Abstract
Antibiotic variation among pediatric oncology patients has not been well-described. Identification of significant variability in antibiotic use within this population would warrant evaluation of its clinical impact. We conducted a retrospective cohort study of newly diagnosed patients with pediatric acute lymophoblastic leukemia (ALL) hospitalized from 1999 to 2009 in 39 freestanding US children’s hospitals within the Pediatric Health Information System. Medication use data were obtained for the first 30 days from each patient’s index ALL admission date. Antibiotic exposure rates were reported as antibiotic days/1000 hospital days. Unadjusted composite broad-spectrum antibiotic exposure rates varied from 577 to 1628 antibiotic days/1000 hospital days. This wide range of antibiotic exposure was unaffected by adjustment for age, gender, race and days of severe illness (adjusted range: 532–1635 days of antibiotic therapy/1000 hospital days). Antibiotic use for children with newly diagnosed ALL varies widely across children’s hospitals and is not explained by demographics or illness severity.
Keywords: Leukemia, pediatrics, benchmarking, drug resistance, microbial
Introduction
Excessive antimicrobial use leads to unnecessary drug-related adverse effects and increased healthcare costs, and promotes the emergence of antimicrobial resistance [1]. Although defining appropriate antimicrobial use is relatively straightforward for clinical conditions that present stereotypically and without complications, such as group A streptococcal pharyngitis or acute otitis media, this task proves more challenging when evaluating more complex conditions. Despite these inherent challenges, it is critically important to identify and subsequently reduce unnecessary antibiotic exposure in these complex clinical settings in order to preserve antibiotic effectiveness.
To this end, benchmarking antibiotic use across hospitals provides a specific metric for hospitals to assess their antibiotic administration practices. Comparing overall antibiotic use across children ‘s hospitals, Gerber et al. identified significant variability in both total and broad-spectrum antibiotic use, despite adjustment for a variety of patient demographic and clinical characteristics [2]. This analysis, however, did not compare use among patients with similar diagnoses. Polk et al. quantified antibiotic use across hospitalized adults, grouping patients by “ service lines “ in an attempt to benchmark antibiotic use within similar patient diagnoses [3]. Although useful, the use of service lines can be limited by their: (1) reliance upon administrative codes, which, if not validated, may misclassify patients with different illnesses; (2) grouping of clinically variable conditions within a given service line; and (3) inability to account for the time since diagnosis for patients with chronic diseases – all of which might impact the indication for antimicrobial therapy.
The use of a more homogeneous patient population would enable more precise benchmarking of disease-specific hospital antibiotic use. Therefore, we compared hospital antibiotic prescribing practices for children hospitalized with newly diagnosed acute lymphoblastic leukemia (ALL). Children with newly diagnosed ALL are an ideal population for benchmarking antibiotic use because: (1) they represent a relatively homogeneous population with a high risk for infection and, thus, frequent, early antibiotic exposures; (2) a large cohort of children with ALL has been assembled and validated using the Pediatric Health Information Systems (PHIS) [4]; and (3) PHIS hospitals serve as major US centers for the care of children with ALL. We hypothesized that, despite this homogeneous patient population and the existence of practice guidelines for antibiotic administration [5], significant variation in antibiotic prescribing practices across these institutions would exist.
Materials and methods
Study design
We performed a retrospective cohort study to compare hospital antibiotic prescribing practices for children with newly diagnosed ALL at PHIS contributing pediatric institutions from 1 January 1999 through 31 December 2009.
Data source
The PHIS database has been described in detail in previous publications [4,6]. In brief, PHIS is a comparative pediatric administrative database including inpatient data from 43 not-for-profit, tertiary children’s hospitals affiliated with the Child Health Corporation of America (Overland Park, KS). PHIS data include demographics, dates of service, discharge disposition, and up to 40 ICD-9 (International Classification of Diseases, 9th Revision) discharge diagnosis and procedure codes for each admission. Additionally, PHIS data provide billed resource utilization information, including all pharmaceuticals, for each patient for every hospital day of service. Patients are assigned a unique identifier in the PHIS database that is preserved for all admissions. Therefore, patient admissions can be followed over time.
