Abstract
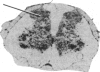
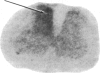
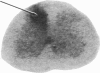
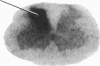
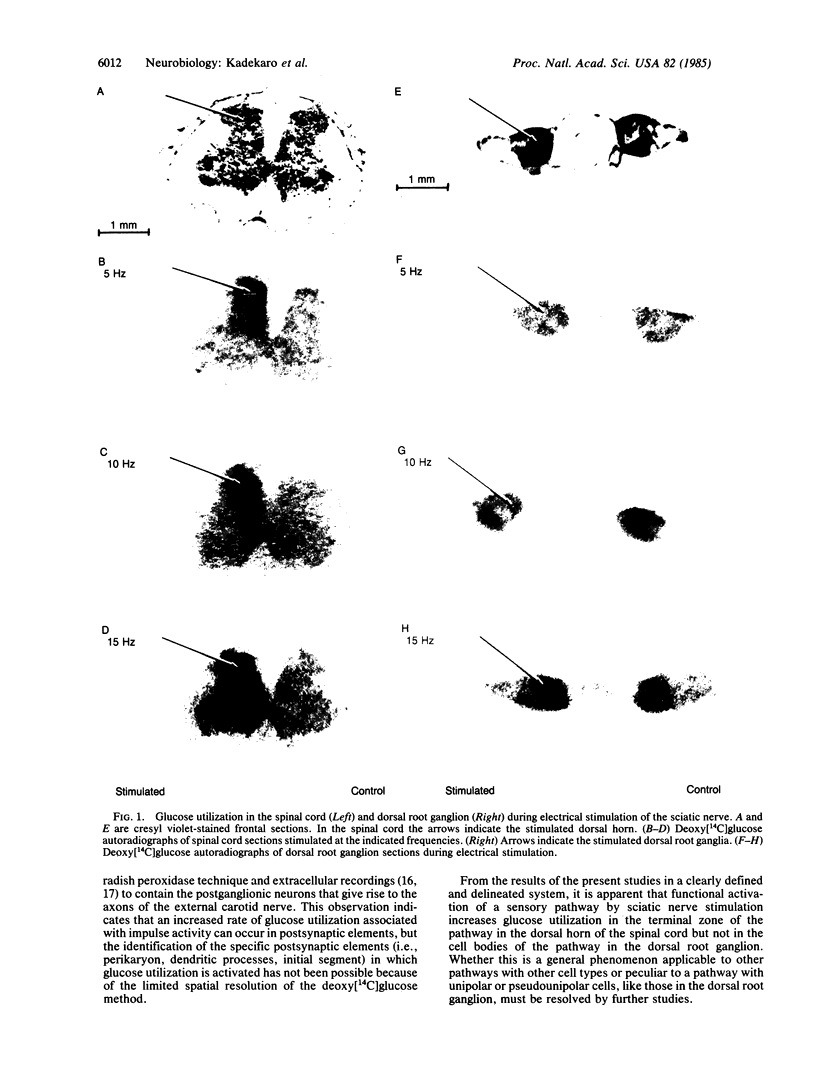
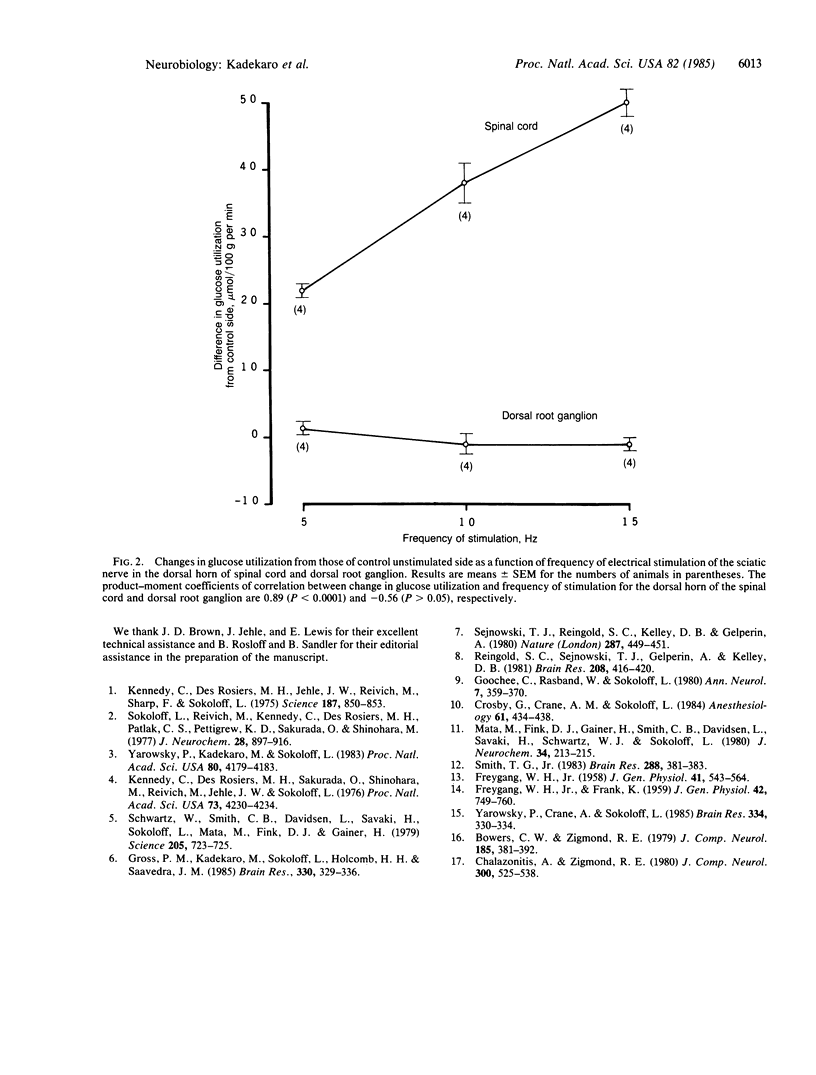
Electrical stimulation of the proximal stump of the transected sciatic nerve produces a frequency-dependent activation of glucose utilization, measured with the autoradiographic deoxy [14C]glucose method, in the dorsal horn of the spinal cord but produces no change in glucose utilization in the dorsal root ganglion cells. These results suggest that axon terminals and not the cell bodies are the sites of enhanced metabolic activity during increased functional activity of this pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers C. W., Zigmond R. E. Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J Comp Neurol. 1979 May 15;185(2):381–391. doi: 10.1002/cne.901850211. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A., Zigmond R. E. Effects of synaptic and antidromic stimulation on tyrosine hydroxylase activity in the rat superior cervical ganglion. J Physiol. 1980 Mar;300:525–538. doi: 10.1113/jphysiol.1980.sp013177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby G., Crane A. M., Sokoloff L. A comparison of local rates of glucose utilization in spinal cord and brain in conscious and nitrous oxide- or pentobarbital-treated rats. Anesthesiology. 1984 Oct;61(4):434–438. doi: 10.1097/00000542-198410000-00012. [DOI] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr An analysis of extracellular potentials from single neurons in the lateral geniculate nucleus of the cat. J Gen Physiol. 1958 Jan 20;41(3):543–564. doi: 10.1085/jgp.41.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, FRANK K. Extracellular potentials from single spinal motoneurons. J Gen Physiol. 1959 Mar 20;42(4):749–760. doi: 10.1085/jgp.42.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goochee C., Rasband W., Sokoloff L. Computerized densitometry and color coding of [14C] deoxyglucose autoradiographs. Ann Neurol. 1980 Apr;7(4):359–370. doi: 10.1002/ana.410070414. [DOI] [PubMed] [Google Scholar]
