copulation investment varied according to familiarity, as individuals coming from the same vial decreased their copulation investment. Moreover, this effect was cancelled when antibiotic was added to the food media, suggesting that the familiar recognition process is influenced by gut bacteria shared by individuals developing in the same vial. It is probable that adults developing as larvae on the same environment acquired their gut bacteria through ‘egg smearing' (that is, eggs are contaminated by the bacteria on the surface, and thus the hatched larvae acquire these bacteria by consuming or probing the eggshell) and ‘coprophagy' (that is, larvae developing on the same environment feed on the excrement of other larvae, thereby reacquiring and exchanging bacteria) (Dillon and Dillon, 2004). This scenario is strengthened by the particular ecology of D. bifasciata, which lives in dense aggregation on sap fluxes and where larvae may exchange bacteria. A mechanism based on the recognition of familiar individual makes sense for species where the probability of encountering related individuals is variable, and thus the potential costs of inbreeding unpredictable.
In D. melanogaster, food type influenced copulation investment of males, suggesting an important role of bacteria associated with food such as the gut bacteria. This result also allows disentangling potential effect of endosymbiotic bacteria as flies tested in this experiment were of identical origin (same laboratory population). The implication of gut bacteria in the reproductive investment of D. melanogaster is more complex than in D. bifasciata. Initially, D. melanogaster, which displays a high rate of dispersal and remating, two traits suggestive of decreased vulnerability to inbreeding depression was also found to be unable to recognise kin. However, the significant interaction between relatedness and food type prompted further investigation. Following the removal of gut microbiota via antibiotics and thus the food-type effect, D. melanogaster decreased copulation duration, and therefore reproductive investment, when mating with a related partner, thus exhibiting kin recognition. It seems that two types of cues are used by D. melanogaster to determine their investment in copulation, the first related to the food eaten during development, ultimately linked to gut bacteria alteration, and the second, relatedness. Our surprising result that gut bacteria masked kin recognition based on relatedness supports the idea that the risk of inbreeding may be low in this species (Tan et al., 2012) or that the costs associated with inbreeding are not high enough to promote the evolution and/or maintenance of inbreeding avoidance through kin recognition (Robinson et al., 2009; Ala-Honkola et al., 2011). This is also supported by the level of polyandry exhibited by this species, with females remating every3–5 days (Markow, 1996, 2002). Finally, this suggests that there could be a conflict of interest between investing in environmentally similar mates while limiting the investment in related individuals (Mack et al., 2002). Preference for environmentally similar mates accounts for the recent findings that gut bacteria specificity can drive assortative mating, and potentially divergence between populations (reviewed in Brucker and Bordenstein, 2012), and this appears to be the case in D. melanogaster (Sharon et al., 2010, 2011).
Our study has demonstrated that ecology determines kin recognition abilities across different Drosophila species. The reproductive system (monandry or polyandry), group-living behaviour (familiarity) and food eaten during development, all affect investment in reproduction at the point of copulation depending on species. Our results demonstrate for the first time that gut bacteria communities directly affect kin recognition. Gut bacteria alteration led to significant changes in reproductive investment, suggesting either that they are involved in mate recognition processes or that they generally alter the physiology of the flies. The use of antibiotics does not allow disentangling between these two explanations, and further experiments are needed. However, our results highlight the fact that diet and environmental conditions should be strictly controlled in sexual selection experiments using flies. Further studies evaluating the mechanisms underlying recognition based on gut bacteria variation, as well as how individual scents are chemically altered, would be highly valuable to enhance our understanding of recognition systems.
Acknowledgments
We are grateful to GDD Hurst and TAR Price and two anonymous reviewers for their useful comments on this manuscript.
The authors declare no conflict of interest.
Footnotes
AL and ZL designed the study, AL and RM performed the research, AL analysed the data, RM wrote the first draft of the manuscript and all authors contributed substantially to revisions.
