Abstract
Theileria annulata infects predominantly macrophages, and to a lesser extent B cells, and causes a widespread disease of cattle called tropical theileriosis. Disease-causing infected macrophages are aggressively invasive, but this virulence trait can be attenuated by long-term culture. Attenuated macrophages are used as live vaccines against tropical theileriosis and via their characterization one gains insights into what host cell trait is altered concomitant with loss of virulence. We established that sporozoite infection of monocytes rapidly induces hif1-α transcription and that constitutive induction of HIF-1α in transformed leukocytes is parasite-dependent. In both infectedmacrophages and B cells induction of HIF-1α activates transcription of its target genes that drive host cells to perform Warburg-like glycolysis. We propose that Theileria-infected leukocytes maintain a HIF-1α-driven transcriptional programme typical of Warburg glycolysis in order to reduce as much as possible host cell H2O2 type oxidative stress. However, in attenuated macrophages H2O2 production increases and HIF-1α levels consequently remained high, even though adhesion and aggressive invasiveness diminished. This indicates that Theileria infection generates a host leukocytes hypoxic response that if not properly controlled leads to loss of virulence.
Introduction
The apicomplexan parasite Theileria annulata infects bovine macrophages and transforms them into aggressively invasive tumours that contribute to tropical theileriosis, a widespread disease endemic in North Africa, the Middle East, India and China (Dobbelaere and Heussler, 1996). In contrast to East Coast fever, caused by T. parva, live vaccines exist to tropical theileriosis (Darghouth, 2008) that are based on multiple-passages of infected macrophages, which become attenuated for virulence i.e. the infected cells have lost the heightened invasiveness virulence trait (Baylis et al., 1995; Hall et al., 1999). As the transformed state of Theileria-infected leukocytes can be reversed by drug-induced parasite death they represent a powerful model to study molecular events related to infection and host cell transformation in an isogenic background, e.g. the same leukocytes can be examined in the transformed and non-transformed states (Chaussepied and Langsley, 1996; Dobbelaere and Heussler, 1996). As attenuated macrophage cell-lines are directly derived from virulent ones they are also isogenic and the same infected macrophage can be examined in virulent and attenuated states. In such a way parasite-dependent TGF-β2 induction was identified as a host cell virulence trait that is lost upon attenuation (Chaussepied et al., 2010).
Otto Warburg observed in 1926 that cancer cells performed glycolysis even in the presence of high oxygen and that pyruvate was converted to lactate, which was secreted from the tumour, an observation that later became known as the Warburg effect (Warburg, 1956). Rapidly dividing tumour cells produce reactive oxygen species (ROS) and poor vasculature of the growing tumour results in low oxygen and hypoxia, where a major mediator of the anti-oxidant response is the hypoxia-inducible factor (HIF) family of transcription factors (Koh and Powis, 2012). HIFs are made up of three major oxygen labile subunits, HIF-1α, HIF-2α and HIF-3α and a constitutive aryl hydrocarbon receptor translocator, or ARNT/HIF-1β (Wang et al., 1995). Under aerobic conditions HIF-1/2α are hydroxylated by specific prolyl hydroxylases (PHDs) and hydroxylation promotes binding of an E3 ubiquitin ligase called von Hippel-Lindau (VHL) that mediates HIF degradation (Koh and Powis, 2012). During hypoxia PHD activity is diminished, VHL binding is consequently ablated and HIF is no longer degraded, leading to the accumulation of the transcription factor.
Infection by microorganisms is known to generate a host cell stress response and in vitro infection of human foreskin fibroblast (HFF) by Toxoplasma gondii provoked elevated levels of HIF-1α (Spear et al., 2006). As stated above, virulent Theileria-infected macrophages also produce TGF-β, however, they differ from T. gondii-infected HFFs, where intracellular growth of the parasite is arrested upon TGF-R blockade by SB505124 (Wiley et al., 2010). Given that Theileria infection of leukocytes transforms them into tumour-like cells we decided to ask whether T. annulata infection also generates a host cell oxidative stress response that activates HIF-1α and what are the consequences of HIF-1α induction on host cell glycolysis. We found that hif-1α transcription is induced within two hours of sporozoite invasion and its constitutive induction confers on infected host cells Warburg-like glycolysis, even though the cells are growing under normoxic conditions. We suggest that host leukocytes switch to Warburg glycolysis in an attempt to control toxic levels of oxidative stress stemming from Theileria infection and the uncontrolled host cell proliferation that ensues. We found that attenuated infected macrophages produce more H2O2 and consequently display greater signs of Warburg-like glycolysis. This observation uncouples HIF-1α induction from aggressive tumour invasiveness and implies that excessive host cell oxidative stress diminishes Theileria-infected macrophage virulence.
