SUMMARY
There is a lack of contemporary prospective data examining the ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) and Stanford V (SV; doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone) regimens in older Hodgkin lymphoma (HL) patients. Forty-four advanced-stage, older HL patients (aged ≥60 years) were treated on the randomized study, E2496. Toxicities were mostly similar between chemotherapy regimens, although 24% of older patients developed bleomycin lung toxicity (BLT), which occurred mainly with ABVD (91%). Further, the BLT-related mortality rate was 18%. The overall treatment-related mortality for older HL patients was 9% versus 0.3% for patients aged <60 years (p<0.001). Among older patients, there were no survival differences between ABVD and SV. According to age, outcomes were significantly inferior for older versus younger patients (5-year failure-free survival: 48% vs 74%, respectively, p=0.002; 5-year overall survival: 58% and 90%, respectively, p<0.0001), while time-to-progression (TTP) was not significantly different (5-year TTP: 68% versus 78%, respectively, p=0.37). Furthermore, considering progression and death without progression as competing risks, the risk of progression was not different between older and younger HL patients (5 years: 30% and 23%, respectively, p=0.30); however, the incidence of death without progression was significantly increased for older HL patients (22% versus 9%, respectively, p<0.0001). Thus, the marked HL age-dependent survival differences appeared attributable primarily to non-HL events.
Keywords: Hodgkin lymphoma, elderly, treatment-related toxicity, bleomycin lung toxicity
Introduction
Survival rates for older patients with Hodgkin lymphoma (HL), typically defined as ≥60 years of age, have been shown to be significantly and disproportionately inferior compared with younger populations (Evens, et al 2008). The majority of studies examining older patients with HL have been retrospective analyses that were reported in the 1980’s–1990’s; these analyses showed 5-year overall survival (OS) rates of approximately 30%–45% (Levis, et al 1996, Mir, et al 1993, Roy, et al 2000, Stark, et al 2002, Weekes, et al 2002) A recent Surveillance, Epidemiology and End Results (SEER) Program report indicated that outcomes for HL in older patients had improved over time (Brenner, et al 2008). It is important to note, however, that survival rates in the earlier era (1980–1984) were exceptionally low (~20%), while the 2000–2004 survival rates remained significantly inferior compared with that seen in younger populations. Moreover, a recent retrospective analysis of older HL patients treated in the contemporary era showed continued overall modest outcomes (Evens, et al 2012).
It remains unclear to what extent the poor outcomes of older HL patients are due to potential biological differences in disease (i.e., higher relapse rate) versus treatment toxicity or other non-progression causes. Several studies have suggested that patients with older HL have biologically different and more aggressive disease compared with younger patients (Enblad, et al 1999, Gandhi, et al 2004, Keegan, et al 2005, Stark, et al 2002). However, most of these studies analysed disease-specific survival (DSS) without considering “competing risks” as part of the analysis. Non-HL related events (e.g., early death due to toxicity) are not fully independent of HL-related events, as patients would have been at risk of relapse (or death) due to HL had the non-HL event not occurred.
The Study of Hodgkin lymphoma In the Elderly/Lymphoma Database (SHIELD) recently reported phase II data using the VEPEMB (vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone, bleomycin, prednisolone) regimen; they reported 3-year progression-free survival (PFS) and OS of 58% and 66%, respectively (Proctor, et al 2012). Despite these recent data, there remains a paucity of prospective clinical trial data examining the outcomes or toxicity of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) for older HL patients in the modern era. Furthermore, there are minimal available data studying the Stanford V regimen (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone) in older patients. We analysed herein patient characteristics, treatment received, tolerability including detailed analysis of toxicity, and outcomes for older HL subjects treated on E2496, a contemporary phase III study that randomized HL patients to ABVD vs Stanford V. Additionally, we compared patient and disease characteristics and survival of older vs younger HL subjects treated on E2496 including survival analyses with competing risks.
