Abstract
Large intergenic non-coding (linc) RNAs constitute a new dimension of post-transcriptional gene regulation. In this issue of Developmental Cell, Wang et al. (2013) find that linc-RoR maintains human embryonic stem cell (ESC) self-renewal by functioning as a sponge to trap miR-145, thus regulating core pluripotency factors Oct4, Nanog, and Sox2.
Non-coding transcripts are emerging as key regulators of diverse biological states and diseases. Recent studies demonstrate the potential ability of thousands of non-coding RNAs to act as ‘microRNA sponges’, i.e., competing endogenous RNAs (ceRNAs) that are able to reduce the amount of microRNAs available to target mRNAs. In this issue of Developmental Cell, Wang et al. (2013) illustrate that a particular ‘microRNA sponge’, linc-RoR, antagonizes miR-145 to critically regulate the levels of pluripotency transcription factors Oct4, Sox2, and Nanog, in order to ensure embryonic stem cell self-renewal.
Ebert et al. introduced the concept of the “microRNA sponge” by chemically synthesizing competitive RNAs with tandem binding sites to a microRNA of interest (Ebert et al., 2007). These competitive RNAs acted as artificial microRNA inhibitors that created a loss-of-function phenotype for an entire microRNA family in cell culture. Simultaneously, the first endogenous microRNA sponge was identified in Arabidopsis thaliana (Franco-Zorrilla et al., 2007), followed by several others in mammalian cells (Ebert and Sharp, 2010). Thus far, three major types of noncoding RNAs have been found to act as microRNA sponges: pseudogene RNAs, circular RNAs (circRNAs), and large intergenic non-coding RNAs (lincRNAs). For example, PTENP1 is a pseudogene of the tumor suppressor gene PTEN. The 3′UTR of PTENP1 mRNA harbors several target sites for microRNAs, which also target the PTEN transcript. Overexpression of the PTENP1 3′UTR leads to increased levels of PTEN transcript and protein, followed by growth inhibition in cancer cells (Tay et al., 2011). CircRNAs, another type of miRNA sponge, presumably result from splicing events and are surprisingly abundant. Two recent studies identified circRNAs as microRNA sponges in the brain, where circRNAs harbor a high density (∼70) of miR-7 seed matches and are resistant to Argonaute protein-mediated degradation (Hansen et al., 2013; Memczak et al., 2013). Furthermore, a testis-specific circRNA, sex-determining region Y(Sry), also functions as a microRNA sponge (Hansen et al., 2013), suggesting that the effect of circRNA as a microRNA sponge is a general phenomenon. Finally, lincRNAs also serve as microRNA sponges. For instance, during myogenesis, lincRNAs protect MAML1 and MEF2C transcripts from degradation, thereby promoting differentiation (Cesana et al., 2011).
Linc-RoR (Regulator of Reprogramming) was first identified as a promoter of reprogramming of human induced pluripotent stem cells (Loewer et al., 2010). Based on the chromosome-modifying functions of many other reported lincRNAs (Guttman et al., 2011), it was previously hypothesized to promote the transcription of core pluripotency factors. Contrary to this hypothesis, in this issue, Wang et al. (2013) demonstrate that linc-RoR actually functions as a microRNA sponge to post-transcriptionally regulate the mRNAs of the core transcriptional factors (TFs) Oct4, Nanog, and Sox2. A direct competition for miR-145 binding occurs between linc-RoR and the mRNAs encoding the core TFs, and this tug of war regulates hESC self-renewal and differentiation (Figure 1).
Figure 1. A competition for miR-145 between linc-RoR and mRNAs encoding the core TFs.
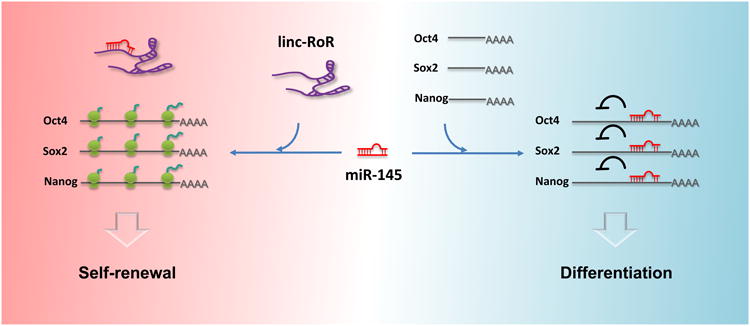
The presence of linc-RoR in hESCs traps miR-145, preventing it from repressing the translation of the core pluripotency factors and ensuring the stem cell fate. The disappearance of linc-RoR in differentiating hESCs releases miR-145, allowing it to repress the translation of core pluripotency factors.