Results
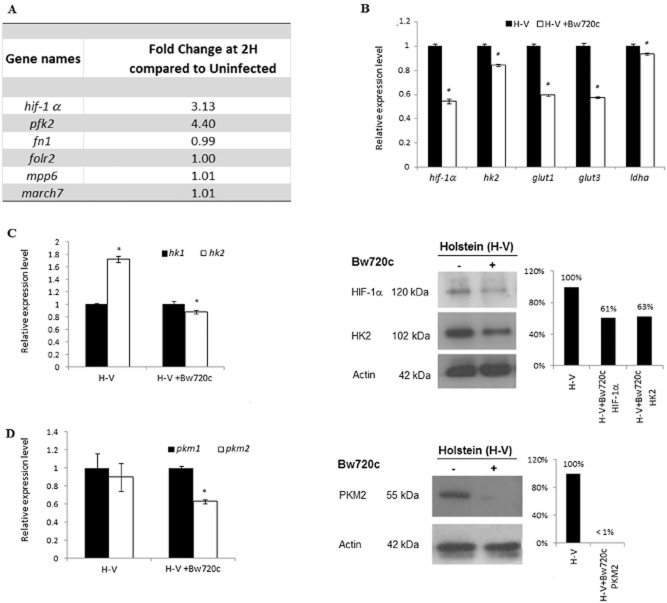
In virulent Holstein-Friesian macrophages hif-1α induction is parasite-dependent and leads to upregulated expression of HIF-1α and its target genes
Monocytes of Holstein-Friesian (H) origin were infected in vitro with T. annulata sporozoites and RNA was isolated and used to probe a bovine macrophage microarray (Jensen et al., 2006; Jensen et al., 2008). As little as 2 h post invasion hif-1α transcript levels and those of a HIF-1α-target gene pfk2 were three- to fourfold higher in H-infected monocytes compared with non-infected monocytes indicating that infection had induced hif-1α transcription (Fig. 1A). The transcription factor HIF-1α plays a key role in regulating the hypoxic response via transcription of a large number of target genes including those involved in glycolysis, particularly those mediating Warburg glycolysis typical of cancer cells (Warburg, 1956; Manalo et al., 2005; Fang et al., 2009). Therefore, in addition to hif-1α we analysed the transcription levels of four known HIF-1α-target genes: glut1, glut3, hk2 and ldha, and observed that their mRNA levels decreased upon drug-induced parasite death (Fig. 1B). As Warburg glycolysis is known to promoter preferential expression of the hexokinase 2 (HK2) and pyruvate kinase 2 (PKM2) isoforms, we verified the parasite dependence of their expression compared with HK1 and PKM1 in virulent (H-V) macrophages. T. annulata infection of H-V induces pronounced expression of hk2 over hk1 and drug-induced parasite death leads to a significant reduction of both HIF-1α and HK2 proteins (Fig. 1C). Similarly, there is pronounced parasite dependence of PKM2 expression (Fig. 1D). Thus, the HIF-1α-driven programme normally associated with hypoxia-induced Warburg glycolysis depends on Theileria-transformed H-V macrophages harbouring live parasites.
Figure 1.
In Holstein-Friesian infected leukocytes HIF-1α activation is parasite-dependent and leads to upregulated expression of HIF-1α target genes.A. Holstein-Friesian (H)-derived resting peripheral monocytes were infected in vitro with T. annulata sporozoites and RNA was isolated and used to probe a bovine macrophage microarray. At 2 h post infection hif-1α transcript levels are threefold higher in infected cells compared with non-infected monocytes. The HIF-1α-target gene pfk2 is upregulated fourfold, whereas 80% of genes show no change, including fn1, folr2, mpp6 and march7.B. In virulent (H-V) macrophages the transcription levels of hif-1α and a selection of its target genes (glut1, glut3, pdk1, ldha and hk2) diminishes upon Bw720c-induced parasite death. *Student’s t-test, highest P-value = 0.0482 < 0.05.C. Bw720c-induced parasite death in H-V macrophages leads to a drop in hk2 transcripts and a reduction in both HIF-1α and HK2 levels. *Student’s t-test, highest P-value = 0.0358 < 0.05.D. PKM2 expression also drops upon Bw720c-induced parasite death. The amount of actin expressed was used as a loading control. *Student’s t-test, P-value = 0.0376 < 0.05.
HIF-1α levels remain high in attenuated macrophages even as their invasiveness decreases
Virulent Holstein-Friesian (H-V) T. annulata-infected macrophages can be attenuated (H-A) by long-term culture and are used to vaccinate against tropical theileriosis. As well as promoting Warburg glycolysis, HIF induction also contributes to tumour aggressiveness due to its capacity to promote transcription of genes involved in angiogenesis, for recent reviews see (Levine and Puzio-Kuter, 2010; Koh and Powis, 2012). As H-A macrophages lose both their adhesive (Fig. 2A) and invasive capacities (Fig. 2B), we expected that these cells would exhibit decreased expression of hif-1α and hif-1β. Surprisingly, hif-1α and hif-1β mRNA levels were largely unchanged (Fig. 2C). Attenuation of Theileria-transformed macrophages and their corresponding loss of tumorigenicity are therefore independent of HIF levels that remain high (Fig. 2D).
Figure 2.
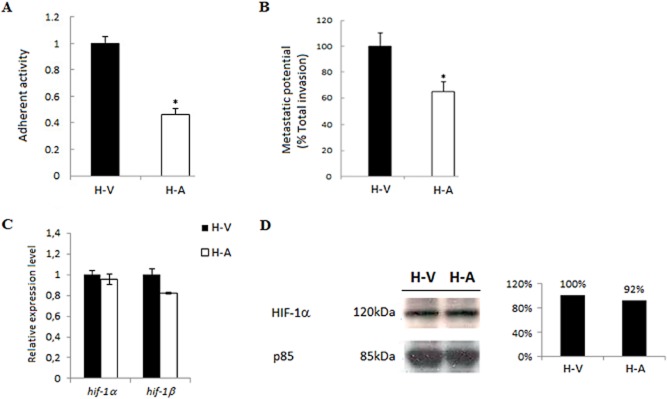
HIF-1α levels remain high in attenuated macrophages even as adhesion and invasiveness decreases.A. Virulent Holstein-Friesian macrophages (H-V) adhere to fibronectin and binding is significantly reduced in attenuated (H-A) macrophages even though attenuated cell spread more than virulent ones.B. H-V macrophages degrade Matrigel better than H-A macrophages.C. Transcription of hif-1α and hif-1β is unchanged in H-V macrophages compared H-A macrophages. Transcript levels were normalized to hprt1 expression. *Student’s t-test, NS P-value > 0.05.D. HIF-1α protein levels also remain largely unchanged in H-V and H-A macrophages. The level of p85 expression was used as a loading control.