Methods
Study eligibility
Eligibility for E2496 included classical HL patients with previously untreated, advanced-stage (III/IV) disease or local disease with bulky mediastinum (Gordon 2012). The latter was defined by a mass over one-third the maximum intrathoracic diameter on a standing posterior-anterior chest x-ray. Histology was determined using central review when available, then local pathology review. Concordance rate was assessed in patients with both central and local pathology review. Patients were randomized to ABVD or Stanford V as was recently reported (Gordon 2012). Of 794 eligible patients, 44 (6%) were aged ≥60 years (n=23 ABVD and n=21 Stanford V). A detailed quality assurance review was performed for all cases; 1 additional subject was deemed ineligible due to baseline computed tomography (CT) scans that were not completed in the required time frame. This patient was included in toxicity analyses. Baseline procedures included assessment of ejection fraction (EF) and pulmonary function testing (PFTs) with diffusing lung capacity for carbon monoxide (DLCO) and forced vital capacity (FVC). Bleomycin lung toxicity (BLT) was defined as the combination of 1) lower-respiratory tract symptoms (e.g., cough, shortness of breath), 2) bilateral infiltrates on chest x-ray or CT, and 3) absence of infection (Evens, et al 2007, Martin, et al 2005, Sleijfer 2001).
Treatment
ABVD was given for 6 or 8 cycles (every 28 days), depending on response by CT scan, while Stanford V was administered for 12 weeks (Gordon 2012). Patients treated on Stanford V received prophylactic antibiotics, which included oral trimethoprim/sulfamethoxazole and ketoconazole while those on ABVD did not. Radiation therapy (RT) was delivered to all patients with bulky mediastinal adenopathy and was scheduled to begin 2 weeks after completion of chemotherapy. RT fields included mediastinum, bilateral hilar and bilateral supraclavicular areas, which were treated at 36 Gy. In addition, for patients who received Stanford V, 36 Gy was delivered to any pretreatment site >5 cm and for macroscopic splenic disease (by CT).
Epstein-Barr virus (EBV) methods
For EBV small RNA (EBER) in situ hybridization (ISH), a tissue microarray was constructed from available formalin-fixed, paraffin-embedded tissue blocks. The array included duplicate 1.5 mm diameter cores of tumour specimens. In situ hybridization for EBER was performed using the INFORM EBER probe (Ventana, Tucson, Arizona). Slides were stained on an automated stainer (Ventana Benchmark XT) using the Ventana ISH/iView Blue detection kit. A known positive control was used. Specimens with Hodgkin-Reed-Sternberg cells with nuclear staining were considered positive.
For DNA extraction and quantitative real-time polymerase chain reaction (PCR), plasma was separated by centrifugation and DNA was isolated from 250 µl of plasma using the QIAamp DNA blood mini kit (Qiagen Inc, Valencia, CA, USA) according to manufacturer instructions. A primer pair and probe corresponding to the BamH-W region of the EBV genome (5’-CCCAACACTCCACCACACC-3’, 5’- TCTTAGGAGCTGTCCGAGGG-3’, 5’-(6-FAM) CACACACTACACACACCCACCCGTCTC (BHQ-1)-3’) were used. Namalwa DNA (Namalwa cell line genomic DNA, ATCC #CRL-1432) was used for calibration.
Statistical analysis
The primary endpoint of E2496 was failure-free survival (FFS), defined as time from randomization to the earlier of progression or relapse, or death. OS was measured from randomization to death of any cause. Time-to-progression (TTP) was defined as the time of randomization to progression, censored at last known progression-free; for death without documented progression, censor at death time. Comparisons were conducted according to intent-to-treat principles among eligible patients with a stratified log-rank test (localized vs. extensive; International Prognostic Score (IPS) 0–2 vs. 3–7), between treatment groups or age groups (<60 vs. ≥60). Toxicity was evaluated on all patients regardless of eligibility. A receiver operating characteristic (ROC) curve was used to determine the cut-off for plasma EBV with optimal sensitivity, specificity, and concordance with EBV status by EBER-ISH. Fishers’ exact and Wilcoxson rank sum tests were used to compare proportions and medians, respectively. Kaplan-Meier and Cox proportional regression models were used to estimate failure rates and hazard ratios. Progression and death without progression were identified as competing risks, and were compared between age groups using the method of cumulative incidence, as implemented in the cmprsk package in R (Gray 1988, Kim 2007). The cumulative incidence of HL-related death (including acute treatment-related toxicity) was similarly estimated considering death due to other cause as a competing risk (Kim 2007).
Results
Demographics and characteristics
Patient characteristics for older HL patients were balanced between ABVD and Stanford V chemotherapy arms (Table I). Median age for older HL patients was 65 years (range, 60–83), 47% had presence of B symptoms, while 18% had an IPS ≥4. There were several differences comparing older patient (n=44) characteristics with patients aged <60 years (n=750). This included increased frequency of mixed cellularity HL (25% vs 10%, respectively, p=0.0005) and inferior Eastern Cooperative Oncology Group (ECOG) performance status (PS) (PS 0: 34% vs 58%, respectively, p=0.003) for older vs younger patients. There was no difference in number of IPS factors (0–2 s. ≥ 3) between age groups (despite all older patients having at least one criterion being age >45 years).
