Abstract
Mycorrhizal fungi have a key role in nitrogen (N) cycling, particularly in boreal and temperate ecosystems. However, the significance of ectomycorrhizal fungal (EMF) diversity for this important ecosystem function is unknown. Here, EMF taxon-specific N uptake was analyzed via 15N isotope enrichment in complex root-associated assemblages and non-mycorrhizal root tips in controlled experiments. Specific 15N enrichment in ectomycorrhizas, which represents the N influx and export, as well as the exchange of 15N with the N pool of the root tip, was dependent on the fungal identity. Light or water deprivation revealed interspecific response diversity for N uptake. Partial taxon-specific N fluxes for ectomycorrhizas were assessed, and the benefits of EMF assemblages for plant N nutrition were estimated. We demonstrated that ectomycorrhizal assemblages provide advantages for inorganic N uptake compared with non-mycorrhizal roots under environmental constraints but not for unstressed plants. These benefits were realized via stress activation of distinct EMF taxa, which suggests significant functional diversity within EMF assemblages. We developed and validated a model that predicts net N flux into the plant based on taxon-specific 15N enrichment in ectomycorrhizal root tips. These results open a new avenue to characterize the functional traits of EMF taxa in complex communities.
Keywords: 15N labeling, beech (Fagus sylvatica), drought, modeling, mycorrhiza, shade
Introduction
The roots of most plant species are associated with mycorrhizal fungi that mediate nutrient exchange between the plants and soil and thus have a central role in biogeochemical cycles (Finlay, 2008). In temperate and boreal forests, fungi that form ectomycorrhizas are the dominant symbiotic life form. Ectomycorrhizal fungi (EMF) encase colonized root tips with a dense hyphal net, termed the mantle, and forage the soil for nutrients by extending extraradical hyphae or hyphal cords (Finlay, 2008). As nitrogen (N) is a major limiting nutrient in many forest ecosystems (LeBauer and Treseder, 2008), the role of EMF in the N nutrition of trees has received considerable attention (Hobbie and Hobbie, 2008; Hobbie and Högberg, 2012). In addition to N delivery, recent studies have suggested that EMF may also limit N transfer to host trees under N-limiting conditions (Näsholm et al., 2013).
Although it has been well established that EMF have key roles in plant nutrition, much less is known about the functions of distinct fungal taxa within complex ectomycorrhizal assemblages for nutrient acquisition and host supply. Ectomycorrhizal communities are usually composed of a diverse flora consisting of several dominant and many infrequent EMF species (Buée et al., 2007; Courty et al., 2010; Pena et al., 2010; Lang et al., 2011; Tedersoo et al., 2012a; Danielsen et al., 2013). EMF community structures are strongly influenced by N deposition (Lilleskov et al., 2011; Kjøller et al., 2012). Stable isotope studies have revealed that EMF species differ in their abilities to exploit different N sources (Hobbie and Högberg, 2012). Furthermore, in situ ectomycorrhizal communities exhibit strong temporal differences in the capability of different EMF taxa to access litter-derived N (Pena et al., 2013a). The experimental manipulation of EMF diversity has shown context-dependent effects for fungal mixtures on plant biomass production and N nutrition (Chu-Chou and Grace, 1985; Jonsson et al., 2001). As the mechanistic concepts that explain the interactions between different EMF taxa in complex assemblages are still missing, the functional relevance of EMF identities for tree nutrition remains enigmatic. Elucidating functional diversity is important for understanding the role of ectomycorrhizal fungi in biogeochemical cycles in a fluctuating environment.
Our study aimed to attribute functions for N acquisition to ectomycorrhizal species identities in root-associated assemblages and uncover taxon-specific responses to environmental stress factors. We used young beech (Fagus sylvatica L.) trees, which are the major tree species of the natural vegetation in Central European temperate forests (Ellenberg and Strutt, 2009). The current beech forest distribution range is endangered as a result of drought stress because of climate change (Weber et al., 2013). Beech trees are tolerant of deep shade in the youth phase (Ellenberg and Strutt, 2009); however, shade-induced carbon limitations have a negative impact on EMF colonization (Druebert et al., 2009). Here, we conducted controlled experiments with beech seedlings cultivated in natural forest soil to develop characteristic EMF communities. The mycorrhizal trees were subsequently grown in sand to permit analysis of intact root systems and supplied with ammonium (NH4+) concentrations similar to those found in beech forest soils (median 0.5 mmol NH4+ kg−1 soil; range: 0.05–2 mmol NH4+ kg−1 soil; Gessler et al., 2005; Göransson et al., 2006; Dannenmann et al., 2009; Andreasson et al., 2012). Subsets of the plants were exposed to full light or shade according to the characteristic light climate in beech forests (median: 150 μmol PAR m−2 s−1, range 25–255 μmol PAR m−2 s−1; Kreuzwieser et al., 1997; Lemoine et al., 2002; Mayer et al., 2002; Fotelli et al., 2003; Gessler et al., 2005; Hertl et al., 2012). Light and shade treatments were combined with sufficient irrigation or water shortage to mimic typical environmental stresses. N acquisition was measured after the application of 15N in root tips associated with distinct EMF species and non-mycorrhizal root tips. We tested the hypotheses that (i) ectomycorrhizal assemblages show taxon-specific differences for NH4+ acquisition and (ii) environmental stress results in functional shifts in EMF species for N acquisition. Partial fluxes for EMF-associated root tips were assessed with whole-plant N uptake for mycorrhizal and non-mycorrhizal plants. We provide evidence that the taxon-specific 15N enrichments in EMF-associated root tips can be used to predict net N flux into the host plant. These results provide a basis for testing functional redundancy and response diversity of EMF assemblages in future field studies.
Materials and methods
Plant cultivation and experimental treatments
Fungicide-treated beech nuts (Fagus sylvatica L., provenance: Forstsaatgutstelle Oerrel, Niedersachsen, Germany) were grown in sterilized or untreated forest soil in individual pots as described previously (Pena et al., 2013b). Ah horizon soil (20 cm depth) collected in the Tuttlingen beech forest (latitude 47°59′N, longitude 8°45′E) was used. The germinated seedlings were maintained in a greenhouse under ambient conditions (20 °C, 55% air humidity) with additional light to achieve a 16-h photoperiod with 200 μmol PAR m−2 s−1 at plant height (lamps series 3071, Schuch, Worms, Germany). After 4 months, eight seedlings per treatment were evaluated for their mycorrhizal status showing that roots of seedlings in untreated soil were 40±4% colonized by EMF, whereas seedlings in sterilized soil were non-mycorrhizal. A total of 120 mycorrhizal and 120 non-mycorrhizal beech seedlings were transplanted without adherent soil individually into 660 ml pots with a sand-peat mixture and supplied daily with 56 ml of nutrient solution containing 0.4 mM NH4+ as the sole N source. This concentration was chosen because it was similar to that in forest soil of the Tuttlingen site (Dannenmann et al., 2009), well above Km values for NH4+ uptake of various EMF (0.005–0.25 mM; Jongbloed et al., 1991; Eltrop and Marschner, 1996) and because preceding analysis with attached beech roots at the Tuttlingen site showed saturation of NH4+ uptake at concentrations above 0.05 mM (Gessler et al., 2005). The seedlings were grown either in full light (200 μmol PAR m−2 s−1) or in the shade (35–40 μmol PAR m−2 s−1 at plant height). The light climate was chosen according to the conditions in thinned and unthinned beech plots in the Tuttlingen forest with mean seasonal light levels of 176 and 25 μmol PAR m−2 s−1, respectively (Gessler et al., 2005). After 2 months, the irrigation solution was reduced to 37% for half of the plants grown under each light regime. After 16 days, when the well-irrigated plants had a predawn leaf water potential of −0.36±0.02 MPa and those subjected to a limited water supply had a predawn leaf potential of −1.34±0.06 MPa, the plants were harvested (Pena et al., 2013b). During the last 3 days before harvest, each beech seedling received a total of 1.864 mg of N in a solution of either non-labeled NH4Cl or 15NH4Cl (99 atom %, Cambridge Isotope Laboratories, Inc., Hampshire, UK). Biomass and whole plant 15N data were determined (Pena et al., 2013b). Ten 15N labeled and three non-labeled plants per treatment were randomly selected and used for the analyses.