- Gross P. M., Kadekaro M., Sokoloff L., Holcomb H. H., Saavedra J. M. Alterations of local cerebral glucose utilization during chronic dehydration in rats. Brain Res. 1985 Mar 25;330(2):329–336. doi: 10.1016/0006-8993(85)90693-6. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Des Rosiers M. H., Jehle J. W., Reivich M., Sharpe F., Sokoloff L. Mapping of functional neural pathways by autoradiographic survey of local metabolic rate with (14C)deoxyglucose. Science. 1975 Mar 7;187(4179):850–853. doi: 10.1126/science.1114332. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Des Rosiers M. H., Sakurada O., Shinohara M., Reivich M., Jehle J. W., Sokoloff L. Metabolic mapping of the primary visual system of the monkey by means of the autoradiographic [14C]deoxyglucose technique. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4230–4234. doi: 10.1073/pnas.73.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M., Fink D. J., Gainer H., Smith C. B., Davidsen L., Savaki H., Schwartz W. J., Sokoloff L. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem. 1980 Jan;34(1):213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- Reingold S. C., Sejnowski T. J., Gelperin A., Kelley D. B. [3H]-2-deoxyglucose autoradiography in a molluscan nervous system. Brain Res. 1981 Mar 16;208(2):416–420. doi: 10.1016/0006-8993(81)90569-2. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Smith C. B., Davidsen L., Savaki H., Sokoloff L., Mata M., Fink D. J., Gainer H. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science. 1979 Aug 17;205(4407):723–725. doi: 10.1126/science.462184. [DOI] [PubMed] [Google Scholar]
- Sejnowski T. J., Reingold S. C., Kelley D. B., Gelperin A. Localization of[3H]-2-deoxyglucose in single molluscan neurones. Nature. 1980 Oct 2;287(5781):449–451. doi: 10.1038/287449a0. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Jr Sites of action potential generation in cultured vertebrate neurons. Brain Res. 1983 Dec 12;288(1-2):381–383. doi: 10.1016/0006-8993(83)90124-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Yarowsky P., Crane A., Sokoloff L. Metabolic activation of specific postsynaptic elements in superior cervical ganglion by antidromic stimulation of external carotid nerve. Brain Res. 1985 May 20;334(2):330–334. doi: 10.1016/0006-8993(85)90226-4. [DOI] [PubMed] [Google Scholar]
- Yarowsky P., Kadekaro M., Sokoloff L. Frequency-dependent activation of glucose utilization in the superior cervical ganglion by electrical stimulation of cervical sympathetic trunk. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4179–4183. doi: 10.1073/pnas.80.13.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]