Table I.
Baseline demographic data of older HL patients.
| Treatment Arms | ||||
|---|---|---|---|---|
| ABVD (n=23) | Stanford V (n=21) | |||
| N | % | N | % | |
| Age (years) | 66 | 64 | ||
| Median | ||||
| Range | 61–83 | 60–83 | ||
| Gender | ||||
| Male | 11 | 49 | 13 | 63 |
| Female | 12 | 52 | 8 | 38 |
| ECOG PS | 6 | 26 | 9 | 43 |
| 0 | ||||
| 1 | 16 | 70 | 12 | 57 |
| 2 | 1 | 4 | . | . |
| B symptoms | 12 | 52 | 11 | 52 |
| No | ||||
| Yes | 11 | 48 | 10 | 48 |
| Disease Stage | 1 | 4 | 2 | 9 |
| II | ||||
| III | 17 | 74 | 13 | 62 |
| IV | 5 | 22 | 6 | 29 |
| Cell type | 2 | 9 | . | . |
| NA | ||||
| Nodular sclerosis | 11 | 48 | 11 | 52 |
| Lymphocyte-rich | . | . | 1 | 5 |
| Mixed cellularity | 7 | 30 | 4 | 19 |
| Classical HL, NOS | 3 | 13 | 5 | 24 |
| No. extra-nodal sites | 18 | 78 | 15 | 71 |
| 0 | ||||
| 1 | 3 | 13 | 4 | 19 |
| 2 | 2 | 9 | . | . |
| 4 | . | . | 1 | 5 |
| 5 | . | . | 1 | 5 |
| Lung involvement | 21 | 91 | 18 | 86 |
| No | ||||
| Yes | 2 | 9 | 3 | 14 |
| Liver involvement | 21 | 91 | 19 | 91 |
| No | ||||
| Yes | 2 | 9 | 2 | 9 |
| Bone marrow involvement | 22 | 96 | 19 | 91 |
| No | ||||
| Yes | 1 | 4 | 2 | 9 |
| Mediastinal involvement | 10 | 44 | 4 | 19 |
| No | ||||
| Yes | 13 | 56 | 17 | 81 |
| IPS risk factors | . | . | 1 | 5 |
| NA | ||||
| 1 | 4 | 17 | 2 | 9 |
| 2 | 10 | 44 | 10 | 48 |
| 3 | 7 | 30 | 2 | 9 |
| 4 | 2 | 9 | 1 | 5 |
| 5 | . | . | 4 | 19 |
| 6 | . | . | 1 | 5 |
Abbreviations: HL, Hodgkin lymphoma; NA, not available; ECOG, Eastern Cooperative Oncology Group; PS, performance status; NOS, not otherwise specified; IPS, International Prognostic Score; ABVD, adriamycin, bleomycin, vinblastine, dacarbazine.
We compared baseline levels of EBV viral load and frequency of EBV(+) tumour between older and younger HL subjects. There was an increased percentage of older patients with EBV(+) detected in tumour compared with younger patients, however this difference was not significant (29% and 15%, respectively, p=0.12). Additionally, plasma EBV viral load was detected in 29% of older patients at baseline compared with 19% of younger patients (p=0.34).
Treatment and toxicity
Adjunctive RT on E2496 was delivered to the mediastinum for all patients with bulky mediastinum on the ABVD arm and for any pretreatment site >5 cm or macroscopic splenic disease detected by CT for patients treated with Stanford V (Gordon 2012). Among older HL patients, 8.7% who received ABVD received RT vs 42.7% of younger subjects (p=0.0007), while 43% of older Stanford V patients received RT vs 77% of younger patients (p=0.002). This probably reflects the lower incidence of bulky stage I-II disease in older HL patients as compared with the whole population (7% vs 35%). There were no differences in RT quality scores between older and younger patients (data not shown).
Chemotherapy dose modifications, as required by protocol, were common with 84% of older HL patients having at least one dose reduction. There were no differences in frequency of dose modifications according to chemotherapy regimen or between older and younger patients (data not shown). Relative dose-intensity for older HL patients was 72%; this information was not available for subjects aged <60 years. Overall, adverse events (AEs) were relatively common among older HL patients (Table II). Besides BLT (discussed below), there were no significant differences in haematological or non-haematological AEs between chemotherapy regimens for older HL patients.