EMF identification and quantification
For each growth regimen, the whole root system of 10 beech seedlings per treatment was inspected using a binocular microscope (Leica M205 FA, Leica Microsystems, Wetzlar, Germany). Within each sample, the root tips were assigned to one of the following fractions: vital ectomycorrhizal (EM), vital non-ectomycorrhizal (NM), dead ectomycorrhizal (DM) and dead non-ectomycorrhizal (DR). Live and dead EM or NM root tips were distinguished as previously described (Downes et al., 1992; Winkler et al., 2010). Vital EM root tips were classified using the morphotyping key of Agerer (1987–2012). The abundance of each morphotype was quantified in the whole root system. The morphotypes were photographed (Leica DFC 420 C, Leica Microsystems), and a scale bar was used to determine ectomycorrhizal lengths. For anatomical analysis, the morphotypes were embedded in styrene-methacrylate (Ducic et al., 2008). Cross-sections with a thickness of 1 μm were cut with an autocut microtome (Ultracut E, Reichert-Jung, Vienna, Austria) and used to measure mantle thickness (Pena et al., 2013a).
Approximately 20 root tips from each morphotype were collected, and aliquots were used for the molecular identification of fungal species by ITS sequencing as previously described (Lang et al., 2011). The sequences obtained were assigned to fungal taxa by BLAST searches carried out against public databases (National Center for Biotechnology Information (NCBI), http://www.ncbi.nlm.nih.gov/ and UNITE, http://unite.ut.ee). The sequences were deposited in NCBI GenBank under the accession numbers HM748636–HM748643 (Supplementary Figure S1).
C, N and 15N measurements in EMF-colonized root tips
Among the fungal species colonizing the beech roots, five were sufficiently abundant for each treatment to be collected for isotope analysis. Depending on fungal morphology, 20 (Tomentella punicea) to 60 root tips (Cenococcum geophilum) were required for a suitable sample for isotope analysis. One replicate comprised the root tips obtained from one individual plant. EM morphotypes, NM, DM and DR root tips of each plant were cut under the binocular microscope. Two different sets of instruments were used to handle non-labeled and labeled plants to avoid cross contamination. EM tips were excised at the last lateral root ramification ensheathed by the hyphal mantle; NM tips were sampled at the youngest and active ‘white' zone (Evert and Eichhorn, 2007). Dead root tips with a shrunken and dry appearance were cut at the same position as the NM tips. The samples were freeze-dried.
Freeze-dried root tips were weighed (0.2–1.1 mg) using a super-micro balance (S4, Sartorius, Göttingen, Germany). Total C, N and 15N concentrations in root tips were determined with an isotope ratio mass spectrometer (IRMS Deltaplus, Thermo Finnigan Mat, Bremen, Germany) coupled to an elemental analyzer (EA 1108, Fisons, Rodano, Italy). The relative 15N abundance was expressed as the following ratio: 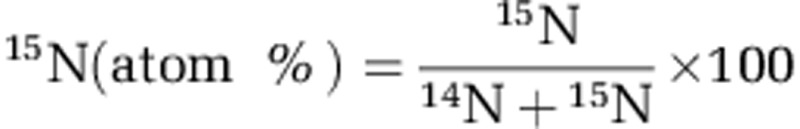
15N content per root tip was calculated as follows:
15N content (ng)=(biomass (g) × N concentration (ng g−1) × 15N atom % excess)/100,
where the biomass represents the mean dry mass of one root tip as determined for each EM fungal species, and 15N atom % excess=15N atom % labeled−15N atom % unlabeled, where labeled and unlabeled refer to samples obtained from plants exposed to 15N and unlabeled nutrient solutions, respectively.
The calculation of partial fluxes and prediction of total N flux is described in the Supplementary information SI1.
Statistical analysis
Statistical analysis was performed using Statgraphics Plus 3.0 (StatPoint, Inc., St Louis, MO, USA). When necessary, data were logarithmically or square root transformed to satisfy the criteria of normal distribution and homogeneity of variance. When transformation of the data did not meet these requirements, the Kruskal–Wallis and Mann–Whitney U-tests were applied instead of analysis of variance. Means or medians were considered to be significantly different from each other when P⩽0.05.
The Shannon–Wiener index of diversity (H′) was calculated with the following equation (Shannon and Weaver, 1949):
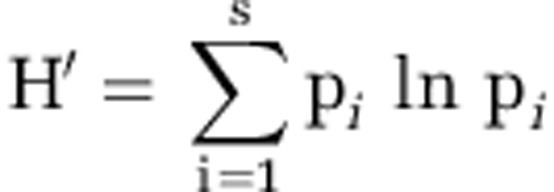
where S is the number of species in the sample and pi is the proportion of species i in the sample.
Evenness was calculated as follows:
E=H′/H(max)
with Hmax=ln (S).
The effects of stress treatments on EMF community composition were analyzed by principal component analysis using the free PAST software package 2.17c (http://folk.uio.no/ohammer/past/, Hammer et al., 2001). A variance–covariance matrix, which centers the data and in which the treatments were incorporated as categorical nominal variables, was used to perform principal component analysis (Jolliffe, 1986). The analysis was followed by a multiple analysis of variance to detect differences between species.
Results
The EM community structure in response to irradiance and water availability
The vital root tips of beech seedlings were 42±4% colonized with 10 different EMF species, regardless of light (F=2.22, P=0.144) or drought treatment (F=2.16, P=0.150). The most abundant species (T. punicea, Cenococcum geophilum, Tuber rufum, Tuber sp.1 and Tuber sp.2) were associated with approximately 95% of colonized root tips, whereas the other detected fungal species (Tomentella badia, unknown EMF (Telephoraceae), Sebacina sp., Cortinarius sp., Tomentella viridula) colonized <5% of the EM root tips (Supplementary Figure S2). All fungal species were previously identified in natural assemblages of beech EMF in the same forest from which the present soil inoculum was used (Pena et al., 2010).
