Abstract
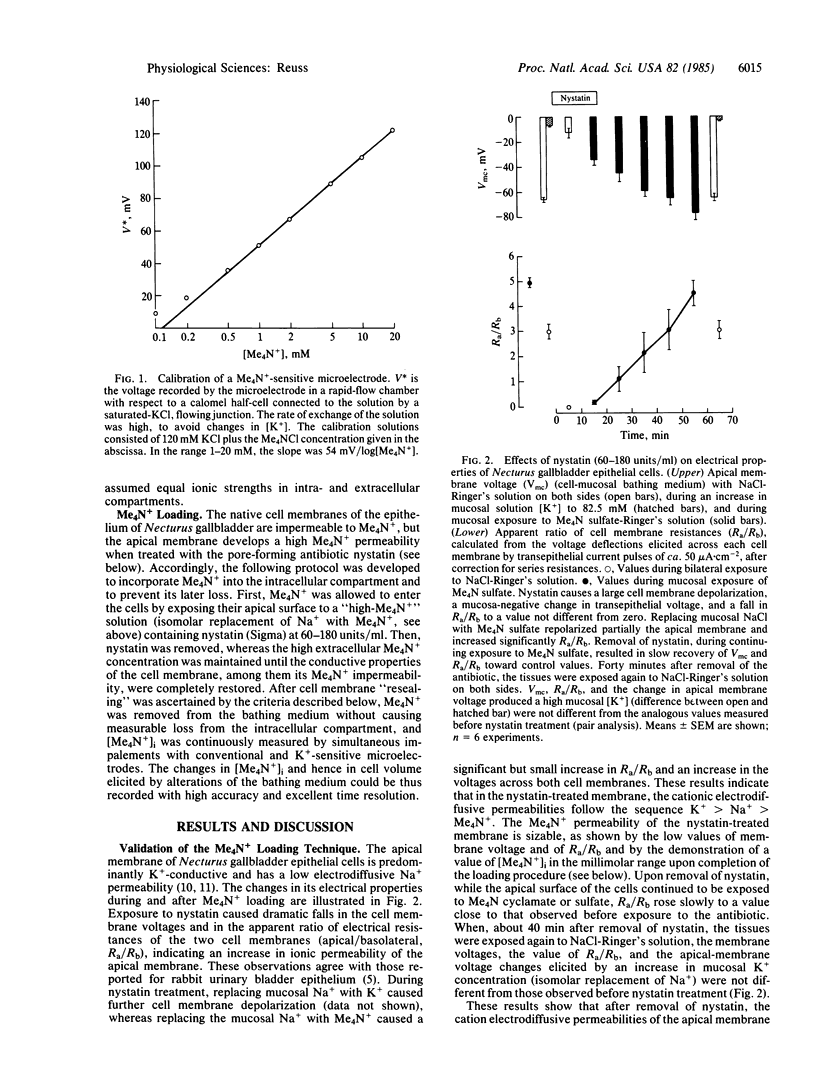
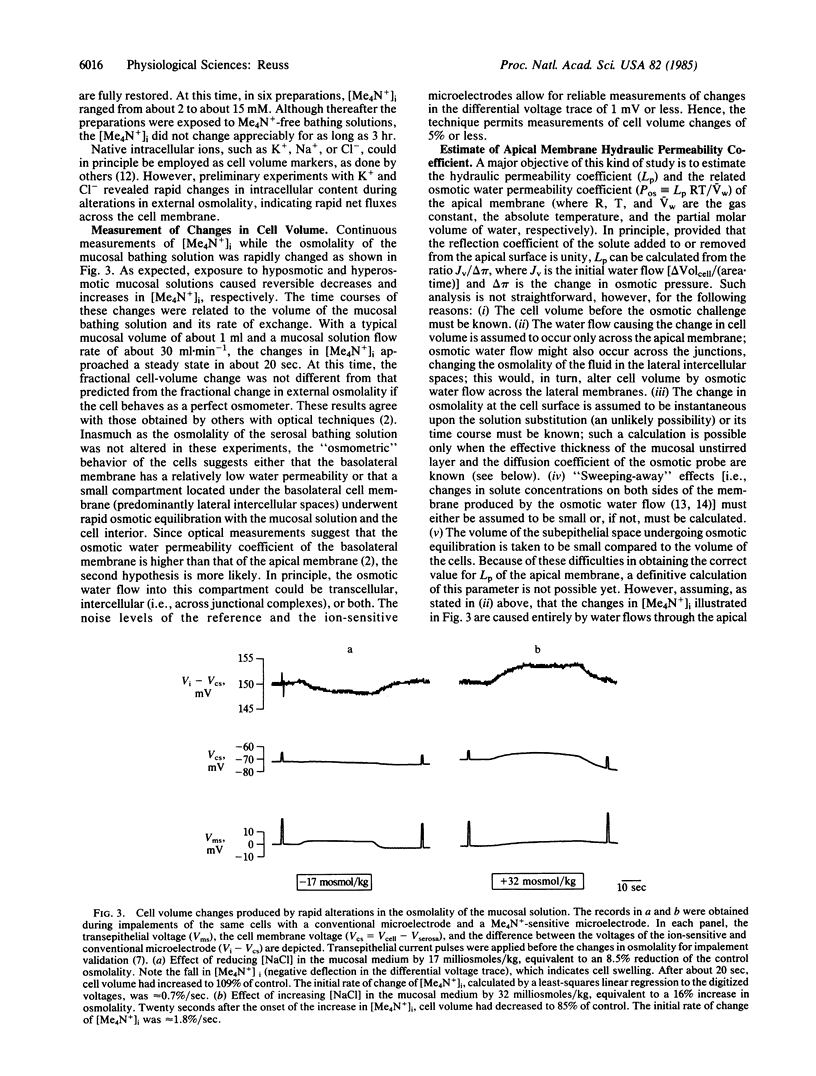
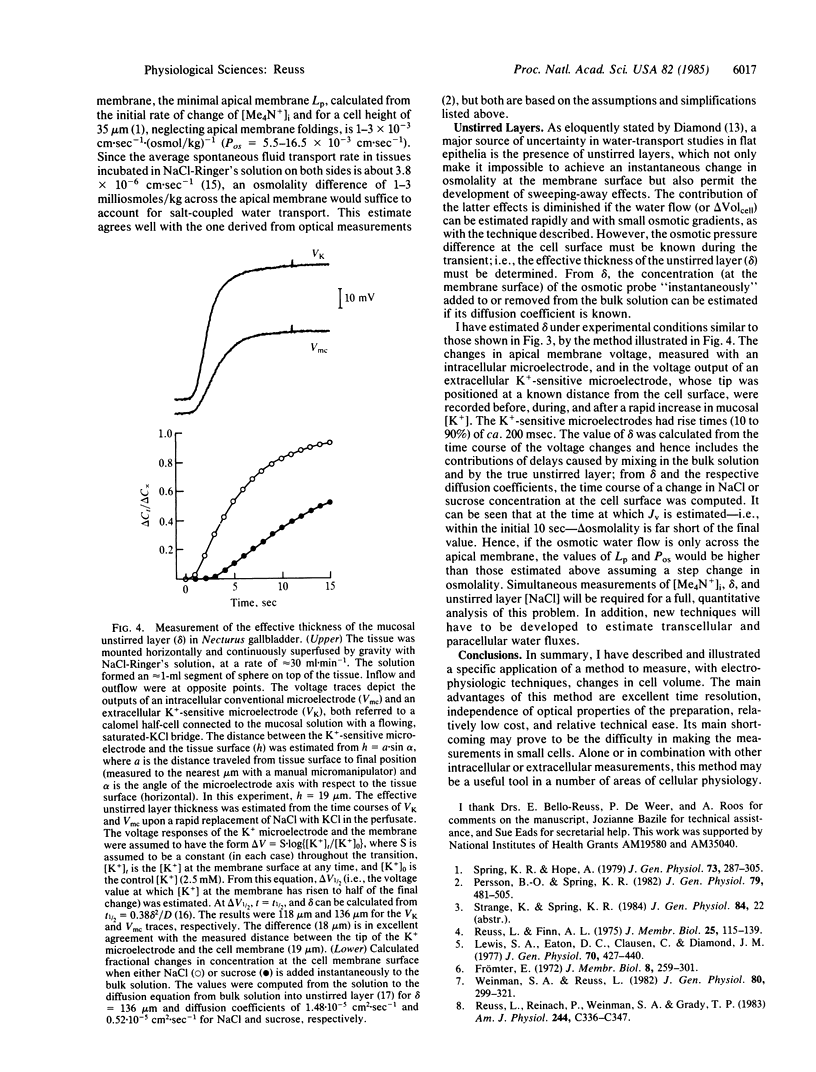
Epithelial cells of the gallbladder of Necturus maculosus were loaded with tetramethylammonium (Me4N+) by transient exposure of the apical (lumen-facing) surface to a solution of high Me4N+ concentration containing also the polyene antibiotic nystatin. Upon removal of nystatin, in the continued presence of Me4N+, spontaneous restoration of the native ionic permeability of the apical cell membrane was observed. At this time, external Me4N+ was removed; intracellular [Me4N+] measured with ion-sensitive microelectrodes was 2-15 mM and remained unchanged for several hours. Changes in cell volume were estimated from the changes in intracellular [Me4N+] produced by alterations in the osmolality of the mucosal bathing solution. Assuming that such changes are caused entirely by water fluxes across the apical membrane, the minimum value of its hydraulic permeability coefficient (Lp) was 1-3 X 10(-3) cm.sec-1.(osmoles/kg)-1, suggesting that an osmolality difference across the apical membrane as small as 1-3 milliosmoles/kg could explain the average rate of transepithelial water transport. These results agree with optical measurements [Persson, B. O. & Spring, K. R. (1982) J. Gen. Physiol. 