Oversight of the methods to maintain PHIS data quality is a joint effort between the Child Health Corporation of America, Thomson Reuters Healthcare (data processing partner) and participating hospitals. After file submission to Thomson Reuters, data quality audits are performed. These audits primarily check for valid entries (e.g. valid ICD-9 diagnosis codes) and reasonable patient information (e.g. birth weight). Known data quality issues are transparently communicated to all PHIS data users.
Study cohort assembly
Individual hospitals served as the primary unit of analysis. PHIS data were interrogated in a three-step process to identify all patients at a hospital with presumed newly diagnosed ALL. This process has been previously described and validated at one of the PHIS institutions [4]. Briefly, the PHIS database was first screened for first admissions containing an ICD-9 discharge diagnosis code consistent with ALL (204.xx). Next, patients were excluded if PHIS data elements suggested an alternative malignancy or receipt of a stem cell transplant during the index ALL admission. Finally, an extensive manual review of chemotherapy billing data in PHIS was performed to identify patients with chemotherapy patterns consistent with ALL induction therapy, the cohort considered for subsequent analyses.
Outcome
Antimicrobial use
The primary goal of this study was to describe the variation in antibiotic use across a cohort of freestanding pediatric hospitals in the United States. The analyses focused on broad-spectrum antimicrobial agents often administered to children hospitalized with ALL. Specifically, data on the following categories of antimicrobial agents were collected: (1) gram-positive agents (vancomycin, daptomycin, clindamycin and linezolid); (2) broad-spectrum penicillins (ampicillin–sulbactam, piperacillin, piperacillin–tazobactam, ticarcillin, ticarcillin–clavulanate); (3) third and fourth generation intravenous cephalosporins (cefotaxime, ceftriaxone, ceftazidime and cefepime); (4) carbapenems (ertapenem, imipenem and meropenem); (5) aminoglycosides (gentamicin, amikacin and tobramycin); fluoroquinolones (ciprofloxacin, levofloxacin and moxifloxacin) and other antimicrobial agents (aztreonam, tigecycline, colistin and metronidazole).
Antibiotic utilization rates were calculated at the hospital level. The unit of measurement was days of antibiotic therapy (DOT) per 1000 inpatient days. On a given hospital day, a patient may have received more than one antibiotic agent; for instance, a patient administered vancomycin, cefepime and gentamicin on one hospital day would have three DOTs per one hospital day. Each hospital’s DOT per 1000 hospital days was calculated as a composite of each antibiotic listed above. Additionally, similar calculations were performed specifically for broad-spectrum gram-positive agents (vancomycin, daptomycin and linezolid) as well as for those anti-pseudomonal agents used in the setting of fever and neutropenia (cetazidime, cefepime, piperacillin, piperacillin–tazobactam, ticarcillin, ticarcillin–tazobactam, aztreonam, gentamicin, tobramycin, amikacin, imipenem, meropenem, ciprofloxacin and colistin).
Variable definitions
Demographics
Demographic data (age, gender and race) were extracted for each patient and summarized by hospital. Gender was coded as a dichotomous variable (male/female), age in years was considered both as a continuous and a categorical variable, and race was collected as a categorical variable (white, black, Asian, Native American, other or missing). Since a substantial number of patients had missing ethnicity data, no analyses by ethnicity were conducted.
Severity of illness
Although the cohort was limited to patients within the first month of ALL diagnosis and adjusted for age, we considered additional measures to address the potential for residual variation in patient acuity across institutions. Therefore, we assessed the utilization of additional healthcare resources that suggest an increased severity of illness by patients within each hospital. The need for any of the following therapeutics was considered in defining an inpatient day as a severe illness day: (1) administration of at least one vasopressor or cardiac support medication (epinephrine, dopamine, nor-epinephrine, dobutamine or milrinone) on current day and day prior; (2) resource or procedure denoting the insertion of or monitoring for an arterial line; (3) resource indicating respiratory support (continuous positive airway pressure, bilevel positive airway pressure, mechanical ventilation, nitric oxide or surfactant therapy) on current day and day prior; (4) resource indicating the need for extracorporeal membrane oxygenation; (5) performance of hemodialysis or peritoneal dialysis; and (6) the presence of a procedure code that suggested close cardiovascular monitoring (e.g. 38.91: arterial catheterization) or actual resuscitation efforts (e.g. 93.93: non-mechanical methods of resuscitation).