Table II.
Adverse event data for older HL patients.
| Toxicity Type | Treatment Arm | |||||
|---|---|---|---|---|---|---|
| ABVD (n=24) | Stanford V (n=21) | |||||
| Grade | Grade | |||||
| 3 | 4 | 5 | 3 | 4 | 5 | |
| (%) | (%) | (%) | (%) | (%) | (%) | |
| Haemoglobin | 13 | 4 | - | 33 | - | - |
| Leucocytes | 50 | 17 | - | 24 | 48 | - |
| Lymphopenia | 71 | - | - | 86 | - | - |
| Neutrophils | 8 | 79 | - | 29 | 48 | - |
| Platelets | 8 | - | - | 5 | - | - |
| Transfusion (PRBCs) | 4 | - | - | 10 | - | - |
| Hypertension | 4 | - | - | - | - | - |
| Cardiac-other | 4 | - | - | - | - | - |
| Fatigue | 17 | - | - | - | - | - |
| Fever | - | - | - | 5 | - | - |
| Constipation | 4 | - | - | - | 5 | - |
| Dehydration | 8 | - | - | - | - | - |
| Dysphagia-esophageal (radiation) | - | - | - | 5 | - | - |
| Nausea | 4 | - | - | 5 | - | - |
| Stomatitis | 4 | - | - | - | - | - |
| Vomiting | 8 | - | - | - | - | - |
| Alkaline phosphatase | 8 | - | - | - | - | - |
| Hypoalbuminemia | 4 | - | - | - | - | - |
| Infection with grade 3 or 4 neutropenia | 4 | 4 | - | 10 | - | 5 |
| Gastrointestinal haemorrhage | - | - | - | - | - | 5 |
| Hyperglycaemia | 8 | - | - | 10 | - | - |
| Muscle weakness | - | - | - | 10 | - | - |
| Neuropathy-motor | - | 8 | - | 14 | - | - |
| Neuropathy-sensory | 4 | 8 | - | - | - | - |
| Syncope | 4 | - | - | - | - | - |
| Abdominal pain | - | - | - | 5 | - | - |
| Arthralgia | 4 | - | - | - | - | - |
| Chest pain | 4 | - | - | - | - | - |
| Myalgia | 8 | - | - | - | 5 | - |
| Deep vein thrombosis | 4 | - | - | - | - | - |
| Pain-other | 4 | - | - | 5 | - | - |
| DLCO decrease | 4 | - | - | - | - | - |
| Dyspnea | 4 | 13 | - | - | - | - |
| Hypoxia | 4 | 4 | - | - | - | - |
| Pneumonitis/pulmonary infiltrates | 8 | - | - | - | - | - |
| Pulmonary-other | - | - | 8 | - | - | - |
| WORST DEGREE | 13 | 79 | 8 | 33 | 57 | 10 |
Abbreviations: HL, Hodgkin lymphoma; PRBC, packed red blood cells; DLCO, diffusion capacity of the lung for carbon monoxide.
Severe haematological AEs (grade 3–4) were more frequent in older vs younger subjects, especially neutropenia (grade 4 or higher: 64% vs 38%, respectively, p=0.0005). The frequency of non-haematological grade 3–4 toxicities on E2496 were not different among older compared with younger patients (Table III). However, the treatment-related mortality (TRM) was significantly higher for older compared with younger HL subjects treated on E2496 (9% vs 0.3%, respectively, p<0.001). Among the grade 5 treatment-related toxicities for older subjects, two (10%) occurred in the Stanford V group (gastrointestinal bleed/renal failure and colitis/sepsis) and two (8%) with ABVD (both due to BLT/pulmonary fibrosis: see below).
Table III.
Severe toxicities on E2496 according to age.*
| Toxicity Type | Age ≥60 years (n=45) | Age <60 years (n=789) | ||||
|---|---|---|---|---|---|---|
| Grade^ | Grade^ | |||||
| 3 | 4 | 5 | 3 | 4 | 5 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Haematological | 11 (24) | 31 (69) | - | 372 (47) | 308 (39) | - |
| Non-Haematological | 14 (31) | 6 (13) | 4 (9) | 322 (41) | 53 (7) | 2 (<1) |
Grade 3, 4, and 5 adverse events (includes all patients who received any protocol treatment, regardless of eligibility status).
Worst degree.