79, 481-505]. The effective thickness of the apical unstirred layer was estimated from the time courses of both the apical membrane voltage and the response of an extracellular K+-sensitive microelectrode to an increase in [K+] in the mucosal bath. Since changes in concentration of the osmotically active solute at the membrane surface were thus shown to be significantly delayed by diffusion, the Lp value, calculated assuming a step-change in osmolality, is an underestimate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry P. H., Diamond J. M. Effects of unstirred layers on membrane phenomena. Physiol Rev. 1984 Jul;64(3):763–872. doi: 10.1152/physrev.1984.64.3.763. [DOI] [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. Osmotic water flow in leaky epithelia. J Membr Biol. 1979 Dec 31;51(3-4):195–216. doi: 10.1007/BF01869084. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Eaton D. C., Clausen C., Diamond J. M. Nystatin as a probe for investigating the electrical properties of a tight epithelium. J Gen Physiol. 1977 Oct;70(4):427–440. doi: 10.1085/jgp.70.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Rapid changes of potassium concentration at the outer surface of exposed single neurons during membrane current flow. J Gen Physiol. 1973 Mar;61(3):385–399. doi: 10.1085/jgp.61.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B. E., Spring K. R. Gallbladder epithelial cell hydraulic water permeability and volume regulation. J Gen Physiol. 1982 Mar;79(3):481–505. doi: 10.1085/jgp.79.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. II. Ionic permeability of the apical cell membrane. J Membr Biol. 1975 Dec 4;25(1-2):141–161. doi: 10.1007/BF01868572. [DOI] [PubMed] [Google Scholar]