Wang et al. (2013) show that, similar to the core TF transcripts, linc-RoR expression is restricted to undifferentiated ESCs. Upon differentiation, the level of linc-RoR rapidly decreases prior to the decline of the core TF transcripts. Overexpression of linc-RoR in hESCs leads to elevated levels of the core TF transcripts regardless of placement in conditions promoting self-renewal or differentiation. To test whether linc-RoR transcriptionally controls the core TFs, the authors used luciferase reporter assays that showed that the Oct4 promoter fails to respond to linc-RoR overexpression, thus pointing to post-transcriptional regulation. Wang et al. (2013) then demonstrated that this regulation is at least partially dependent upon Dicer, suggesting a microRNA-dependent mechanism.
The study by Wang et al. (2013) strongly supports that linc-RoR acts as a microRNA sponge. Linc-RoR modulates miR-145 levels, a sits overexpression diminishes endogenous miR-145 in self-renewing hESCs and drastically delays the increase in miR-145 upon hESC differentiation. These data are consistent with the previous finding that miR-145 represses the translation of the core TF mRNAs, thereby facilitating the differentiation program (Xu et al., 2009). The expression level of mature miR-145 was inversely proportional to the expression levels of the wild-type linc-RoR but not to mutant linc-RoR lacking specific miR-145 seed matches, suggesting that linc-RoR negatively regulates miR-145 through specific binding sites. In particular, linc-RoR only affects mature miR-145 but not its precursors, demonstrating a post-transcriptional control mechanism.
To further investigate whether linc-RoR could protect the core TF mRNAs from miR-145-mediated suppression, the authors found that ectopic linc-RoR efficiently abolished the miR-145-induced reduction of luciferase activity in reporter assays. Consistent with its sponge effect, linc-RoR copy number is much higher than that of miR-145 (>100 vs. 10-20 copies/cell) in self-renewing hESCs compared to differentiating hESCs (20 vs. >500 copies/cell). The sponge effect of linc-RoR may therefore vanish after hESC differentiation. Finally, in the self-renewal state, suppression of linc-RoR by shRNA leads to spontaneous differentiation while in the differentiated state, forced expression of linc-RoR restore score TF expression, leading to a resistance of cells to differentiate.
In summary, this study suggests a mechanism of regulating cellular pluripotency by linking three RNA components--lincRNAs, microRNAs, and mRNAs of core TFs. The balanced regulation of these three components at the post-transcriptional level ensures appropriate self-renewal and differentiation of hESCs.
An interesting question remains: is linc-RoR regulated by miR-145? Studies of previously identified ceRNAs indicate that the effects of microRNAs on ceRNAs should be less profound than those on the target mRNAs. For example, PTENP1 is expressed at much higher levels than PTEN (100-fold higher) to increase its efficacy (Tay et al., 2011). CiRS-7, a circRNA in the brain, harbors ∼70 microRNA target sites and is resistant to microRNA-mediated destabilization (Hansen et al., 2013; Memczak et al., 2013). This, however, does not seem to be the case for linc-RoR, the level of which decreases even prior to the decline of core TFs upon cellular differentiation. Although this could be due to decreased transcription of linc-RoR regulated by a miR-145-independent mechanism, it is possible that miR-145 targets linc-RoR and leads to its down-regulation. If so, do linc-RoR and miR-145 associate with each other at a specific subcellular location? Potentially, novel RNA and/or protein partners of linc-RoR may be critical in regulating how it interacts with microRNAs in a spatially and temporally specific manner. Of course, it is also possible that hESCs only need a limited level of ceRNAs to ensure a rapid response to differentiation cues.
By beginning to explore a role for ceRNA in hESCs, this study raises intriguing questions about exactly what, and how extensive, these roles might be in various types of stem cells. For example, does this mode of action hold true for adult stem cells? How conserved is this mechanism during evolution? Do ceRNAs regulate other small RNAs such as endo-siRNAs or piRNAs? These questions will require much effort to answer but are crucialto understanding this new paradigm of gene regulation.
This is a commentary on article Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H.Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25(1):69-80.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR, Sharp PA. Nat Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA. Curr Biol. 2010;20:R858–861. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, García JA, Paz-Ares J. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Nature. 2013 doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al. Nat Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Nature. 2013 doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, et al. Cell. 2011;147:344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]