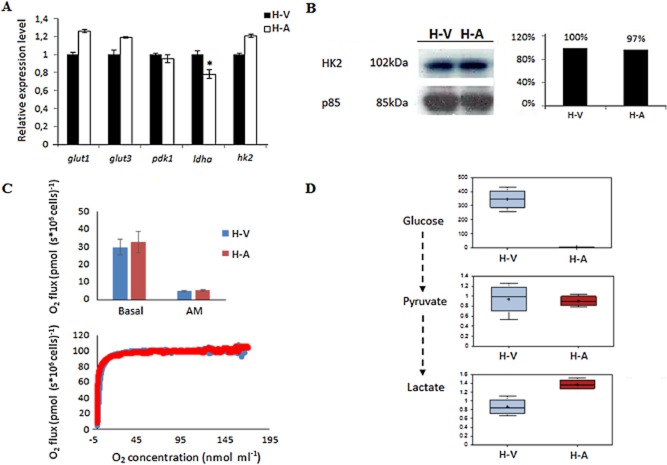
Theileria-transformed macrophages display signs of aerobic glycolysis (Warburg effect)
As neither hif-1α nor hif-1β levels changed significantly the expression of a selection of HIF-target genes also remained upregulated in attenuated T. annulata-infected macrophages, with only ldha transcription being somewhat dampened (Fig. 3A). Attenuated H-A infected macrophages maintain HK2 protein levels (Fig. 3B), consume oxygen (Fig. 3C), consume glucose and produce lactate (Fig. 3D), all traits typically associated with Warburg type glycolysis. Greater glucose consumption with higher lactate output is also true for a B-cell line (BL3) infected with T. annulata (TBL3, Fig. S1C). Taken together, all data concur and suggest that following invasion T. annulata-transformed leukocytes perform Warburg-like glycolysis.
Figure 3.
HIF-1α-target gene expression in the presence of high O2 consumption and lactate output.A. Transcription levels of five hif-1α target genes; glut1, glut3, pdk1, ldha and hk2, were investigated in virulent (H-V) and attenuated T. annulata-infected (H-A) macrophages. Only ldha levels are lower in H-A macrophages. *Student’s t-test, highest P-value = 0.0074 < 0.05.B. HK2 protein levels remained equivalent in H-V and H-A macrophages. The amounts of p85 were used as a loading control.C. Upper panel, three independent Oroboros measurements show O2 fluxes are equivalent in both H-A and H-V macrophages and respiration is largely mitochondrial, since upon antimycin (AM) inhibition of complex III O2 flux is markedly decreased. Lower panel, O2 consumption over time is the same in H-V and H-A macrophages in a closed Oroboros chamber, which eventually becomes hypoxic. Between each experiment Oroboros chambers were washed 3 times and incubated for at least 5 min with ethanol then rinsed with water.D. Metabolomic analyses highlighting the intracellular metabolite levels stemming from glycolytic activity of H-V and H-A macrophages. H-A macrophages consume more glucose and produce more lactate than H-V macrophages, whereas pyruvate levels are equivalent.
Infection induces H2O2 type oxidative stress, and attenuation of virulence increases H2O2 output
Figures 3 show that HIF-1α, HK2 and other HIF-1α-target genes involved in aerobic glycolysis are expressed even though Theileria-infected H-V and H-A macrophages are consuming O2 and growing under normoxic conditions. As oxidative stress has been linked to HIF-1α activation (Bonello et al., 2007; Pialoux et al., 2009), we measured the H2O2 output of T. annulata-infected macrophages of different origins (Fig. 4). Attenuated macrophages of pure breed (H-A), or cross-breed (S-A) origin produced more H2O2 compared with NO, than their virulent counterparts (Fig. 4A–C). H2O2 output from macrophages is parasite-dependant, as it diminishes on Bw720c treatment (Fig. 4A). Similarly, H2O2 levels are also significantly higher in infected TBL3 compared with non-infected BL3 lymphocytes (Fig. S1C). Bw720c-induced parasite death does not diminish the capacity of the host macrophage to produce H2O2, as output is sustained by aminotriazole (AT) inhibition of macrophage catalase that converts H2O2 to 2 H2O (Fig. 4D). Consistently, the antioxidant NAC reduces H2O2 output even in the presence of live parasites (Fig. 4E). Reducing high H2O2 output by attenuated H-A macrophages increases their transwell migration and conversely, increasing H2O2 output by H-V macrophages dampens their transwell migration (Fig. 4F). Theileria infection of macrophages therefore induces H2O2 type oxidative stress that underpins their invasive capacity.
Figure 4.
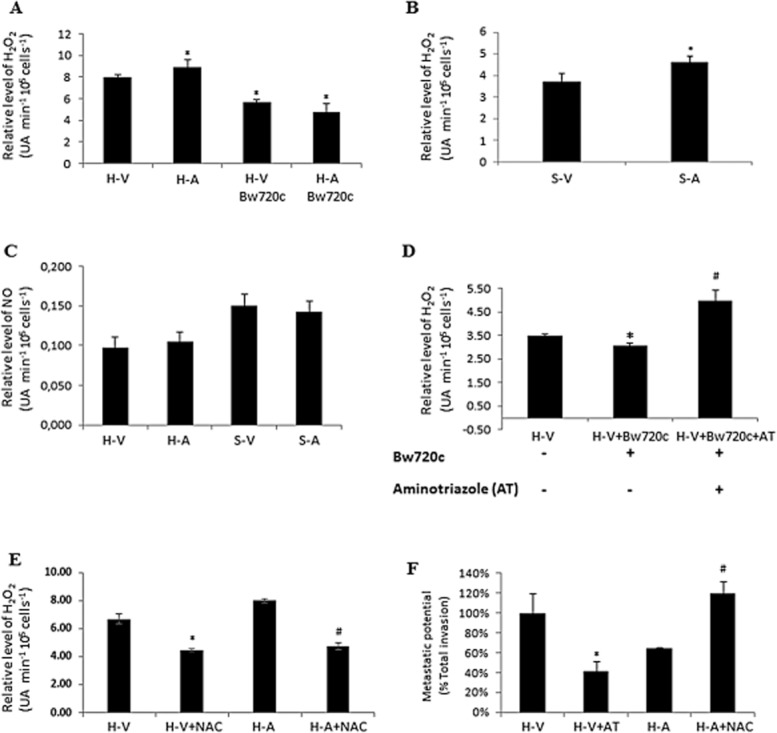
Infection induces H2O2 type oxidative stress and attenuation increases H2O2 output.A. Virulent (H-V) T. annulata-infected macrophages produce less H2O2 than attenuated (H-A) T. annulata-infected macrophages and the amount of H2O2 is reduced upon Bw720c-induced parasite death.B. Virulent Sahiwal/Holstein-Friesian (S-V) T. annulata-infected macrophages produce less H2O2 than attenuated (S-A) macrophages.C. The levels of NO are unchanged between virulent and attenuated T. annulata-infected macrophages of H and S origin, even though Sahiwal/Holstein-Friesian infected macrophages produce more NO.D. Bw720c does not incapacitate macrophage production of H2O2 when host cells are stressed by AT treatment.E. The anti-oxidant NAC reduces H2O2 output by H-V and H-A macrophages.F. Increasing H2O2 output by AT blockade of catalase reduces transwell migration by H-V and conversely, reducing H2O2 levels with NAC increases transwell migration of H-A macrophages. #, *Student’s t-test, highest P-value = 0.04 < 0.05.