Similar to antibiotic utilization rates, days of severe illness were indexed to total inpatient days at the hospital level. A hospital day that included multiple resources for severe illness would only be counted as a single severe illness day. The unit of measure was days of severe illness (DSI) per 1000 inpatient days. Finally, inpatient mortality rates by hospital were calculated as the number of inpatient deaths during the study period per total number of patients at that hospital.
Statistical analysis
Summary statistics for each of the demographic variables and for DSI for the entire cohort and within each of the institutions were constructed using frequencies and proportions for categorical data elements and means and medians for continuous variables. Unadjusted DOT were calculated as DOT per 1000 hospital days within each institution.
Poisson multivariate regression analysis was used to establish rates of DOT per 1000 hospital days adjusted for variation in demographics (age, gender and race) as well as variation in the rate of DSI per 1000 hospital days at each hospital. Poisson regression assumes that the conditional mean of the outcome (DOT per 1000 hospital days) is equal to the conditional variance. In the event that this assumption was violated (referred to as over-dispersion) then the same regression model using a negative binomial distribution was performed.
Measures of correlation for variation of a hospital’s DOT per 1000 hospital days with their variation in DSI per 1000 hospital days as well as with their variation in inpatient mortality rates were performed using the Spearman correlation coefficient.
The above described data compilation, management and analyses were performed using Microsoft Excel, SAS version 9.2 (Cary, NC) and Stata statistical software version 11.0 (College Station, TX).
Human subjects oversight
The conduct of this study was approved by the Child Health Corporation of America and received an exemption status from the Committee for Protection of Human Subjects at the Children’s Hospital of Philadelphia.
Results
Between 1 January 1999 and 31 December 2009, 8733 patients with suspected newly diagnosed ALL were identified from 39 of the 43 PHIS contributing institutions [4]. From this parent cohort, 443 patients (5.1%) were excluded, either because of a lack of complete 30-day follow-up (310 patients), or because additional review of their chemotherapy regimens suggested that they were not newly diagnosed ALL cases (133 patients), resulting in a final cohort of 8290 patients.
Table I displays the demographic characteristics for the cohort by institution. The inpatient case fatality rate in the first 30 days was 1%. Composite unadjusted total DOT per 1000 hospital days varied from 577 to 1628 DOT per 1000 hospital days (median: 845 DOT per 1000 hospital days).
Table I.
Demographic characteristics, antibiotic exposure rates and rates of severe illness for the entire ALL cohort and by hospital.