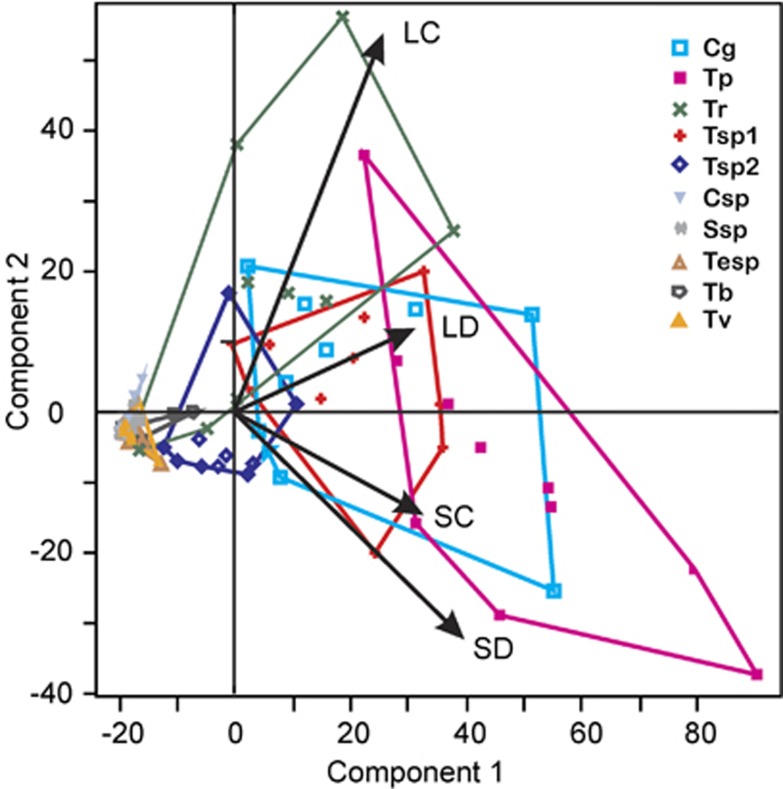
Principal component analysis revealed shifts in the EMF community structure under light or shade with sufficient or limited water availability (Figure 1). The first two components of the principal component analysis represented 96% of the variation in EMF species abundance in response to drought and shade (Supplementary Table S1a). All variables (LC, LD, SC and SD) were highly loaded onto PC1 (0.850<r<0.951), with vectors of similar lengths and directions suggesting that all sets of variables were correlated and equally important in explaining variation in EMF abundance. LC treatment was the main source of variation for PC2 (r=0.472). The minimum hulls revealed the contrasting influence of light and shade on fungal abundance. Multiple analysis of variance confirmed differences between fungal species (F=60.5, Pspecies<0.001) and revealed significant interaction between light and EMF species (F=2.7, P(species x light)=0.005, Supplementary Table S1b); T. rufum increased in full light (LC), whereas T. punicea and Tuber sp. 1 increased in response to shade (Figure 1). The abundance shifts in EM fungal species composition did not affect the Shannon diversity index H′ or Evenness of the assemblages (Supplementary Table S2).
Figure 1.
Biplot of the principal component analysis (PCA) ordination for similarities in the relative abundances of ectomycorrhizal fungal species associated with the roots of young beech trees (Fagus sylvatica). LC=full light, well-irrigated; LD=full light, drought; SC=shade, well-irrigated; SD=shade, drought. The abbreviations refer to the following fungi: Cg, Cenococcum geophilum; Tp, Tomentella punicea; Tr, Tuber rufum; Tsp1, Tuber sp.1; Tsp2, Tuber sp.2; Csp, Cortinarius sp.; Ssp, Sebacina sp.; Tesp, Telephoraceae; Tb, Tomentella badia; Tv, Tomentella viridula. Species clusters are indicated by minimum hulls.
Fungal species identities determine ectomycorrhizal N acquisition in response to environmental stress
We reasoned that specific N enrichment (measured as 15N atom–% above natural abundance) is the result of N influx and export, as well as the exchange of 15N with the N pool of the root tip. Therefore, specific N enrichment is an indicator of the physiological activity of N metabolism in a given type of root tip if it exceeds the 15N enrichment of dead root tips (DR and DM) and natural abundance of 15N. In DM and DR, the 15N signature was significantly higher than the natural 15N abundance (0.3661±0.0002; Figure 2). This unexpected enrichment in DR or DM might have been caused by soaking with the nutrient solution, by very low physiological activity of root tips or root tips that were initially active but died during the labeling phase or by activities of microfungi and bacteria colonizing the mycorrhizosphere (Heinonsalo et al., 2001; Calvaruso et al., 2007; Izumi and Finlay, 2011). Only 15N enrichment that was above the DR and DM threshold was considered to reflect active 15N uptake.
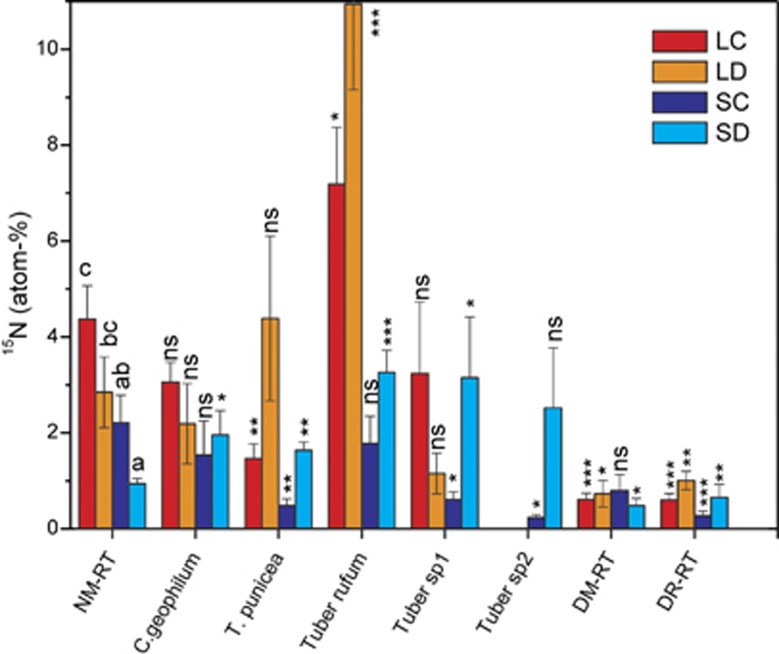
Figure 2.