H2O2 output by infected macrophages regulates HIF-1α levels
In Figs 1 and 4 we show that HIF-1α levels and H2O2 output depend on live parasites raising the question as to whether it is the parasite, or H2O2 that is responsible for HIF-1α expression. The parasite was killed by Bw720c treatment and H2O2 output sustained by AT inhibition of catalase and under these conditions the level of HIF-1α expression is maintained (Fig. 5A). It follows that reducing H2O2 by NAC treatment also diminishes HIF-1α expression (Fig. 5B) and consequently HK2 levels (data not shown). Consistently, NAC treatment also reduced transcription of HIF-target genes in both H-V and H-A macrophages (Fig. 5C and D). Clearly, it is H2O2 that induces the HIF-1α-driven Warburg-like programme of parasite-transformed macrophages.
Figure 5.
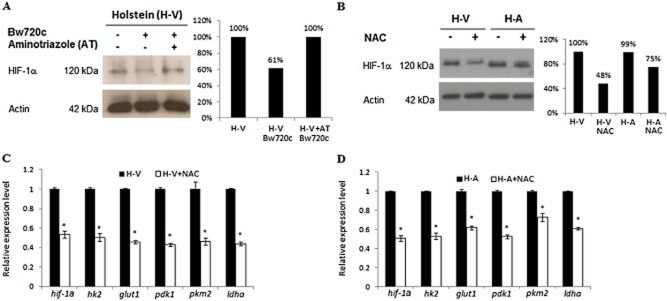
H2O2 output by infected macrophages regulates HIF-1α levels.A. Bw720c treatment reduces HIF-1α levels, but does not inhibit the host cell HIF-1α response when H-V macrophages are stressed by aminotriazole (AT) treatment.B. Lowering H2O2 levels by NAC treatment also reduces HIF-1a expression in both H-V and H-A macrophages.C. NAC treatment of H-V reduces the expression of hif-1α and 4 different hif-1α-target genes.D. Similarly, NAC treatment of H-A reduces the expression of hif-1α and 4 different hif-1α-target genes. *Student’s t-test, highest P-value = 0.013 < 0.05.
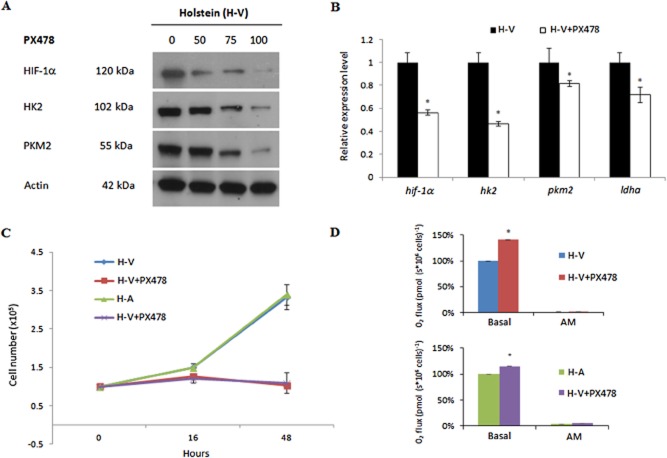
Loss of HF-1α provokes a shift to more oxidative phosphorylation and reduced macrophage proliferation
To directly link HIF-1α to HK2, PKM2 and LDHA expression, infected Holstein-Friesian macrophages were treated with increasing doses of the specific HIF-1α inhibitor PX478 (Fig. 6). HIF-1α levels were progressively lost with increasing concentrations of PX478 and consequently HK2 and PKM2 diminished according (Fig. 6A). Complete loss of HIF-1α occurred after treatment with 100 μM PX478 for 16 h and this reduced transcription of hif-1α, hk2, pkm2 and ldha (Fig. 6B) and initiated an arrest of infected macrophage proliferation and prolonged inhibition leads to a complete block in growth (Fig. 6C). Upon PX478-induced loss of HIF-1α both H-V and H-A macrophages consume more oxygen indicating that they are more OXPHOS-like (Fig. 6C).
Figure 6.
Loss of HIF-1α provokes a shift to more oxidative phosphorylation and reduced macrophage proliferation.A. 16 h treatment of H-V macrophages with increasing concentrations of PX478 results in reduced levels of HIF-1α, HK2 and PKM2 compared with actin.B. Treatment with PX748 at 100 μM for 16 h reduces expression of hif-1a, hk2, pkm2 and ldha.C. 16 h of PX748 at 100 μM initiates a proliferation arrest that becomes pronounced at 48 h.D. Three independent Oroboros measurements of both H-A and H-V macrophages treated for 16 h with PX748 show O2 fluxes increase and respiration is more mitochondrial, as upon antimycin (AM) inhibition of complex III O2 flux is strongly diminished. *Student’s t-test, highest P-value = 0.03395 < 0.05.