BLT
Among the n=45 older HL patients enrolled on E2496, 11 (24%) developed BLT, of whom 2/11 (18%) died due to acute pulmonary fibrosis/respiratory failure (Table IV). Furthermore, 10/11 (91%) BLT cases occurred with/during ABVD (BLT incidence: 43% with ABVD vs 5% Stanford V, p=0.04). This toxicity appeared to occur later in the chemotherapy course, however, the two BLT-related deaths occurred during cycle 3 of ABVD. We did not identify any factors that predicted the development of BLT or death due to BLT. Granulocyte growth factor was given the vast majority of patients, thus it was not analysed as a risk factor. Additionally, as detailed in Table IV, the median age and baseline/pre-treatment levels of EF, FVC, and DLCO in the 11 older HL patients who developed BLT (69, 65%, 89%, 83%, respectively) were not significantly different than the 34 patients who did not (64, 61%, 85%, 77%, respectively).
Table IV.
Bleomycin lung toxicity characteristics.
| Case | Age (years)/sex | Treatment | Tobacco history (status at entry) |
Baseline EF FVC, and DLCO |
Timing of BLT |
Initial CTCAE code |
Death due to BLT |
HL disease status |
|---|---|---|---|---|---|---|---|---|
| 1 | 72/M | ABVD | 46 pack-years (active) |
72%, 84%, and 47% | Cycle 5 | Grade 3 hypoxia |
No | DWOD* |
| 2 | 61/F | ABVD | None | 49%, 66%, and 54% | Cycle 5 | Grade 2 SOB | No | DOD |
| 3 | 66/M | ABVD | 40 pack-years (none in 20 years) |
55%, 74%, and 84% | Cycle 5 | Grade 2 cough | No | AWOD |
| 4 | 64/F | ABVD | None | 45%, 102%, and 87% | Cycle 5 | Grade 1 cough, Grade 1 PI |
No | AWOD |
| 5 | 69/M | Stanford V | 50-pack years (active) |
62%, 89%, and 90% | Month 3 | Grade 4 dyspnea |
No | AWOD |
| 6 | 66/M | ABVD | None | 67%, 92%, and 24% | Cycle 4 | Grade 2 cough | No | AWOD |
| 7 | 72/F | ABVD | None | 78%, 95%, and 83% | Cycle 6 | Grade 2 cough | No | AWOD |
| 8 | 78/F | ABVD | None | 73%, 75%, and 77% | Cycle 3 | Grade 4 pulmonary |
Yes | DWOD^ |
| 9 | 62/F | ABVD | 60-pack years (none in 20 years) |
NA, 72%, and 63% | Cycle 6 | Grade 2 cough | No | AWOD |
| 10 | 77/F | ABVD | 5 pack-years (none in 40 years) |
79%, 106%, and 92% | Cycle 4 | Grade 1 cough, Grade 1 PI |
No | AWOD |
| 11 | 69/M | ABVD | None | 55%, 104%, and 115% | Cycle 3 | Grade 3 pulmonary |
Yes | DWOD^ |
Abbreviations: M, male; F, female; FVC, forced vital capacity; DLCO, diffusion of lung capacity of carbon monoxide; EF, ejection fraction; CTCAE, Common Terminology Criteria for Adverse Events; BLT, bleomycin lung toxicity; HL, Hodgkin lymphoma; SOB, shortness of breath; AWOD, alive without disease; DOD, dead as a result of disease; DWOD, dead without disease; PI, pulmonary infiltrates.
Death due to lung cancer.
Death due to acute pulmonary fibrosis (during chemotherapy).