The specific 15N enrichment of young beech root tips (RT) colonized by different ectomycorrhizal fungal species (Cenococcum geophilum, Tuber rufum, Tomentella punicea, Tuber sp 1 and Tuber sp 2). Data show the means (n=5±s.e., except n=4 for T. rufum and Tuber sp.2 under SC, n=3 for T. rufum under SD and n=10 for NM and DR). Different letters for NM-RT indicate significant differences at P⩽0.05 between treatments. The asterisks indicate significant differences (*P<0.05, **P<0.01, ***P<0.001) compared with NM-RT. DM, dry ectomycorrhizal; DR, dry non-mycorrhizal; NM, non-mycorrhizal; LC, full light, well-irrigated; LD, full light, drought; SC, shade, well-irrigated; SD, shade, drought; NS, not significant.
The specific 15N enrichment of vital NM root tips of non-mycorrhizal beech trees was similar to that of NM root tips of inoculated beeches (P=0.228, means shown in Figure 2) and well above that of dead root tips (Figure 2). Specific 15N enrichment in NM root tips decreased in response to drought and shade compared with well-irrigated and irradiated plants (Figure 2), indicating that the physiological activity of NM root tips is highly sensitive to environmental stress.
The specific 15N enrichment of root tips colonized with C. geophilum was similar to that of NM root tips under LC conditions but was unresponsive to stress and therefore exceeded the 15N enrichment of NM root tips under low light and low water availability (Figure 2).
Notably, the specific 15N enrichment of root tips from well-irrigated beech plants colonized with T. punicea was even lower than that of NM root tips, regardless of the light level, and almost as low as in DR (Figure 2). This finding suggests that EMF formed with T. punicea did not significantly participate in active N acquisition under LC and SC conditions (Figure 2). However, in drought-stressed beech plants, 15N accumulation in T. punicea-colonized root tips was increased moderately in LD root tips and strongly in SD root tips and therefore significantly exceeded the 15N enrichment of dead or NM root tips (Figure 2).
Root tips colonized with Tuber sp. 1 and Tuber sp. 2 behaved similarly to those colonized with T. punicea (Figure 2). In shaded, well-irrigated plants, the specific 15N enrichment of the Tuber-colonized root tips was similar to that of DR but was significantly enriched when the shaded plants were drought stressed (Figure 2).
The strongest specific 15N enrichment was found in root tips of light-exposed beech plants colonized with T. rufum, particularly when the plants were exposed to drought stress (Figure 2). However, in contrast with the other two Tuber species, the physiological activity of T. rufum-colonized root tips was very sensitive to shade (P=0.03).
We repeated the experiment under stronger drought stress and confirmed the behavior of the EMF species with regard to the 15N enrichment in response to the stress treatments (Supplementary Figure S3).
To test whether the 15N enrichment of ectomycorrhizas was related to fungal biomass, the fungal mantle volumes of distinct EMF species were calculated and related to the total amount of 15N in colonized root tips. A negative relationship was found between the amount of 15N in root tips and the fungal mantle volume, suggesting that thicker fungal mantle structures led to a dilution rather than an enrichment of the 15N label (Supplementary Figure S4). Greater 15N enrichment is therefore not a result of greater ectomycorrhizal biomass.
Modeling plant N uptake by partial N flux assessment for different ectomycorrhizal taxa
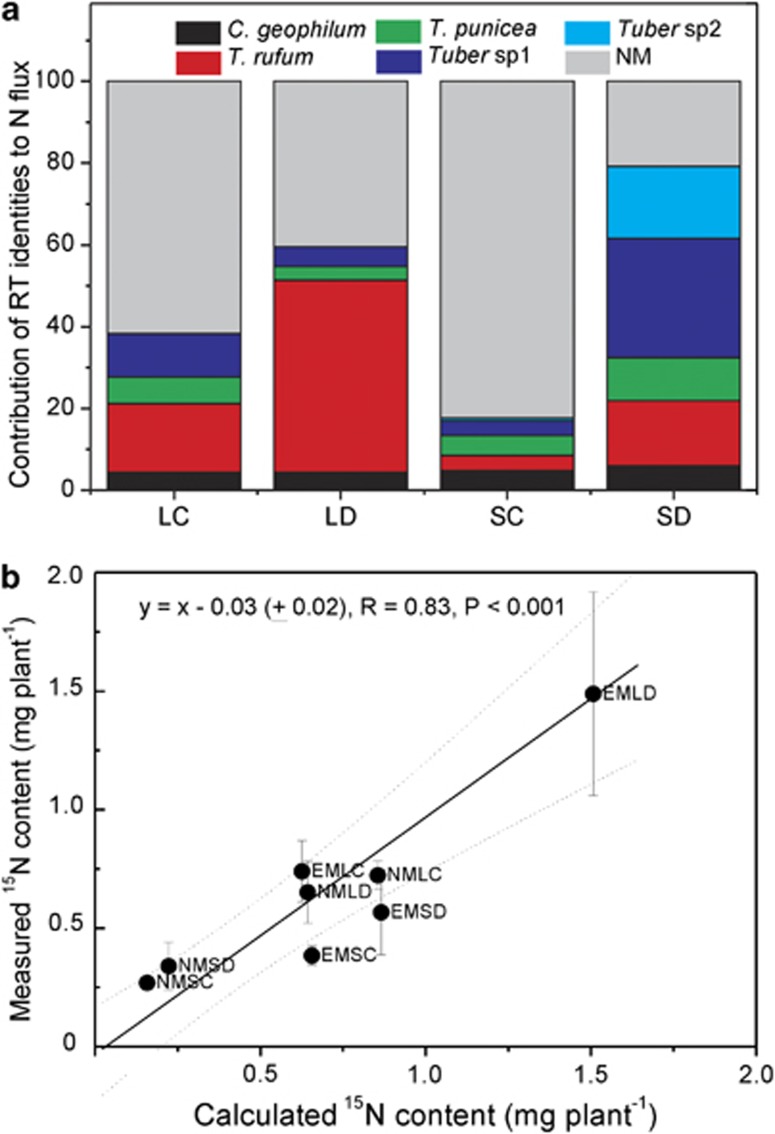
As the beech plants received 15N-labeled ammonium as the sole N source during the last 3 days before harvest, the 15N content of the entire plant was the result of the net N flux through all active root tips during this time period. We assumed that the formation of new root tips and death of old root tips were negligible within our 3-day labeling period. Based on the total number of vital root tips and whole plant 15N content, we calculated mean fluxes of 3.3 and 1.6 ng N h−1 root tip−1 for well-irradiated and shaded plants, respectively (Supplementary Table S3). However, the previous analyses of specific 15N enrichment (Figure 2) implied that not all vital root tips were actively involved in N metabolism to the same extent under different experimental treatments. We reasoned that it should be possible to predict total plant 15N content by assessing the contributions of different categories of root tips to total uptake if the specific 15N enrichment of a given root tip category was proportional to the N flux through this type of root tip. Based on this assumption, we introduced activity coefficients for each root tip category, which were normalized relative to the 15N enrichment of the root tips of NM plants (Supplementary Information SI1). With the NM plants, independent flux measurements were obtained and used to predict the fluxes of EM plants. Assessment of the relative contributions of the different root tip categories to the total flux clearly revealed that EMF contributed less to N flux than NM root tips in well-irrigated beeches, but their relative importance increased strongly under drought conditions (Figure 3a). The relative contribution of EMF to N flux was highest under SD conditions (Figure 3a).