Discussion
We have shown that monocyte invasion by T. annulata sporozoites activates hif-1α transcription and that HIF-1A remains induced even as transformed macrophage virulence is lost. However, it is possible that the pathways leading to HIF-1α activation immediately following invasion differ slightly to those that maintain HIF-1α induction in established Theileria-transformed cell lines. The hif-1a promoter is known to have binding sites for HIF-1α, NF-κB and AP-1 (Frede et al., 2007). As both NF-κB and AP-1 are induced by Theileria infection (Palmer et al., 1997; Botteron and Dobbeldeare, 1998; Chaussepied et al., 1998), it is likely that these two transcription factors participate in the rapid induction of hif-1α transcription post sporozoite invasion. Once induced HIF-1α can contribute to maintaining its own transcription and the loss of hif-1α message upon PX478 inhibition of HIF-1α (Fig. 6) is entirely consistent with this notion. Indeed, this may compensate for the loss of AP-1-driven transcription that characterizes attenuated vaccine lines (Baylis et al., 1995; Adamson et al., 2000).
We examined also the expression profiles of 6 genes (glut1, glut3, hk2, pdk1, ldha and pkm2) known to be under the control of HIF-1α (Keith et al., 2012). This showed that Theileria infection has activated a leukocyte transcriptional programme typical of Warburg glycolysis (e.g. higher expression of hk2 and pkm2), which is normally associated with cells experiencing hypoxia (Levine and Puzio-Kuter, 2010). This was unexpected for Theileria-infected macrophages that are consuming O2 (Fig. 3C). We therefore performed global metabolomic analyses on virulent (H-V) compared with attenuated (H-A) T. annulata-infected macrophages cells and compared the levels of intracellular metabolites associated with HK2, PKM2 and LDHA (Fig. 3D). This confirmed that Theileria infection confers on its host leukocyte (macrophage and B cell) high glucose consumption and lactate output (only intracellular lactate was measured so the levels in Fig. 3D and Fig. S1D do not account for secreted lactate), characteristics typical of Warburg-like glycolysis. We considered that the metabolome of infected TBL3 cells is composed of metabolites coming not only from leukocyte glycolysis, but also from the parasite glycolytic pathway, as apicomplexan parasites are known to produce abundant lactate (Ginsburg, 2006). However, HK2 expression is typical of mammalian cells performing Warburg glycolysis (Wolf et al., 2011) and only infected leukocytes express HK2 (see Fig. S1B).
Attenuated T. annulata-infected macrophages are used as live vaccines against tropical theileriosis, because the cells have lost their virulent hyper-invasiveness (Hall et al., 1999). When we examined virulent compared with attenuated T. annulata-infected macrophages we expected to find reduced HIF-1α levels, since HIF-1α activation is frequently associated with aggressive tumours and poor prognosis (Jubb et al., 2010). Instead, HIF-1α levels were unchanged in attenuated infected macrophages and consequently HK2 levels also remained constant (Fig. 2D). Since, expression of HIF-1α and its target-genes remained high, even as infected macrophages lose virulence, we compared production and consumption of glycolysis metabolites between virulent and attenuated infected macrophages (Fig. 3D). Attenuated macrophages consume more glucose and produce more lactate than virulent infected macrophages indicating that upon attenuation glycolytic activity increased i.e. in spite of being attenuated the cells have become more Warburg-like. Since the virulent versus attenuated metabolomes were derived on the same background (the same Holstein-Friesian macrophages infected with Theileria annulata), the differences observed are due to host cell metabolites, as live intracellular parasites are always present.
Combined, the data indicate that T. annulata infection drives its host cell to perform Warburg-like glycolysis even though the 2 different infected (H and S) macrophage lines examined here are proliferating under normoxic conditions. Why Theileria-infected macrophages should maintain a HIF-1α-driven transcriptional programme typical of Warburg glycolysis is curious, but as infection induces hif-1α transcription within two hours of sporozoite invasion it is conceivable that the infected host cell switches to Warburg-like glycolysis in order to keep the level infection-induced ROS under toxic levels. However, a few Theileria-infected cell lines like TBL3 grow in suspension as clusters that could result in them experiencing hypoxia that would contribute to HIF-1α activation. Indeed, a recent TBL3-based study showed that HIF-1α inhibition led to a reduced formation of colonies in soft agar (Medjkane et al., 2013), whereas as in attenuated macrophages HIF-1α is maintained even though virulence is lost. This implies that in attenuated T. annulata-infected macrophages HIF-1α is driving a transcriptional programme unrelated to transformed leucocyte invasiveness, such as Glut1-mediated glucose uptake necessary for infected leucocyte proliferation. Specific PX478 inhibition of HIF-1α confirmed its role in macrophage proliferation (Fig. 6C) and it is possible that the failure of TBL3 cells to form colonies in soft agar upon HIF-1α inhibition (Medjkane et al., 2013) is associated with a proliferation defect. Comparing the HIF-1α transcriptional programmes of virulent compared with attenuated T. annulata-infected macrophages should allow the identification of the subset of HIF-1α-target genes regulating hyper invasiveness of Theileria-transformed macrophages.
We have shown that two independent attenuated macrophage lines are more stressed their virulent counterparts. Attenuation is concomitant with loss of JNK activity (Chaussepied and Langsley, 1996; Galley et al., 1997; Chaussepied et al., 1998) and JNK activation can mediate an anti-oxidant response (Shen and Liu, 2006; Fang et al., 2009). Loss of JNK activity might render attenuated macrophages less capable of controlling oxidative stress and hence, they produce more H2O2. Furthering our understanding of how varying levels of H2O2 output is linked to Theileria-induced virulence and eventual attenuation will stimulate future studies.