Outcomes for older patients
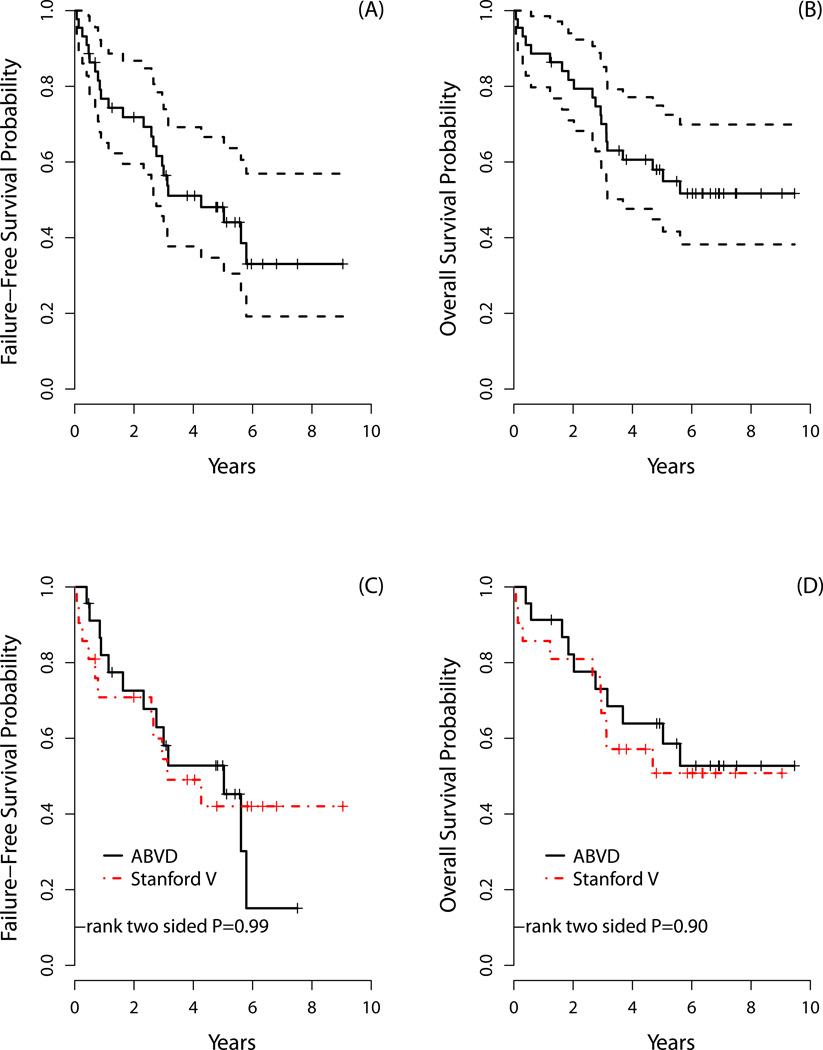
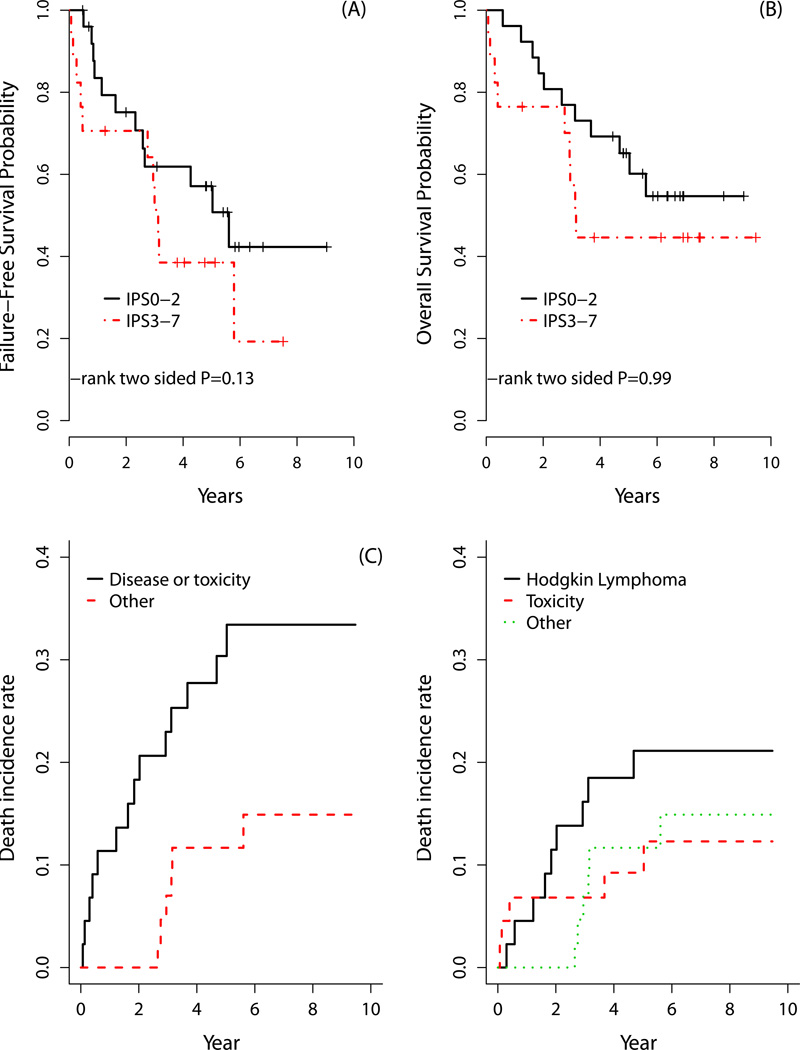
The overall response (ORR) and complete response (CR) rates for older HL patients were 68% and 64%, respectively. As noted in Table V, ORR did not differ between the two chemotherapy arms for older subjects. The 3-year FFS and OS rates were 56% and 70%, respectively, while the 5-year FFS and OS rates were 48% and 58%, respectively (Figure 1). FFS and OS did not significantly differ by chemotherapy regimen. Outcomes for older HL patients were also analysed according to the IPS. There was no significant difference between two IPS groups for FFS or OS among older patients (Figure 2); this included analysing IPS as a continuous variable (0–7; FFS p=0.17 and OS p=0.29). Nevertheless, this analysis may be underpowered.