| Hospital site | Number of patients (n) | Age (mean, years) | Gender (male, %) | Race* (non-white, %) | Patients receiving any antibiotic † (%) | DOT per 1000 hospital days | DSI per 1000 hospital days |
|---|---|---|---|---|---|---|---|
| Entire cohort | 8290 | 7.1 | 56.0 | 22.7 | 83 | 884.9 | 23.7 |
| 1 | 294 | 6.8 | 54.4 | 25.2 | 86.1 | 691.1 | 15.4 |
| 2 | 345 | 6.9 | 58.0 | 20.0 | 79.4 | 864.1 | 11.9 |
| 3 | 230 | 5.8 | 54.4 | 12.2 | 81.3 | 801.1 | 12.5 |
| 4 | 210 | 7.2 | 59.1 | 35.2 | 83.3 | 985.1 | 28.7 |
| 5 | 280 | 7.0 | 56.1 | 9.6 | 80.0 | 710.2 | 29.6 |
| 6 | 373 | 7.4 | 56.6 | 21.2 | 79.9 | 951.3 | 21.8 |
| 7 | 140 | 8.2 | 65.0 | 8.6 | 76.4 | 849.8 | 19.2 |
| 8 | 275 | 6.7 | 53.5 | 95.3 | 90.6 | 868.6 | 17.7 |
| 9 | 100 | 6.5 | 58.0 | 35.0 | 78.0 | 819.1 | 19.7 |
| 10 | 201 | 7.4 | 54.7 | 11.4 | 81.6 | 728.3 | 22.4 |
| 11 | 174 | 7.0 | 58.1 | 10.9 | 87.4 | 969.8 | 10.0 |
| 12 | 284 | 7.1 | 54.2 | 14.4 | 87.3 | 761.2 | 10.0 |
| 13 | 93 | 7.3 | 60.2 | 0.0 | 77.4 | 802.9 | 11.6 |
| 14 | 78 | 7.5 | 61.5 | 21.8 | 88.5 | 863.2 | 18.8 |
| 15 | 238 | 7.3 | 57.1 | 18.9 | 78.2 | 862.1 | 8.1 |
| 16 | 299 | 7.1 | 56.5 | 12.0 | 86.3 | 832.0 | 19.7 |
| 17 | 103 | 6.3 | 57.3 | 31.1 | 94.2 | 1099.2 | 48.6 |
| 18 | 138 | 7.5 | 50.7 | 20.3 | 74.6 | 704.8 | 10.5 |
| 19 | 80 | 7.8 | 51.3 | 23.8 | 86.3 | 762.2 | 5.3 |
| 20 | 277 | 7.0 | 55.2 | 16.6 | 81.6 | 839.5 | 10.6 |
| 21 | 591 | 6.4 | 54.8 | 21.7 | 87.3 | 1075.8 | 35.8 |
| 22 | 160 | 6.9 | 53.1 | 11.3 | 86.9 | 1021.2 | 7.5 |
| 23 | 189 | 7.1 | 56.1 | 10.1 | 82.0 | 1118.1 | 10.1 |
| 24 | 555 | 7.5 | 57.3 | 22.2 | 82.0 | 892.1 | 5.7 |
| 25 | 395 | 6.8 | 56.5 | 26.3 | 76.2 | 956.3 | 24.8 |
| 26 | 308 | 7.5 | 58.4 | 33.8 | 80.8 | 918.6 | 15.1 |
| 27 | 231 | 7.1 | 52.8 | 18.2 | 83.1 | 940.2 | 15.5 |
| 28 | 236 | 6.5 | 53.4 | 30.5 | 82.2 | 1002.3 | 18.8 |
| 29 | 105 | 7.7 | 61.9 | 40.0 | 81.0 | 722.2 | 18.5 |
| 30 | 177 | 7.7 | 66.1 | 17.0 | 67.2 | 576.5 | 20.1 |
| 31 | 77 | 8.0 | 62.3 | 44.2 | 98.7 | 1263.3 | 9.3 |
| 32 | 194 | 7.0 | 51.6 | 16.5 | 88.7 | 1628.1 | 12.4 |
| 33 | 140 | 7.0 | 60.7 | 32.2 | 92.9 | 1046.4 | 4.9 |
| 34 | 162 | 7.1 | 58.6 | 17.3 | 80.9 | 1025.3 | 9.1 |
| 35 | 111 | 7.8 | 55.0 | 23.4 | 94.6 | 970.9 | 16.9 |
| 36 | 20 | 7.6 | 30.0 | 5.0 | 80.0 | 691.0 | 13.4 |
| 37 | 52 | 8.3 | 38.5 | 28.9 | 86.5 | 925.1 | 6.9 |
| 38 | 137 | 6.7 | 53.3 | 20.4 | 83.9 | 1014.0 | 8.3 |
| 39 | 238 | 6.8 | 52.1 | 10.5 | 75.6 | 946.4 | 14.7 |
Race was dichotomized as white and non-white.
Includes only those antibiotics listed in the “Methods” section.
ALL, acute lymphoblastic leukemia; DOT, days of therapy; DSI, days of severe illness.