Experimental procedures
Theileria-infected cell lines
Holstein-Friesian monocytes were prepared and infected with T. annulata sporozoites, as described in Jensen et al. (2008). The characterization of the Holstein-Friesian Ode vaccine line from India has been reported previously (Singh, 1990). In this study virulent Holstein-Friesian (H-V) corresponds to Ode passage 61–70 and attenuated (H-A) to Ode passage 309–322. BL3 and TBL3 cells used in this study have been characterized (Theilen et al., 1968; Moreau et al., 1999). In this study the virulent Sahiwal/Holstein-Friesian crossbreed-derived T. annulata-infected cell-line (S-V) corresponds to cloned Jed4 passage 18 and the attenuated cell-line (S-A) to cloned Jed4 passage 333 (Darghouth et al., 1996). All cultures were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 100 μg ml−1 penicillin, 100 UI ml−1 streptomycin, 2 mM l-glutamine, 10 mM Hepes and 5% 2-mercapthoethanol for BL3 and TBL3. Holstein-Friesian cells were treated 72 h with the drug buparvaquone (Bw720c) to eliminate the parasite as described in Lizundia et al. (2006); 24 h with 3-Amino-1,2,4-triazole (Sigma A8056-10G) to block catalase activity (Bayliak et al., 2008; Walton and Pizzitelli, 2012); 24 h with N-acetylcysteine (NAC, Sigma) and for up to 16 h with PX478 to inhibit HIF-1α (MedKoo Biosciences).
Western blot analysis
After indicated treatments cells were harvested and were extracted with lysis buffer (Hepes 20 mM, pH 8; NaCl 150 mM; EDTA 2 mM; Nonidet P40 1%; SDS 0.1%; Sodium deoxycholate 0.5% containing proteases inhibitors (Complete mini EDTA free, Roche) and phosphatase (PhosSTOP, Roche). Lysates were centrifuged at 13 000 r.p.m. for 15 min at 4°C, and supernatants collected. Equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose membrane (Protran, Whatman) at 30 V overnight at 4°C and blocked with 4% skimmed milk for 1 h. The antibodies used in immunoblotting were as follows: anti-HIF-1α (Abcam, ab2185); anti-HK2 (Cell Signaling, #2867S); Anti-PI3-K (p85) antibody (Merck Millipore, 06-195); anti-PKM2 (Cell Signaling #3198S) and anti-actin (I-19, Santa Cruz Biotechnology).
Total RNA extraction
Total RNA was isolated from each of the T. annulata-infected cell lines using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. The quality and quantity of the resulting RNA was determined using a Nanodrop spectrophotometer. mRNA was reverse transcribed to first-strand cDNA and the relative levels of each transcript were quantified by real-time PCR using SYBR Green detection. The detection of a single product was verified by dissociation curve analysis and relative quantities of mRNA calculated using the method described by (Pfaffl, 2001). Hprt1 was used to normalize mRNA levels.
Primer sequences use for qRT-PCR:
| Sense | Antisense | |
|---|---|---|
| Hif-1a | GCTTGCTCATCAGTTGCCAC | AGCTGATGGTGAGCCTCATA |
| Hif-1b | GGAACCACGACCTTCACTGT | GCCCATCTCCAGGGATAAAT |
| Glut1 | TCTCCGTGGGCCTTTTTGTT | AGGCCAGCAGGTTCATCATC |
| Glut3 | ACTTTGGAAGAGCGGTCAGA | AAGGACCACAGGGATGTGAG |
| Hk1 | GGGACGCTCTACAAGCTTCA | CAGTTCCTTCACGGTTTGGT |
| Hk2 | CCGGGAAGCAACTATTTGAA | TCACCAGGATAAGCCTCACC |
| Pdk1 | CCGCCTATTCAGGTCCATGT | ACCTCCTCGGTCACTCATCT |
| Pkm1 | CCTGATAGCTCGTGAGGCTG | GCTCGCGCAAGTTCTTCAAA |
| Pkm2 | GGAATGAATGTGGCTCGTCT | ATGGTCTCCGCATGGTACTC |
| Ldha | GTTGCTGGTGTCTCCCTGAA | TGTGAACCGCTTTCCACTGT |
Intracellular levels of hydrogen peroxide (H2O2)
Holstein-Friesian (H) T. annulata-infected macrophages (1 × 105 cells per well) were seeded in 96-well plates and incubated 18 h in complete medium. Cells were washed in PBS and incubated with 100 μl per wells of 5 μM H2-DCFDA diluted in PBS (Molecular Probes). H2O2 levels were assayed by spectrofluorimetry on a fusion spectrofluorimeter (PackardBell). Fluorescence intensity was recorded every hour over a period of 5 h. Excitation and emission wavelengths used for H2O2 were 485 and 530 nm. The number of cells was evaluated by the crystal violet assay. Cells were stained in 0.05% crystal violet and 2% ethanol in PBS for 30 min at room temperature. After four washes in PBS, the stain was dissolved in methanol and measured at 550 nm on Fusion. The level of H2O2 was calculated in each sample as follows: reactive oxygen species rate (arbitrary units min−1 105 cells−1) = [fluorescence intensity (arbitrary units) at T300 minutes − fluorescence intensity (arbitrary units) at T0] per 60 min per number of cells as measured by the crystal violet assay.
Adhesion assay
A 96-well plate was coated with bovine fibronectin (Sigma #F1141), 2 μg cm−2 diluted in double distilled water overnight at 4°C. The plate was then washed twice with 100 μl 0.1% BSA in RPMI-1640 and blocked for 1 h at 37°C by 0.5% BSA in RPMI-1640. After two washes, 1 × 104 cells were added to each well and incubated at 37°C, 5% CO2 for 30 min. Non-adherent cells were removed by washing the wells three times before fixing with 100 μl 4% paraformaldehyde for 10 min at room temperature. Following one further wash, wells were stained with 100 μl crystal violet (1 mg ml−1) for 10 min at room temperature. Wells were extensively washed with distilled water and air-dried. Samples were re-suspended by 30 min incubation at room temperature in 100 μl 2% SDS, 2% ethanol before reading the optical density at 595 nm.