Figure 3.
Relative contribution of different root tip categories to the total N flux (a) and correlation of the predicted and measured N uptake of young beech (Fagus sylvatica) trees (b). EM, ectomycorrhizal plants; NM, non-mycorrhizal plants; LC, full light, well-irrigated; LD, full light, drought; SC, shade, well-irrigated; SD, shade, drought. Data indicate means (n=5).
The 15N content in EM plants was predicted by the sum of the partial fluxes for each experimental treatment (LC, LD, SC and SD) (Supplementary Information SI1). Using a complementary approach, the 15N of NM plants was predicted based on the data for EM plants (Supplementary Information SI1). As the predicted 15N in EM plants was based on measured 15N values in NM plants and vice versa, the two data sets yielded independent predictions of 15N and therefore could be used to validate each other. A curvilinear relationship established for the N uptake of NM plants predicted the calculated N uptake of EM plants with a highly significant correlation (P=0.006, R(adjusted)=92%). The measured values for the 15N content of the plants were plotted against the calculated values for total 15N uptake (Figure 3b). A linear regression model with a slope of 1 was highly significant (Figure 3b). This result supports the notion that the specific enrichment of 15N in root tips is an indicator of flux through the root tip.
Discussion
EMF assemblages show interspecific differences for N acquisition and response diversity to stress
We documented clear differences in the N acquisition by ectomycorrhizas of different identities and provided evidence that uptake through ectomycorrhizas increased under drought stress and decreased in strongly shaded plants, with pronounced interspecific differences. These results support our initial hypotheses and may have wider ecological implications for ecosystem functions when anthropogenic disturbances lead to species loss. For example, strong dominance of the universal species C. geophilum in drought-stressed environments may be less beneficial with regard to N nutrition than associations with T. punicea.
Previous analyses of natural carbon and N isotope discrimination have mainly focused on functional differences of EMF species for the utilization of different resources (Zeller et al., 2007; Högberg et al., 2008; Tedersoo et al., 2012b). In contrast, studies investigating the utilization of the same resource by distinct taxa in the same environment are scarce, but this information is important for our understanding of the functional redundancy of EMF as contributors to ecosystem resilience. For example, in a forest community, most ectomycorrhizas, regardless of the fungal species, showed early enrichment of litter-derived N, likely from released solutes (Pena et al., 2013a). However, EMF with emanating hyphae and known saprotrophic capacities, such as Cortinarius sp. and Tomentella viridis, ultimately accumulated more N from degrading leaf litter compared with EMF with short extraradical mycelium, indicating spatiotemporal differentiation for access to a complex organic N source (Pena et al., 2013a). In our study, the aforementioned species were also present but were rare, suggesting that beech trees may foster fungal species without immediate benefits because the supply with NH4+ does not require the degradation of organic matter. Furthermore, long distance transport of N via fungal rhizomorphs is carbohydrate demanding (Ekblad et al., 2013). Therefore, long distance EMF are probably less favored by small plants with limited light resources than short distance EMF.
In temperate forests, NH4+ is an important soluble inorganic N source, rapidly taken up by beech trees (Gessler et al., 1998). In our experiment, we used an NH4+ concentration similar to that found in the Tuttlingen forest (Dannenmann et al., 2009), where the soil for beech mycorrhizal inoculation was sampled. The beech trees in the Tuttlingen forest are colonized by a characteristic EMF flora, including all species present in this study (Buée et al., 2005; Pena et al., 2010; Lang et al., 2011). Intact roots of these trees showed saturation of NH4+ uptake above a threshold of 50 μM NH4+ when exposed to feeding solutions with increasing NH4+ concentrations (Gessler et al., 2005). As the plants in our experiment were acclimated to NH4+ concentrations well above this threshold, the pronounced interspecific differences in EMF NH4+ accumulation were unexpected. T. rufum and T. punicea exhibited the greatest contrast in N acquisition. T. punicea forms rhizomorphs (Agerer, 2001), which have typically been associated with water transport and improvement of host water status (Duddridge et al., 1980; Brownlee et al., 1983; Plamboeck et al., 2007). Here, we showed that in addition to possible functions in water transport, T. punicea is important for nutrient supply under stress in shaded plants. In contrast, T. rufum, which displayed the highest specific N enrichment under full light, was highly sensitive to shade. Similar to our results, this species was frequently found on the root tips of sun-exposed beech trees and completely lost after long-term shade exposure (Druebert et al., 2009). As T. rufum has a thin mantle, it is unlikely that carbohydrate demand for colonization and maintenance is responsible for this behavior (Markkola et al., 2004).
The ascomycetes, C. geophilium and three truffle species actively participated in NH4+ uptake but showed divergent behavior for N acquisition in response to drought and shade. Although the small number of species precludes numerical analyses, our results support the notion that lineage-specific classification is unsuitable as a proxy for functional traits (Tedersoo et al., 2012b). In ecological terms, our findings reflect functional redundancy and response diversity (Mori et al., 2013). However, more extensive analyses, particularly under field conditions and along environmental gradients, are required to characterize distinct EMF traits in in situ assemblages and their adaptive importance for plant nutrition.
15N-specific enrichment in root tips as a proxy for N flux and the buffering functions of ectomycorrhizas in response to environmental stress
The high concordance of measured and predicted uptake with activity coefficients lends support to the suggestion that specific N enrichment is a proxy for N transport activities at the level of the root tip. This is an important result because it implies that stable isotope applications under field conditions can be used to assess the relative taxon-specific contributions of EMF in assemblages to plant N nutrition after correction for unspecific N enrichment. Whether this model is also valid for other N sources, such as nitrate, amino acids or complex compounds, must be further investigated.
Ectomycorrhizas did not increase N uptake or biomass compared with non-mycorrhizal plants under propitious environmental conditions (Pena et al., 2013b). The EMF colonization rate and fungal species richness of the beech trees in our study were similar to those reported in other studies with young trees (Bledsoe et al., 1982; Wilson and Harley, 1983; Högberg, 1989; Dahlberg, 2001; Izzo et al., 2006) but were much lower than in old-growth forests, where usually 95% to 99% of the vital root tips are colonized by EMF (Pena et al., 2010; Lang and Polle, 2011; Näsholm et al., 2013). Therefore, the mean N fluxes, which are similar to those reported by others (for example, Högberg, 1989: approximately 4 ng N h−1 RT−1), may be confounded by NM root tips. Our data suggest that fully mycorrhizal, well-irrigated and irradiated trees would even exhibit lower N uptake than that observed because of the low activity coefficients for NH4+ of the prevailing EMF. This reasoning contrasts with the widely accepted beneficial implications of EMF for plant nutrition but supports results obtained in a boreal forest (Näsholm et al., 2013). The authors concluded that when N but not carbon was the limiting factor, EMF could aggravate N limitation in trees (Näsholm et al., 2013). EMF control of the host N supply may also be a reason for the decreased N/C ratios commonly found in EM compared with NM plants (Ducic et al., 2008; Druebert et al., 2009; Jones et al., 2009; Pena et al., 2013b).