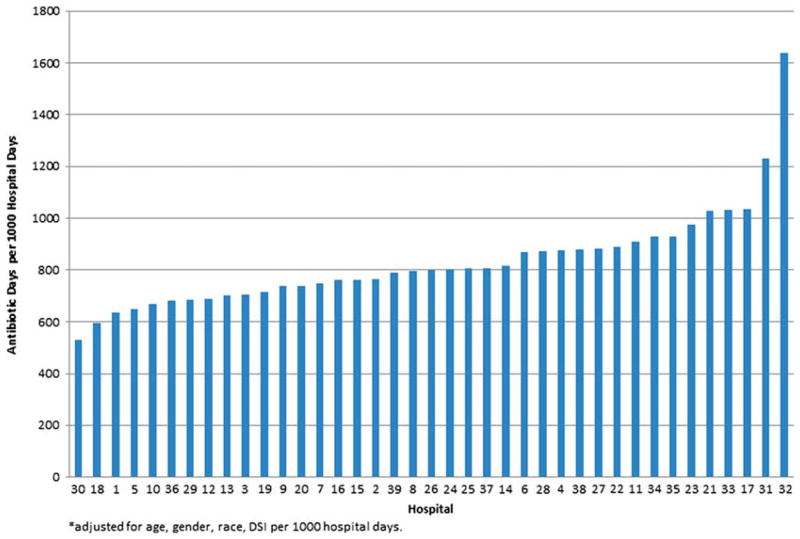
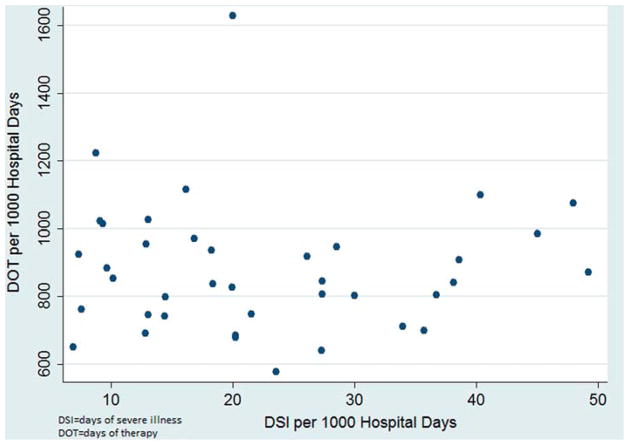
To control for patient-level factors that may impact the decision to prescribe antibiotics, we performed multivariable modeling to adjust for patient age, gender, race and severity of illness (DSI/1000 hospital days) across institutions; since the conditional mean of the outcome was equal to the conditional variance, we employed a negative binomial distribution to establish adjusted rates of DOT per 1000 hospital days by hospital. Adjusted antibiotic use varied minimally from unadjusted estimates for individual hospitals, and the scope of the variability (Figure 1) across hospitals was similar (unadjusted range: 577–1628 DOT per 1000 hospital days; versus adjusted range: 532–1635 DOT per 1000 hospital days; median: 758 DOT per 1000 hospital days). Although differences in DSI per 1000 hospital days existed between hospitals (Table I and Figure 2), this variation did not explain nor did it correlate with the variation of DOT per 1000 hospital days (Spearman’s correlation coefficient: rho=−0.04, p = 0.8). Similar to DSI per 1000 hospital days, the in-patient mortality rates at each hospital varied during the study period, ranging from 0 to 2.9% with a median of 0.71%. However, this variation in hospital in-patient mortality also did not correlate positively or negatively with variation of DOT per 1000 hospital days (Spearman’s correlation coefficient: rho=0.09, p =0.6).
Figure 1.
Variation in antibiotic use among children with ALL across 39 children’s hospitals, adjusted for age, gender, race, DSI per 1000 hospital days.
Figure 2.
Scatter plot highlighting the lack of correlation for a Hospital’s days of severe illness and Days of antibiotic therapy.