Table V.
Response data.
| Response | Older HL Treatment Arms | |||
|---|---|---|---|---|
| ABVD | Stanford V | |||
| N | % | N | % | |
| Complete response | 6 | 26 | 4 | 19 |
| Clinical complete response | 9 | 39 | 9 | 43 |
| Partial response | 2 | 9 | - | - |
| No change/stable | 4 | 17 | 2 | 10 |
| Progression | - | - | 2 | 10 |
| Unevaluable | 2 | 9 | 3 | 14 |
| Missing | - | - | 1 | 4 |
| Response | HL Age Group | |||
| < 60 years | ≥ 60 years | |||
| N | % | N | % | |
| Complete response | 122 | 16 | 10 | 23 |
| Clinical complete response | 411 | 55 | 18 | 41 |
| Partial response | 58 | 8 | 2 | 4 |
| No change/stable | 69 | 9 | 6 | 14 |
| Progression | 7 | 1 | 2 | 5 |
| Unevaluable | 61 | 8 | 5 | 11 |
| Missing | 22 | 3 | 1 | 2 |
Abbreviations: HL, Hodgkin lymphoma; N, number; ABVD, adriamycin, bleomycin, vinblastine, dacarbazine.
Figure 1. Older Hodgkin lymphoma (HL) patient survival.
The (A) failure-free survival (FFS) and (B) overall survival (OS) for all older HL patients. The (C) 3- and 5-year FFS for older patients who received ABVD was 58% and 53%, respectively, which compared with 54% and 42%, respectively, for patients who received Stanford V (p=0.99); while the (D) 3- and 5-year OS for older patients who received ABVD was 73% and 64%, respectively, which compared with 67% and 51%, respectively, for patients who received Stanford V (p=0.90).
Figure 2. Survival for older Hodgkin lymphoma (HL) patients based on International Prognostic Score (IPS) and including competing risk analysis.
There were no significant differences in (A) failure-free survival and (B) overall survival according to IPS for older HL patients. The (C) cumulative incidence of death due to HL/progression and death due to HL treatment/toxicity (i.e., treatment-related mortality) vs death incidence rate due to all other causes. The associated cumulative incidence of death at 3 and 5 years for older HL patients was 23% and 30%, respectively vs 7% and 12%, respectively, for all other causes. The (D) cumulative incidence of death due to HL treatment/toxicity was plotted separately. The associated 3- and 5-year incidences of death was 16% and 21%, respectively, due to HL/progression, 7% and 9%, respectively, due to toxicity, and 7% and 12%, respectively, due to other causes.
Treatment arms were pooled and stratified for exploratory analyses. When analysing all deaths due to HL and HL-related therapy (i.e., death due to disease/progression and TRM combined), the cumulative incidence of death at 3 and 5 years for older HL patients was 23% and 30%, respectively, considering death due to other causes as competing risk (Figure 2C); this compared with 7% and 10% death due to any cause, respectively, for HL subjects aged <60 years. The 3- and 5-year incidences of death directly due only to HL (i.e., disease progression) for older subjects were 16% and 21%, respectively (Figure 2D).