Notably, the impact of EMF on host N supply is mitigated by moderate drought. Episodes during which roots are exposed to water limitations frequently occur in the upper soil layer (Holst et al., 2009), where the majority of root tips reside (Meinen et al., 2009). EMF colonization delays the drought-induced decrease of the plant water potential (Beniwal et al., 2010; Pena et al., 2013b). Although the high N flux rates of unstressed NM plants imply that EMF is not necessary for plant N nutrition, the susceptibility of NM root tips to water limitation underlines the ecological significance of EMF in buffering plant nutrition against fluctuating environmental conditions. The underlying physiological or molecular mechanisms of this advantage are unclear but may be related to improved water retention by hyphae (Lehto and Zwiazek, 2011) or fungal-induced activation of osmotic solutes in roots (Luo et al., 2009a, 2009b). Furthermore, fungal stress tolerance is important. Among the fungi in our study, C. geophilum is known as a drought-tolerant species (Coleman et al., 1989; Di Pietro et al., 2007). It is abundant on beech roots in dry habitats (Jany et al., 2003; Pena et al., 2010), but its function for forest tree N nutrition has been disputed (Herzog et al., 2012; Kipfer et al., 2012). The current results indicate a buffering function for C. geophilum against varying environmental conditions but also suggest that other EMF species provide greater host benefits under water-limiting conditions.
In conclusion, this study shows that the analyzed ectomycorrhizal taxa use NH4+ in in situ assemblages to strongly diverging degrees. As NH4+ is an important N source across temperate forest ecosystems (Gobert and Plassard, 2008), its uptake must be granted under different environmental conditions. We provide evidence that the benefits of the ectomycorrhizal assemblage for N uptake and host transfer are mainly realized under environmental constraints. The physiological and molecular bases for the diverging responses within and between fungal species are currently unknown. With the advent of mycorrhizal genome projects (Marmeisse et al., 2013), novel opportunities to uncover the molecular processes that are involved in response diversity are expected. The use of molecular tools together with stable isotope incorporation will unravel the functions of EMF assemblages, and we expect great progress in expanding our understanding of ecosystem resilience, which depends on both diversity and functional redundancy.
Acknowledgments
We thank M Fastenrath, M Franke-Klein and T Klein for their excellent technical assistance. We are grateful to J Dyckmans for measuring 15N (Centre for Stable Isotopes, KOSI, University of Göttingen). We gratefully acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG, Po362/19).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Agerer R. Colour Atlas of Ectomycorrhizae. Einhorn-Verlag: Schwäbisch Gmünd, Germany; 1987–2012. [Google Scholar]
- Agerer R. Exploration types of ectomycorrhizae: a proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza. 2001;11:107–114. [Google Scholar]
- Andreasson F, Balsberg-Pahlsson AM, Bergkvist B. Differences in soil organic matter, extractable nutrients, and acidity in European beech (Fagus sylvatica L.) forest soils related to the presence of ground flora. J For Res. 2012;17:333–342. [Google Scholar]
- Beniwal RS, Langenfeld-Heyser R, Polle A. Ectomycorrhiza and hydrogel protect hybrid poplar from water deficit and unravel plastic responses of xylem anatomy. Environ Exp Bot. 2010;69:189–197. [Google Scholar]
- Bledsoe CS, Tennyson K, Lopushinsky W. Survival and growth of outplanted Douglas-fir seedlings inoculated with mycorrhizal fungi. Can J Forest. 1982;12:720–723. [Google Scholar]
- Brownlee C, Duddrige JA, Malibari A, Read DJ. The structure and function of mycelia systems of ectomycorrhizal roots with special reference to their role in assimilate and water transport. Plant Soil. 1983;71:433–443. [Google Scholar]
- Buée M, Courty PE, Mignot D, Garbaye J. Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal fungal community. Soil Biol Biochem. 2007;39:1947–1955. [Google Scholar]
- Buée M, Vairelles D, Garbaye J. Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus sylvatica) forest subjected to two thinning regimes. Mycorrhiza. 2005;15:235–245. doi: 10.1007/s00572-004-0313-6. [DOI] [PubMed] [Google Scholar]
- Calvaruso C, Turpault MP, Leclerc E, Frey-Klett P. Impact of ectomycorrhizosphere on the functional diversity of soil bacterial and fungal communities from a forest stand in relation to nutrient mobilization processes. Microbial Ecol. 2007;54:567–577. doi: 10.1007/s00248-007-9260-z. [DOI] [PubMed] [Google Scholar]
- Chu-Chou M, Grace LJ. Comparative efficiency of the mycorrhizal fungi Laccaria laccata, Hebeloma crustuliniforme and Rhizopogon species on growth of radiata pine seedlings. New Zeal J Bot. 1985;23:417–424. [Google Scholar]
- Coleman MD, Bledsoe CS, Lopushinsky W. Pure culture response of ectomycorrhizal fungi to imposed water stress. Can J Botany. 1989;67:29–39. [Google Scholar]
- Courty PE, Buée M, Diedhiou AG, Frey-Klett P, Le Tacon F, Rineau F, et al. The role of ectomycorrhizal communities in forest ecosystem processes: new perspectives and emerging concepts. Soil Biol Biochem. 2010;42:679–698. [Google Scholar]
- Dahlberg A. Community ecology of ectomycorrhizal fungi: an advancing interdisciplinary field. New Phytol. 2001;150:555–562. [Google Scholar]
- Danielsen L, Lohaus G, Sirrenberg A, Karlovsky P, Bastien C, Pilate G, et al. Ectomycorrhizal colonization and diversity in relation to tree biomass and nutrition in a plantation of transgenic poplars with modified lignin biosynthesis. PLoS One. 2013;8:e59207. doi: 10.1371/journal.pone.0059207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenmann M, Simon J, Gasche R, Holst J, Naumann PS, Kögel-Knabner I, et al. Tree girdling provides insight in the role of labile carbon in the competitive balance of N partitioning between soil microorganisms and adult European beech. Soil Biol Biochem. 2009;41:1622–1631. [Google Scholar]
- Di Pietro M, Churin JL, Garbaye J. Differential ability of ectomycorrhizas to survive drying. Mycorrhiza. 2007;17:547–550. doi: 10.1007/s00572-007-0113-x. [DOI] [PubMed] [Google Scholar]