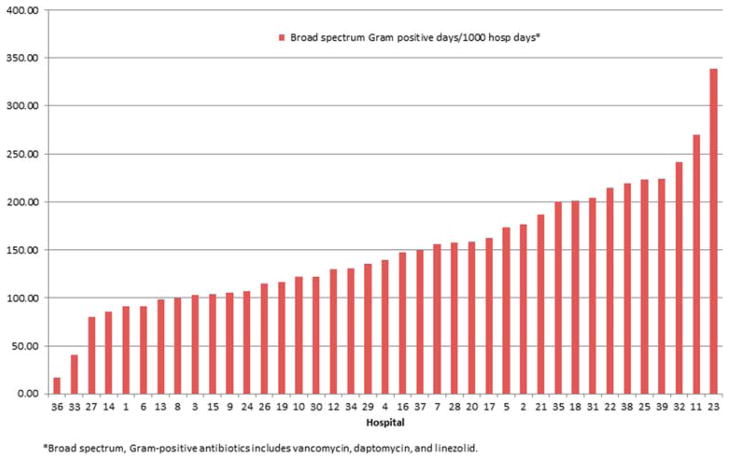
To further explore the variation in DOT between hospitals, we analyzed prescribing rates of specific antibiotic subgroups. Given that adjustment via multivariable modeling had no effect on analyses of overall antibiotic use, these subgroup analyses are shown as unadjusted rates. Figure 3 displays the composite DOT per 1000 hospital days for anti-pseudomonal agents commonly used in this patient population. These analyses demonstrated broad variation in both the predominant choice for an anti-pseudomonal agent as well as the rate of overall anti-pseudomonal antibiotic use across institutions (range: 378–1071 DOT per 1000 hospital days; median: 639 DOT per 1000 hospital days). Likewise, broad-spectrum gram-positive antibiotic use (vancomycin, daptomycin and linezolid) varied considerably across institutions (range: 17–339 DOT per 1000 hospital days; median: 139 DOT per 1000 hospital days) (Figure 4).
Figure 3.
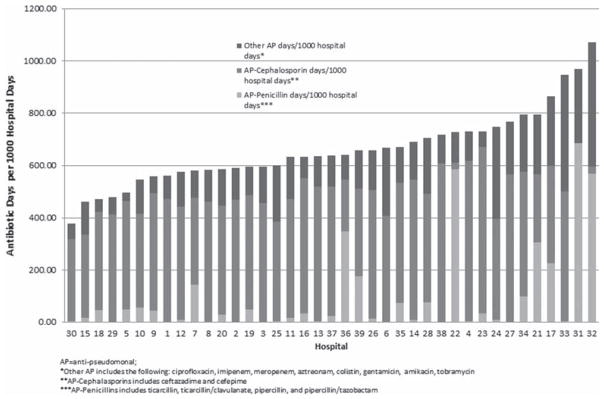
Variation in anti-pseudomonal (AP) antibiotic use across 39 children’s hospitals. *Other AP includes ciprofloxacin, imipenem, meropenem, aztreonam, colistin, gentamicin, amikacin, tobramycin. ** AP-cephalosporins include ceftazidime, cefepime. ***AP-penicillins include ticarcillin, ticarcillin–clavulanate, piperacillin, piperacillin–tazobactam.
Figure 4.
Variation in broad-spectrum gram-positive antibiotic use among children with ALL across 39 children’s hospitals. Broad-spectrum gram-positive antibiotics include vancomycin, daptomycin, linezolid.
Discussion
This is the first report describing variation in antibiotic use for hospitalized children with the same illness, in this case newly diagnosed ALL. Freestanding children’s hospitals – a common setting for the treatment of children with leukemia – varied broadly in both the amount and choice of antibiotic administration. Moreover, this variation in antibiotic prescribing practices could not be attributed to variation in patient level of acuity, or in-hospital mortality rates (1%).