References
- Ala-Honkola O, Manier MK, Lupold S, Pitnick S. No Evidence for postcopulatory inbreeding avoidance in Drosophila melanogaster. Evolution. 2011;65:2699–2705. doi: 10.1111/j.1558-5646.2011.01317.x. [DOI] [PubMed] [Google Scholar]
- Archie EA, Theis KR. Animal behaviour meets microbial ecology. Anim Behav. 2011;82:425–436. [Google Scholar]
- Averhoff WW, Richardson RH. Pheromonal control of mating patterns in Drosophila melanogaster. Behav Genet. 1974;4:207–225. doi: 10.1007/BF01074155. [DOI] [PubMed] [Google Scholar]
- Averhoff WW, Richardson RH. Multiple pheromone system controlling mating in Drosophila melanogaster. Proc Natl Acad Sci USA. 1976;73:591–593. doi: 10.1073/pnas.73.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa F. Males responding to sperm competition cues have higher fertilization success in a soldier fly. Behav Ecol. 2012;23:815–819. [Google Scholar]
- Boyse EA, Beauchamp GK, Yamazaki K. The genetics of body scent. Trends Genet. 1987;3:97–102. [Google Scholar]
- Bretman A, Fricke C, Chapman T. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. P Roy Soc Lond B. 2009;276:1705–1711. doi: 10.1098/rspb.2008.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretman A, Fricke C, Hetherington P, Stone R, Chapman T. Exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav Ecol. 2010;21:317–321. [Google Scholar]
- Bretman A, Gage MJG, Chapman T. Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol Evol. 2011;26:467–473. doi: 10.1016/j.tree.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Brucker RM, Bordenstein SR. Speciation and symbiosis. Trends Ecol Evol. 2012;27:443–451. doi: 10.1016/j.tree.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Chandler JA, Morgan Lang J, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host–microbe model system. PLoS Genet. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Dodd DMB. Reproductive isolation as a consequence of adaptive divergence in Drosophila pseudoobscura. Evolution. 1989;43:1308–1311. doi: 10.1111/j.1558-5646.1989.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Evans AT. Some observations on the breeding habits of the common house-fly. J Econ Entomol. 1916;9:354–362. [Google Scholar]
- Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- Firman RC, Simmons LW. Polyandry, sperm competition, and reproductive success in mice. Behav Ecol. 2008;19:695–702. [Google Scholar]
- Gadagkar R. Kin recognition in social insects and other animals. A review of recent finding and consideration of their relevance for the theory of kin selection. Proc Indian Acad Sci -Anim Sci. 1985;94:587–621. [Google Scholar]
- Gorman ML, Nedwell DB, Smith RM. Analysis of contents of anal scent pockets of Herpestes auropunctatus (Carnivora-Viverridae) J Zool. 1974;172:389–399. [Google Scholar]
- Gorman ML. A mechanism for individual recognition by odour in Herpestes auropunctatus (Carnivora: Viverridae) Anim Behav. 1976;24:141–145. [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I, II. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Species differences in the diurnal rhythmicity of courtship behaviour within the melanogaster group of the genus Drosophila. Anim Behav. 1972;20:170–174. doi: 10.1016/s0003-3472(72)80188-x. [DOI] [PubMed] [Google Scholar]
- Hepper PG. Kin Recognition. Cambridge university Press: Cambridge, UK; 1991. [Google Scholar]
- Holmes WG. The early history of Hamiltonian based research on kin recognition. Ann Zool Fenn. 2004;41:691–711. [Google Scholar]
- Hongoh Y, Deevong P, Inoue T, Moriya S, Trakulnaleamsai S, Ohkuma M, et al. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl Environ Microbiol. 2005;71:6590–6599. doi: 10.1128/AEM.71.11.6590-6599.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- Inoue R, Ushida K. Vertical and horizontal transmission of intestinal commensal bacteria in the rat model. Fems Microbiol Ecol. 2003;46:213–219. doi: 10.1016/S0168-6496(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Jaenike J. Induction of host preference in Drosophila melanogaster. Oecologia. 1983;58:320–325. doi: 10.1007/BF00385230. [DOI] [PubMed] [Google Scholar]