- Downes GM, Alexander IJ, Cairney JWG. A study of ageing of spruce [Picea sitchensis (Bong.) Carr.] ectomycorrhizas. I. Morphological and cellular changes in mycorrhizas formed by Tylospora fibrillosa (Burt. Donk) and Paxillus involutus (Batsch. ex Fr.) Fr. New Phytol. 1992;122:141–152. doi: 10.1111/j.1469-8137.1992.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Druebert C, Lang C, Valtanen K, Polle A. Beech carbon productivity as driver of ectomycorrhizal abundance and diversity. Plant Cell Environ. 2009;32:992–1003. doi: 10.1111/j.1365-3040.2009.01983.x. [DOI] [PubMed] [Google Scholar]
- Ducic T, Parlade J, Polle A. The influence of the ectomycorrhizal fungus Rhizopogon subareolatus on growth and nutrient element localisation in two varieties of Douglas fir (Pseudotsuga menziesii var. menziesii and var. glauca) in response to manganese stress. Mycorrhiza. 2008;18:227–239. doi: 10.1007/s00572-008-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddridge JA, Malibari A, Read DJ. Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature. 1980;287:834–836. [Google Scholar]
- Ekblad A, Wallander H, Godbold DL, Cruz C, Johnson D, Baldrian P, et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: role in carbon cycling. Plant Soil. 2013;366:1–27. [Google Scholar]
- Ellenberg H, Strutt GK.2009Vegetation Ecology of Central Europe Cambridge University Press: Cambridge; p756 [Google Scholar]
- Eltrop L, Marschner H. Growth and mineral nutrition of non-mycorrhizal and mycorrhizal Norway spruce (Picea abies) seedlings grown in semi-hydroponic sand culture. I. Growth and mineral nutrient uptake in plants supplied with different forms of nitrogen. New Phytol. 1996;133:469–478. [Google Scholar]
- Evert RF, Eichhorn SE. Esau's Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. John Wiley & Sons, Inc.: Holboken, NJ, USA; 2007. [Google Scholar]
- Finlay RD. Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot. 2008;59:1115–1126. doi: 10.1093/jxb/ern059. [DOI] [PubMed] [Google Scholar]
- Fotelli MN, Rennenberg H, Holst T, Mayer H, Gessler A. Carbon isotope composition of various tissues of beech (Fagus sylvatica) regeneration is indicative of recent environmental conditions within the forest understorey. New Phytol. 2003;159:229–244. doi: 10.1046/j.1469-8137.2003.00782.x. [DOI] [PubMed] [Google Scholar]
- Gessler A, Jung K, Gasche R, Papen H, Heidenfelder A, Börner E, et al. Climate and forest management influence nitrogen balance of European beech forests: microbial N transformations and inorganic N net uptake capacity of mycorrhizal roots. Eur J Forest Res. 2005;124:95–111. [Google Scholar]
- Gessler A, Schneider S, Von Sengbusch D, Weber P, Hanemann U, Huber C. Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytol. 1998;138:275–285. doi: 10.1046/j.1469-8137.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- Gobert A, Plassard C.2008The beneficial effect of mycorrhizae on N utilization by the host-plant: myth or realityIn: Varma A, (ed)Mycorrhiza-State of the Art, Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematics Springer-Verlag: Berlin, Germany; 209–240. [Google Scholar]
- Göransson H, Wallander H, Ingerslev M, Rosengren U. Estimating the relative nutrient uptake from different soil depths in Quercus robur, Fagus sylvatica and Picea abies. Plant Soil. 2006;286:87–97. [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. PAST: palaeontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9–17. [Google Scholar]
- Heinonsalo J, Jorgensen KS, Sen R. Microcosm-based analyses of Scots pine seedling growth, ectomycorrhizal fungal community structure and bacterial carbon utilization profiles in boreal forest humus and underlying illuvial mineral horizons. FEMS Microbial Ecol. 2001;36:73–84. doi: 10.1111/j.1574-6941.2001.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Hertl C, Leuchner M, Rötzer T, Menzel A. Assessing stand structure of beech and spruce from measured spectral radiation properties and modeled leaf biomass parameters. Agr Forest Meteorol. 2012;165:82–91. [Google Scholar]
- Herzog C, Peter M, Pritsch K, Günthardt-Goerg MS, Egli S. Drought and air warming affects abundance and exoenzyme profiles of Cenococcum geophilum associated with Quercus robur, Q. petraea, and Q. pubescens. Plant Biol. 2012;15:230–237. doi: 10.1111/j.1438-8677.2012.00614.x. [DOI] [PubMed] [Google Scholar]
- Hobbie EA, Hobbie JE. Natural abundance of N-15 in nitrogen-limited forests and tundra can estimate nitrogen cycling through mycorrhizal fungi: a review. Ecosystems. 2008;11:815–830. [Google Scholar]
- Hobbie EA, Högberg P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012;196:367–382. doi: 10.1111/j.1469-8137.2012.04300.x. [DOI] [PubMed] [Google Scholar]
- Högberg P. Growth and nitrogen inflow rates in mycorrhizal and non-mycorrhizal seedlings of Pinus sylvestris. For Ecol Manage. 1989;28:7–17. [Google Scholar]
- Högberg P, Högberg MN, Göttlicher SG, Betson NR, Keel SG, Metcalfe DB, et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol. 2008;177:220–228. doi: 10.1111/j.1469-8137.2007.02238.x. [DOI] [PubMed] [Google Scholar]
- Holst J, Grote R, Offermann C, Ferrio JP, Gessler A, Mayer H, et al. Water fluxes within beech stands in complex terrain. Int J Biometeorol. 2009;54:23–36. doi: 10.1007/s00484-009-0248-x. [DOI] [PubMed] [Google Scholar]
- Izumi H, Finlay RD. Ectomycorrhizal roots select distinctive bacterial and ascomycete communities in Swedish subarctic forests. Environ Microbiol. 2011;13:819–830. doi: 10.1111/j.1462-2920.2010.02393.x. [DOI] [PubMed] [Google Scholar]
- Izzo A, Nguyen DT, Bruns TD. Spatial structure and richness of the ectomycorrhizal resistant propagule community colonizing hosts with differing seedling establishment patterns. Mycologia. 2006;98:374–383. doi: 10.3852/mycologia.98.3.374. [DOI] [PubMed] [Google Scholar]
- Jany JL, Martin F, Garbaye J. Respiration activity of ectomycorrhizas from Cenococcum geophilum and Lactarius sp. in relation to soil water potential in five beech forests. Plant Soil. 2003;255:487–494. [Google Scholar]
- Jolliffe IT. Principal Component Analysis. Springer Verlag: New York, NY, USA; 1986. [Google Scholar]
- Jones MD, Grenon F, Peat H, Fitzgerald M, Holt L, Philip LJ, et al. Differences in 15N uptake amongst spruce seedlings colonized by three pioneer ectomycorrhizal fungi in the field. Fungal Ecol. 2009;2:110–120. [Google Scholar]
- Jongbloed RH, Clement JMAM, Borst-Pauwels GWFH. Kinetics of NH4+ and K+ uptake by ectomycorrhizal fungi. Effect of NH4+ on K+ uptake. Physiol Plant. 1991;83:427–432. [Google Scholar]