Invasion assays of H-transformed cells
The invasive capacity of the Holstein-Friesian T. annulata-infected macrophage cell-line was assessed in vitro using Matrigel migration chambers: culture Coat 96 well medium BME cell invasion assay was obtained from Culturex Instructions (3482-096-K). After 24 h of incubation at 37°C, the top chamber was washed once in buffer and placed back on the receiver plate. One hundred microlitres of cell dissociation solution/Calcein AM was added to the bottom chamber of each well, incubated at 37°C for 1 h to fluorescently label cells and dissociate them from the membrane before reading at 485 nm excitation, 520 nm emission using the same parameters as the standard curve.
Metabolome data
Virulent and attenuated T. annulata-infected H macrophages together with BL3 and TBL3 B cells were prepared according to the Metabolon (http://www.metabolon.com/) protocol. Briefly, leucocytes were flash frozen and shipped on dry ice to Metabolon for profiling. The analyses were performed in quadruplicate and 334 different metabolites were detected. Intracellular metabolites stemming from glycolytic activity associated with hexokinase, pyruvate kinase and lactate dehydrogenase were extracted from the global metabolome for this purpose of this study.
Oroboros measurements of O2 consumption
O2 concentration and consumption by H-V and H-A infected macrophages was measured by a high-resolution respirometer (Oroboros Oxygraph-2k). Both electrodes were calibrated at 37°C and 100% oxygen before adding 2.5 ml of cells (2 × 106 cells ml−1) to each chamber. Antimycin was added at the basal level to block complex III, as an estimate of mitochondrial contribution to overall cell respiration. When the chambers are left closed, cells gradually consume all the O2 present in the media until they become hypoxic and consequently the flux of O2 consumption decreases to 0 [pmole (s*ml)−1].
Acknowledgments
We thank Carole Peyssonnaux for advice and gift of reagents and Souhila Medjkane and Jonathan Weitzman for growing BL3 and TBL3 cells. M.M. is a recipient of a PhD fellowship from the University Paris-Diderot. N.E. is a recipient of a Wellcome Trust PhD training fellowship award and M.C. a Wellcome Trust post-doctoral fellowship. This work was supported by a Wellcome Trust Special Initiative grant (075820/A/04/Z ) for an integrated approach for the development of sustainable methods to control topical theileriosis to G.L. and E.J.G. and by an ANR grant (11 BSV3 01602) to G.L. who also recognises Inserm, the Cnrs and the Labex ParaFrap ANR-11-LABX-0024 for support. E.J.G. acknowledges a BBSRC Institute Strategic Grant.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher’s web-site:
Fig S1 Infected BL3 lymphocytes display signs of aerobic glycolysis.
A. T. annulata-infected TBL3 cells express HIF-1α to greater levels than non-infected BL3 lymphocytes. Actin amounts were used as a loading control.
B. T. annulata infection strongly induces HK2 expression in TBL3 lymphocytes, whereas as in non-infected BL3 cells HK2 is below the level of detection. Actin was used as a loading control.
C. Non-infected BL3 lymphocytes produce less H2O2 than infected TBL3 lymphocytes.
D. Metabolomic analyses highlighting intracellular metabolites stemming from glycolytic activity associated with hexokinase, pyruvate kinase and lactate dehydrogenase in BL3 compared with TBL3 lymphocytes. TBL3 consume more glucose and produce more lactate than BL3, whereas pyruvate levels remain approximately equivalent. The intracellular lactate levels do not take into account lactate secreted into the culture medium.
References
- Adamson R, Logan M, Kinnaird J, Langsley G, Hall R. Loss of matrix metalloproteinase 9 activity in Theileria annulata-attenuated cells is at the transcriptional level and is associated with differentially expressed AP-1 species. Mol Biochem Parasitol. 2000;106:51–61. doi: 10.1016/s0166-6851(99)00213-3. [DOI] [PubMed] [Google Scholar]
- Bayliak M, Gospodaryov D, Semchyshyn H, Lushchak V. Inhibition of catalase by aminotriazole in vivo results in reduction of glucose-6-phosphate dehydrogenase activity in Saccharomyces cerevisiae cells. Biochemistry. 2008;73:420–426. doi: 10.1134/s0006297908040068. [DOI] [PubMed] [Google Scholar]
- Baylis HA, Megson A, Hall R. Infection with Theileria annulata induces expression of matrix metalloproteinase 9 and transcription factor AP-1 in bovine leukocytes. Mol Biochem Parasitol. 1995;69:211–222. doi: 10.1016/0166-6851(94)00216-a. [DOI] [PubMed] [Google Scholar]
- Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, et al. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- Botteron C, Dobbeldeare D. AP-1 and ATF-2 are constitutively activated via the JNK pathway in Theileria parva-transformed T-cells. Biochem Biophys Res Commun. 1998;246:418–421. doi: 10.1006/bbrc.1998.8635. [DOI] [PubMed] [Google Scholar]
- Chaussepied M, Langsley G. Theileria transformation of bovine leukocytes: a parasite model for the study of lymphoproliferation. Res Immunol. 1996;147:127–138. doi: 10.1016/0923-2494(96)83165-8. [DOI] [PubMed] [Google Scholar]
- Chaussepied M, Lallemand D, Moreau MF, Adamson R, Hall R, Langsley G. Upregulation of Jun and Fos family members and permanent JNK activity lead to constitutive AP-1 activation in Theileria-transformed leukocytes. Mol Biochem Parasitol. 1998;94:215–226. doi: 10.1016/s0166-6851(98)00070-x. [DOI] [PubMed] [Google Scholar]
- Chaussepied M, Janski N, Baumgartner M, Lizundia R, Jensen K, Weir W, et al. TGF-b2 induction regulates invasiveness of Theileria-transformed leukocytes and disease susceptibility. PLoS Pathog. 2010;6:e1001197. doi: 10.1371/journal.ppat.1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darghouth MA. Review on the experience with live attenuated vaccines against tropical theileriosis in Tunisia: considerations for the present and implications for the future. Vaccine. 2008;26(Suppl. 6):G4–G10. doi: 10.1016/j.vaccine.2008.09.065. [DOI] [PubMed] [Google Scholar]