- Kimura MT, Toda MJ, Beppu H, Watabe H. Breeding sites of Drosophilid flies in and near Sapporo, Northern Japan, with supplementary notes on the adult feeding habits. Kontyû. 1977;45:571–582. [Google Scholar]
- Koch H, Schmid-Hempel P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc Natl Acad Sci USA. 2011;108:19288–19292. doi: 10.1073/pnas.1110474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukou K, Pavlikaki H, Kilias G, Werren JH, Bourtzis K, Alahiotis SN. Influence of antibiotic treatment and Wolbachia curing on sexual isolation among Drosophila melanogaster cage populations. Evolution. 2006;60:87–96. [PubMed] [Google Scholar]
- Kuhn F, Natsch A. Body odour of monozygotic human twins: a common pattern of odorant carboxylic acids released by a bacterial aminoacylase from axilla secretions contributing to an inherited body odour type. J R Soc Interface. 2009;6:377–392. doi: 10.1098/rsif.2008.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaise D, Cariou ML, David JR, Lemeunier F, Tsacas L, Ashburner M. Historical biogeography of the Drosophila melanogaster species subgroup. Evol Biol. 1988;22:159–225. [Google Scholar]
- Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizé A, Doff RJ, Smaller EA, Lewis Z, Hurst GDD. Perception of male-male competition influences Drosophila copulation behaviour even in species where females rarely remate. Biol Lett. 2012a;8:35–38. doi: 10.1098/rsbl.2011.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizé A, McKay R, Lewis Z. Gut microbiota and kin recognition. Trends Ecol Evol. 2013;28:325–326. doi: 10.1016/j.tree.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Lizé A, Price TAR, Marcello M, Smaller EA, Lewis Z, Hurst GDD. Males do not prolonge copulation duration in response to competitor males in the polyandrous fly Drosophila bifasciata. Physiol Entomol. 2012b;37:227–232. [Google Scholar]
- Lutz FE. Biological notes concerning Drosophila ampelophila. J N Y Entomol Soc. 1914;22:134–138. [Google Scholar]
- Mack PD, Hammock BA, Promislow DEL. Sperm competitive ability and genetic relatedness in Drosophila melanogaster: similarity breeds contempt. Evolution. 2002;56:1789–1795. doi: 10.1111/j.0014-3820.2002.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Manning CJ, Wakeland EK, Potts WK. Communal nesting patterns in mice implicate MHC genes in kin recognition. Nature. 1992;30:581–583. doi: 10.1038/360581a0. [DOI] [PubMed] [Google Scholar]
- Markov AV, Lazebny OE, Goryacheva II, Antipin MI, Kulikov AM. Symbiotic bacteria affect mating choice in Drosophila melanogaster. Anim Behav. 2009;77:1011–1017. [Google Scholar]
- Markow TA. Evolution of Drosophila mating systems. Evol Biol. 1996;29:73–106. [Google Scholar]
- Markow TA. Female remating, operational sex ratio, and the arena of sexual selection in Drosophila. Evolution. 2002;59:1725–1734. doi: 10.1111/j.0014-3820.2002.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Matsuura K. Nestmate recognition mediated by intestinal bacteria in a termite, Reticulitermes speratus. Oikos. 2001;92:20–26. [Google Scholar]
- Maynard-Smith J. Fertility, mating behaviour and sexual selection in Drosophila subobscura. J Genet. 1956;54:261–279. doi: 10.1007/BF02715886. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA.1989Generalized Linear Models2nd edn.Chapman & Hall: London, UK [Google Scholar]
- Michalczyk L, Millard AL, Martin OY, Lumley AJ, Emerson BC, Chapman T, et al. Inbreeding promotes female promiscuity. Science. 2011;333:1739–1742. doi: 10.1126/science.1207314. [DOI] [PubMed] [Google Scholar]