- Jonsson LM, Nilsson MC, Wardle DA, Zackrisson O. Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos. 2001;93:353–364. [Google Scholar]
- Kipfer T, Wohlgemuth T, van der Heijden MGA, Ghazoul J, Egli S. Growth response of drought-stressed Pinus sylvestris seedlings to single- and multi-stress inoculation with ectomycorrhizal fungi. PLoS One. 2012;7:e35275. doi: 10.1371/journal.pone.0035275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjøller R, Nilsson L-O, Hansen K, Schmidt IK, Vesterdal L, Gundersen P. Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelia production along a stand-scale nitrogen deposition gradient. New Phytol. 2012;194:278–286. doi: 10.1111/j.1469-8137.2011.04041.x. [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Herschbach C, Stulen I, Wiersema P, Vaalburg W, Rennenberg H. Interactions of NH4+ with NO3− transport processes of non-mycorrhizal Fagus sylvatica roots. J Exp Bot. 1997;48:1431–1438. [Google Scholar]
- Lang C, Polle A. Ectomycorrhizal fungal diversity, tree diversity and root nutrient relations in a mixed Central European forest. Tree Physiol. 2011;31:531–538. doi: 10.1093/treephys/tpr042. [DOI] [PubMed] [Google Scholar]
- Lang C, Seven J, Polle A. Host preferences and differential contributions of deciduous tree species shape mycorrhizal species richness in a mixed Central European forest. Mycorrhiza. 2011;21:297–308. doi: 10.1007/s00572-010-0338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89:371–379. doi: 10.1890/06-2057.1. [DOI] [PubMed] [Google Scholar]
- Lehto T, Zwiazek J. Ectomycorrhizas and water relations of trees: a review. Mycorrhiza. 2011;21:71–90. doi: 10.1007/s00572-010-0348-9. [DOI] [PubMed] [Google Scholar]
- Lemoine D, Cochard H, Granier A. Within crown variation in hydraulic architecture in beech (Fagus sylvatica L): evidence for a stomatal control of xylem embolism. Ann For Sci. 2002;59:19–27. [Google Scholar]
- Lilleskov EA, Hobbie EA, Horton TR. Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol. 2011;4:174–183. [Google Scholar]
- Luo ZB, Janz D, Jiang XN, Gobel C, Wildhagen H, Tan YP, et al. Upgrading root physiology for stress tolerance by ectomycorrhizas: insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiol. 2009a;151:1902–1917. doi: 10.1104/pp.109.143735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZB, Li K, Jiang XN, Polle A. Ectomycorrhizal fungus (Paxillus involutus) and hydrogels affect performance of Populus euphratica exposed to drought stress. Ann For Sci. 2009b;66:106. [Google Scholar]
- Markkola AM, Kuikka K, Rautio P, Härmä E, Roitto M, Tuomi J. Defoliation increase carbon limitation in ectomycorrhizal symbiosis of Betula pubescens. Oecologia. 2004;140:234–240. doi: 10.1007/s00442-004-1587-2. [DOI] [PubMed] [Google Scholar]
- Marmeisse R, Nehls U, Opik M, Selosse M-A, Pringle A. Bridging mycorrhizal genomics, metagenomics and forest ecology. New Phytol. 2013;198:343–346. doi: 10.1111/nph.12205. [DOI] [PubMed] [Google Scholar]
- Mayer H, Holst T, Schindler D. Microclimate within beech stands – part I: photosynthetically active radiation. Forstwiss Centralb. 2002;121:301–321. [Google Scholar]
- Meinen C, Hertel D, Leuschner C. Biomass and morphology of fine roots in temperate broad-leaved forests differing in tree species diversity: is there evidence of below-ground overyielding. Oecologia. 2009;161:99–111. doi: 10.1007/s00442-009-1352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori AS, Furukawa T, Sasaki T. Response diversity determines the resilience of ecosystems to environmental change. Biol Rev. 2013;88:349–364. doi: 10.1111/brv.12004. [DOI] [PubMed] [Google Scholar]
- Näsholm T, Högberg P, Franklin O, Metcalfe D, Keel SG, Campbe C, et al. Are ectomycorrhizal fungi alleviating or aggravating nitrogen limitation of tree growth in boreal forests. New Phytol. 2013;198:214–221. doi: 10.1111/nph.12139. [DOI] [PubMed] [Google Scholar]
- Pena R, Offermann C, Simon J, Naumann PS, Gessler A, Holst J, et al. Girdling affects ectomycorrhizal fungal (EMF) diversity and reveals functional differences in EMF community composition in a beech forest. Appl Environ Microbiol. 2010;76:1831–1841. doi: 10.1128/AEM.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena R, Tejedor J, Zeller B, Dannenman M, Polle A. Interspecific temporal and spatial differences in the acquisition of litter-derived nitrogen by ectomycorrhizal fungal assemblages. New Phytol. 2013a;199:520–528. doi: 10.1111/nph.12272. [DOI] [PubMed] [Google Scholar]
- Pena R, Simon J, Rennenberg H, Polle A. Ectomycorrhiza affect the architecture of, and nitrogen partitioning and allocation in beech (Fagus sylvatica L.) seedlings under shade and drought. Environ Exp Bot. 2013b;87:207–217. [Google Scholar]
- Plamboeck AH, Dawson TE, Egerton-Warburton LM, North M, Bruns TD, Querejeta JI. Water transfer via ectomycorrhizal fungal hyphae to conifer seedlings. Mycorrhiza. 2007;17:439–447. doi: 10.1007/s00572-007-0119-4. [DOI] [PubMed] [Google Scholar]
- Shannon CE, Weaver W. A Mathematical Theory of Communication. University of Illinois Press: Urbana, IL, USA; 1949. [Google Scholar]
- Tedersoo L, Bahram M, Toots M, Diédhiou AG, Henkel TW, Kjøller R. Towards global patterns in the diversity and community structure of ectomycorrhizal fungi. Mol Ecol. 2012a;21:4160–4170. doi: 10.1111/j.1365-294X.2012.05602.x. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Naadel T, Bahram M, Pritsch K, Buegger F, Leal M., et al. Enzymatic activities and stable isotope patterns of ectomycorrhizal fungi in relation to phylogeny and exploration types in an afrotropical rain forest. New Phytol. 2012b;195:832–843. doi: 10.1111/j.1469-8137.2012.04217.x. [DOI] [PubMed] [Google Scholar]
- Weber P, Bugmann H, Pluess AR, Walthert L, Rigling A. Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees. 2013;27:171–181. [Google Scholar]
- Wilson JW, Harley JL. The development of mycorrhiza on seedlings of Fagus sylvatica L. New Phytol. 1983;95:673–695. [Google Scholar]
- Winkler JB, Dannemann M, Simon J, Pena R, Offermann C, Sternad W, et al. Carbon and nitrogen balance in beech roots under competitive pressure of soil-borne microorganisms induced by girdling, drought and glucose application. Funct Plant Biol. 2010;37:879–889. [Google Scholar]
- Zeller B, Brechet C, Maurice JP, Le Tacon F. 13C and 15N isotopic fractionation in trees, soil and fungi in a natural forest stand and a Norway spruce plantation. Ann For Sci. 2007;64:419–429. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.