- Darghouth MA, Ben Miled L, Bouattour A, Melrose TR, Brown CG, Kilani M. A preliminary study on the attenuation of Tunisian schizont-infected cell lines of Theileria annulata. Parasitol Res. 82:647–655. doi: 10.1007/s004360050179. [DOI] [PubMed] [Google Scholar]
- Dobbelaere D, Heussler V. Transformation of leukocytes by Theileria parva and T. annulata. Annu Rev Microbiol. 1996;53:1–42. doi: 10.1146/annurev.micro.53.1.1. 1999. [DOI] [PubMed] [Google Scholar]
- Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–419. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- Galley Y, Hagens G, Glaser I, Davis W, Eichhorn M, Dobbelaere D. Jun NH2-terminal kinase is constitutively activated in T cells transformed by the intracellular parasite Theileria parva. Proc Natl Acad Sci USA. 1997;94:5119–5124. doi: 10.1073/pnas.94.10.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H. Progress in in silico functional genomics: the malaria Metabolic Pathways database. Trends Parasitol. 2006;22:238–240. doi: 10.1016/j.pt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Hall R, Ilhan T, Kirvar E, Wilkie G, Preston PM, Darghouth M, et al. Mechanism(s) of attenuation of Theileria annulata vaccine cell lines. Trop Med Int Health. 1999;4:A78–A84. doi: 10.1046/j.1365-3156.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- Jensen K, Talbot R, Paxton E, Waddington D, Glass EJ. Development and validation of a bovine macrophage specific cDNA microarray. BMC Genomics. 2006;7:224. doi: 10.1186/1471-2164-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Paxton E, Waddington D, Talbot R, Darghouth MA, Glass EJ. Differences in the transcriptional responses induced by Theileria annulata infection in bovine monocytes derived from resistant and susceptible cattle breeds. Int J Parasitol. 2008;38:313–325. doi: 10.1016/j.ijpara.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Jubb AM, Buffa FM, Harris AL. Assessment of tumour hypoxia for prediction of response to therapy and cancer prognosis. J Cell Mol Med. 2010;14:18–29. doi: 10.1111/j.1582-4934.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MY, Powis G. Passing the baton: the HIF switch. Trends Biochem Sci. 2012;37:364–372. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- Lizundia R, Chaussepied M, Huerre M, Werling D, Di Santo JP, Langsley G. c-Jun NH2-terminal kinase/c-Jun signaling promotes survival and metastasis of B lymphocytes transformed by Theileria. Cancer Res. 2006;66:6105–6110. doi: 10.1158/0008-5472.CAN-05-3861. [DOI] [PubMed] [Google Scholar]
- Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- Medjkane S, Perichon M, Marsolier J, Dairou J, Weitzman JB. Theileria induces oxidative stress and HIF1α activation that are essential for host leukocyte transformation. Oncogene. 2013 doi: 10.1038/onc.2013.134. Advance online publication 13 May 2013. doi: 10.1038/onc.2013.134. [DOI] [PubMed] [Google Scholar]
- Moreau MF, Thibaud JL, Miled LB, Chaussepied M, Baumgartner M, Davis WC, et al. Theileria annulata in CD5(+) macrophages and B1 B cells. Infect Immun. 1999;67:6678–6682. doi: 10.1128/iai.67.12.6678-6682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer GH, Machado J, Jr, Fernandez P, Heussler V, Perinat T, Dobbelaere DA. Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection. Proc Natl Acad Sci USA. 1997;94:12527–12532. doi: 10.1073/pnas.94.23.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux V, Mounier R, Brown AD, Steinback CD, Rawling JM, Poulin MJ. Relationship between oxidative stress and HIF-1 alpha mRNA during sustained hypoxia in humans. Free Radic Biol Med. 2009;46:321–326. doi: 10.1016/j.freeradbiomed.2008.10.047. [DOI] [PubMed] [Google Scholar]
- Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Singh DK. Methods currently used for the control of Theileria annulata: their validity and proposals for future control strategies. Parassitologia. 1990;32:33–40. [PubMed] [Google Scholar]
- Spear W, Chan D, Coppens I, Johnson RS, Giaccia A, Blader IJ. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–352. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- Theilen GH, Rush JD, Nelson-Rees WA, Dungworth DL, Munn RJ, Switzer JW. Bovine leukemia: establishment and morphologic characterization of continuous cell suspension culture, BL-1. J Natl Cancer Inst. 1968;40:737–749. [PubMed] [Google Scholar]
- Walton PA, Pizzitelli M. Effects of peroxisomal catalase inhibition on mitochondrial function. Front Physiol. 2012;3:108. doi: 10.3389/fphys.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- Wiley M, Sweeney KR, Chan DA, Brown KM, McMurtrey C, Howard EW, et al. Toxoplasma gondii activates hypoxia-inducible factor (HIF) by stabilizing the HIF-1alpha subunit via type I activin-like receptor kinase receptor signaling. J Biol Chem. 2010;285:26852–26860. doi: 10.1074/jbc.M110.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1 Infected BL3 lymphocytes display signs of aerobic glycolysis.
A. T. annulata-infected TBL3 cells express HIF-1α to greater levels than non-infected BL3 lymphocytes. Actin amounts were used as a loading control.
B. T. annulata infection strongly induces HK2 expression in TBL3 lymphocytes, whereas as in non-infected BL3 cells HK2 is below the level of detection. Actin was used as a loading control.
C. Non-infected BL3 lymphocytes produce less H2O2 than infected TBL3 lymphocytes.
D. Metabolomic analyses highlighting intracellular metabolites stemming from glycolytic activity associated with hexokinase, pyruvate kinase and lactate dehydrogenase in BL3 compared with TBL3 lymphocytes. TBL3 consume more glucose and produce more lactate than BL3, whereas pyruvate levels remain approximately equivalent. The intracellular lactate levels do not take into account lactate secreted into the culture medium.