- Miller PM, Saltz JB, Cochrane VA, Marcinkowski CM, Mobin R, Turner TL. Natural variation in decision-making behavior in Drosophila melanogaster. PLoS One. 2011;6:e16436. doi: 10.1371/journal.pone.0016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley N, Fujita A, Brune A, Kirchner WH. Nest specificity of the bacterial community in termite guts (Hodotermes mossambicus) Insectes Soc. 2006;53:339–344. [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Natsch A, Derrer S, Flachsmann F, Schmid J. A Broad diversity of volatile carboxylic acids, released by a bacterial aminoacylase from axilla secretions, as candidate molecules for the determination of human-body odor type. Chem Biodiv. 2006;3:1–20. doi: 10.1002/cbdv.200690015. [DOI] [PubMed] [Google Scholar]
- Ödeen A, Moray CM. Drosophila melanogaster virgins are more likely to mate with strangers than familiar flies. Naturwissenschaften. 2008;95:253–256. doi: 10.1007/s00114-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Kennington WJ, Simmons LW. No evidence for optimal fitness at intermediate levels of inbreeding in Drosophila melanogaster. Biol J Linn Soc. 2009;98:501–510. [Google Scholar]
- Robinson SP, Kennington WJ, Simmons LW. Assortative mating for relatedness in a large naturally occurring population of Drosophila melanogaster. Evol Biol. 2012;25:716–725. doi: 10.1111/j.1420-9101.2012.02466.x. [DOI] [PubMed] [Google Scholar]
- Scott D, Shields A, Straker M, Dalrymple H, Dhillon PK, Harbinder S. Variation in the male pheromones and mating success of wild caught Drosophila melanogaster. PloS One. 2011;6:e23645. doi: 10.1371/journal.pone.0023645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell JM, Vaglio S, Abbott KM, Moggi-Cecchi J, Boscaro F, Pieraccini G, et al. Odour signals major histocompatibility complex genotype in an Old World monkey. P Roy Soc Lond B. 2011;278:274–280. doi: 10.1098/rspb.2010.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. P Natl Acad Sci USA. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Segal D, Zilber-Rosenberg I, Rosenberg E. Symbiotic bacteria are responsible for diet-induced mating preference in Drosophila melanogaster, providing support for the hologenome concept of evolution. Gut Microbes. 2011;2:190–192. doi: 10.4161/gmic.2.3.16103. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Parker GA, Stockley P. Sperm displacement in the yellow dung fly, Scatophaga stercoraria: an investigation of male and female processes. Am Nat. 1999;153:302–314. doi: 10.1086/303171. [DOI] [PubMed] [Google Scholar]
- Singer TL. Roles of hydrocarbons in the recognition systems of insects. Amer Zool. 1998;38:394–405. [Google Scholar]
- Singh PB, Herbert J, Roser B, Arnott L, Tucker DK, Brown RE. Rearing rats in a germ-free environment eliminates their odors of individuality. J Chem Ecol. 1990;16:1667–1682. doi: 10.1007/BF01014099. [DOI] [PubMed] [Google Scholar]
- Spiess EB.1987Discrimination among prospective mates in DrosophilaIn: Fletcher DJC, Michener CD (eds)Kin recognition in animals John Wiley and Sons: Chichester, UK; 75–119. [Google Scholar]
- Tan CKW, Løvlie H, Pizzari T, Wigby S. No evidence for precopulatory inbreeding avoidance in Drosophila melanogaster. Anim Behav. 2012;83:1433–1441. [Google Scholar]
- Tompkins L, Hall JC. Sex pheromones enable Drosophila males to discriminate between conspecific females from different laboratory stocks. Anim Behav. 1984;32:349–352. [Google Scholar]
- Vander Meer RK, Breed MD, Winston ML, Espelie KE. Pheromone communication in social insects: ants, wasps, bees and termites. Westview: Boulder, CO, USA; 1998. [Google Scholar]
- Waldman B. Mechanisms of kin recognition. J Theor Biol. 1987;128:159–185. [Google Scholar]
- Waldman B, Frumhoff PC, Sherman PW. Problems of kin recognition. Trends Ecol Evol. 1988;3:8–13. doi: 10.1016/0169-5347(88)90075-4. [DOI] [PubMed] [Google Scholar]
- Wedekind C, Seebeck T, Bettens F, Paeke AJ. MHC-dependent mate preferences in humans. P Roy Soc Lond B. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- Wedell N, Gage MJG, Parker GA. Sperm competition, male prudence and sperm limited females. Trends Ecol Evol. 2002;17:313–320. [Google Scholar]