This inequity in antibiotic utilization for a specific patient population at a similar point in their disease across similar hospitals is concerning. Although these analyses cannot determine “appropriate” antibiotic prescribing, the more than two-fold differences in total, anti-pseudomonal and broad-spectrum gram-positive antibiotic prescribing across centers suggests that either (1) some hospitals are under-treating patients with antibiotics, or (2) some hospitals are exposing patients to excessive quantities of these drugs and, therefore, subjecting patients to unnecessary risks. Although the low mortality rate of this cohort is consistent with the latter, more detailed analyses are necessary to confirm this hypothesis. Given the extensively documented association of increasing rates of resistant infections with increased administration of inpatient antibiotics [1,7–11], such studies are critical. In an attempt to curb inpatient antibiotic utilization, the Infectious Diseases Society of America has published and revised guidelines regarding inpatient antimicrobial administration [1,12,13] followed-up by specific guidelines to help standardize antibiotic prescribing practices in patients with cancer [5]. Despite the presence of these guidelines, our data suggest that, even in a homogeneous population of pediatric patients in a defined period of disease, antibiotic use is far from standardized.
Evaluating antibiotic prescribing practices across hospitals is of long-standing interest. In 1970, Scheckler and Bennett described variation in the prevalence of antibiotic use across seven community hospitals and between various hospital services [14]. Recent studies have focused on establishing benchmarks for inpatient antibiotic prescribing practices. In 2008, Kuster et al. compared their inpatient antibiotic utilization rate to rates published at other institutions [15]. Although interesting, these comparisons were limited by differences in how antibiotic utilization rates were calculated, and did not adjust for patient diagnoses or level of acuity. More recent studies have employed unique approaches to report risk-adjusted antibiotic prescribing practices across hospitals. Polk et al. provided standardized antibiotic utilization rates for hospitalized adults by “service lines,” but were unable to compare specific diagnoses and did not have particularly robust severity of illness adjustment [3]. Gerber et al. established risk-adjusted rates of antibiotic prescribing across children’s hospitals, accounting for demographic and clinical characteristics, but their analyses did not assess variability within specific diagnoses [2].
Although these studies provide an important foundation for comparing antimicrobial use across institutions, the establishment of risk-adjusted antibiotic utilization indices within a specific patient population at a similar time point in their illness is an important additional step forward in evidence based pediatric practice. Specifically, pediatric oncologists at academic children’s hospitals can use these data to compare their antibiotic prescribing practices with peer institutions. Ideally, a system that provides institutions with this information and provides continuous feedback over time may help establish more appropriate antibiotic utilization practices for this and other patient populations.
Our study has limitations. The reported rates of DOT per 1000 hospital days are technically only externally generalizable to a freestanding academic children’s hospital. However, the majority of pediatric cancer patients are likely admitted to an academic, freestanding children ‘ s hospital. Additionally, administrative billing data were used to establish both antibiotic use and severity of illness. Although billing data are not synonymous with actual patient receipt of medications or hospital resources, the reported estimates should provide useful comparative estimates. Finally, when interpreting variation in antibiotic prescribing practices, we adjusted for variation in DSI across institutions but other possibilities such as variation in central catheter utilization and administration of antibiotic prophylaxis could also potentially explain the differences in DOT. Unfortunately, it is difficult to establish the presence or absence of a central catheter or to declare that certain antibiotics were administered as prophylaxis from the primary data source. Therefore, the impact of these factors on antibiotic utilization could not be fully assessed.
While these data support the presence of significant variation in antibiotic prescribing practices, the etiology of such variation is not known. It is plausible to speculate that variation in the frequency of certain pathogens and hospital resistance patterns for these pathogens may result in differential antibiotic use. However, one might expect that if a hospital had an increased frequency of invasive bacterial pathogens that explained an increased use in antibiotics then there would have been an increase in the DSI in those same institutions, which was not the case. If a hospital had higher rates of resistant pathogens one might expect the choices of antibiotics to vary between centers but not necessarily the duration of antibiotics. Regardless, there is a concern that at least a proportion of the differences in antibiotic prescribing practices are the result of unnecessary administration.