- Reuss L. Independence of apical membrane Na+ and Cl- entry in Necturus gallbladder epithelium. J Gen Physiol. 1984 Sep;84(3):423–445. doi: 10.1085/jgp.84.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Reinach P., Weinman S. A., Grady T. P. Intracellular ion activities and Cl-transport mechanisms in bullfrog corneal epithelium. Am J Physiol. 1983 May;244(5):C336–C347. doi: 10.1152/ajpcell.1983.244.5.C336. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Hope A. Fluid transport and the dimensions of cells and interspaces of living Necturus gallbladder. J Gen Physiol. 1979 Mar;73(3):287–305. doi: 10.1085/jgp.73.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman S. A., Reuss L. Na+-H+ exchange at the apical membrane of Necturus gallbladder. Extracellular and intracellular pH studies. J Gen Physiol. 1982 Aug;80(2):299–321. doi: 10.1085/jgp.80.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Relations between intracellular ion activities and extracellular osmolarity in Necturus gallbladder epithelium. J Membr Biol. 1982;66(2):109–121. doi: 10.1007/BF01868487. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Slegers J. F. The electrical potential profile of gallbladder epithelium. J Membr Biol. 1975 Dec 4;24(3-4):341–363. doi: 10.1007/BF01868631. [DOI] [PubMed] [Google Scholar]