Furthermore, we were unable to directly assess the potential clinical implications of differential rates of antibiotic use in this study population. The association between unnecessary antibiotic use and adverse outcomes (e.g. increased resistance and increased risk for Clostridium difficile infection) has been well established in other clinical scenarios [9– 11,16]. An attempt was made to associate an increased use of antibiotic administration with an increase of C. difficile infections. However, the frequency of cases of C. difficile during this study’s brief follow-up period was not substantial enough to execute such an analysis. Efforts are ongoing to establish a larger cohort with longer follow-up time to further evaluate the negative impact of increased antibiotic use relative to C. difficile.
In summary, hospital antibiotic prescribing practices for children with newly diagnosed ALL varies broadly across institutions, despite adjustment for patient demographics and severity of illness. Benchmarking antimicrobial use for specific patient populations provides the opportunity for institutions to compare the management of similar patients at peer institutions. Future efforts should focus on establishing a prospective surveillance system with hospital level feedback on the rates of antibiotic prescribing for specific patient populations to encourage more judicious antibiotic use.
Acknowledgments
This study was supported by NIH grant RO1 CA133881-01 (R.A.) and a Canuso Foundation Innovation Award (A.E.S.). Both funding sources provided salary support for the respective investigators and had no involvement in this project.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 2.Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children’s hospitals. Pediatrics. 2010;126:1067–1073. doi: 10.1542/peds.2010-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polk RE, Hohmann SF, Medvedev S, et al. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis. 2011;53:1100–1110. doi: 10.1093/cid/cir672. [DOI] [PubMed] [Google Scholar]
- 4.Fisher BT, Harris T, Torp K, et al. Establishment of an 11-year cohort of 8733 pediatric patients hospitalized at United States free-standing children’s hospitals with de novo acute lymphoblastic leukemia from health care administrative data. Med Care. 2012 Mar 8; doi: 10.1097/MLR.0b013e31824deff9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 6.Fisher BT, Aplenc R, Localio R, et al. Cefepime and mortality in pediatric acute myelogenous leukemia: a retrospective cohort study. Pediatr Infect Dis J. 2009;28:971–975. doi: 10.1097/INF.0b013e3181a75939. [DOI] [PubMed] [Google Scholar]
- 7.McGowan JE. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983;5:1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- 8.Monroe S, Polk R. Antimicrobial use and bacterial resistance. Curr Opin Microbiol. 2000;3:496–501. doi: 10.1016/s1369-5274(00)00129-6. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL. “Collateraldamage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38(Suppl 4):S341–S345. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa TM, Levy SB. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat. 2000;3:303–311. doi: 10.1054/drup.2000.0167. [DOI] [PubMed] [Google Scholar]
- 11.Polk RE, Johnson CK, McClish D, et al. Predicting hospital rates of fluoroquinolone-resistant Pseudomonas aeruginosa from fluoroquinolone use in US hospitals and their surrounding communities. Clin Infect Dis. 2004;39:497–503. doi: 10.1086/422647. [DOI] [PubMed] [Google Scholar]
- 12.Marr JJ, Moffet HL, Kunin CM. Guidelines for improving the use of antimicrobial agents in hospitals: a statement by the Infectious Diseases Society of America. J Infect Dis. 1988;157:869–876. doi: 10.1093/infdis/157.5.869. [DOI] [PubMed] [Google Scholar]
- 13.Shlaes DM, Gerding DN, John JF, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol. 1997;18:275–291. doi: 10.1086/647610. [DOI] [PubMed] [Google Scholar]
- 14.Scheckler WE, Bennett JV. Antibiotic usage in seven community hospitals. JAMA. 1970;213:264–267. [PubMed] [Google Scholar]
- 15.Kuster SP, Ruef C, Ledergerber B, et al. Quantitative antibiotic use in hospitals: comparison of measurements, literature review, and recommendations for a standard of reporting. Infection. 2008;36:549–559. doi: 10.1007/s15010-008-7462-z. [DOI] [PubMed] [Google Scholar]
- 16.Courcol RJ, Pinkas M, Martin GR. A seven year survey of antibiotic susceptibility and its relationship with usage. J Antimicrob Chemother. 1989;23:441–451. doi: 10.1093/jac/23.3.441. [DOI] [PubMed] [Google Scholar]