Outcomes by age
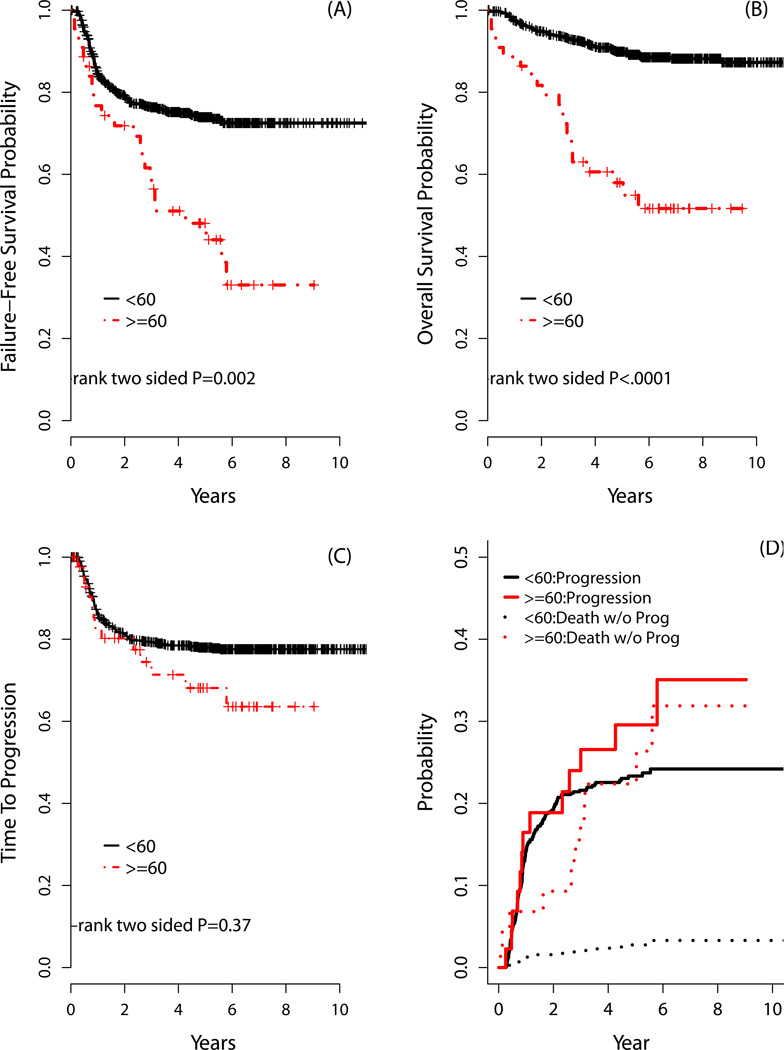
In comparing outcomes by age, there was a trend for improved response in younger compared with older HL patients, though this was not significant (ORR: 79% vs 68%, respectively, p=0.13; CR rates: 71% vs 64%, respectively, p=0.31). Three-year and 5-year FFS and OS, however, were significantly inferior for the older HL population (Table VI and Figure 3). As part of this sub-group analysis (i.e., older vs younger HL patients), there was no significant interaction between age and treatment for FFS (p=0.95) or OS (p=0.56). Interestingly, the rates of TTP were not different according to age (Figure 3C). Further, a ‘competing risk’ survival analyses considering death without progression a competing risk for progression, showed no differences in risk of progression by age groups. Even when including analysis with competing risks, there we no differences detected in progression by age groups. However, the incidence rate of death without progression was significantly higher for older compared with younger HL patients (Figure 3D).
Table VI.
Outcomes according to age.
| Age < 60 years | Age ≥ 60 years | p-value (log-rank) |
||
|---|---|---|---|---|
| FFS | 3-year | 76% | 56% | 0.002 |
| 5-year | 74% | 48% | ||
| Median | Not reached | 4.7 years | ||
| OS | 3-year | 93% | 70% | <0.0001 |
| 5-year | 90% | 58% | ||
| Median | Not reached | Not reached | ||
Abbreviations: FFS, failure-free survival; OS, overall survival.
Figure 3. Outcomes comparing older HL with younger patients.
The (A) 3- and 5-year failure-free survival for patients aged ≥60 years was 56% and 48%, respectively, which compared with 76% and 74%, respectively, for patients aged <60 years (p=0.002); while (B) the 3- and 5-year overall survival for patients aged ≥60 years was 70% and 58%, respectively, which compared with 93% and 90%, respectively, for patients aged <60 years (p<0.0001). (C) The 2- and 5-year time-to-progression (TTP) for patients aged ≥60 years was 80% and 68%, respectively; this compared with 81% and 78%, respectively, for patients aged <60 years (p=0.37). (D) The rates of progression were determined with competing risk analysis because death without progression is a competing risk for disease progression. The incidence rates of progression including competing risks for patients aged ≥60 years at 2 and 5 years were 19% and 30%, respectively, compared with 19% and 23%, respectively, for patients aged <60 years (p=0.30); however, the incidence rates of death without progression for patients aged ≥60 years at 2 and 5 years were 13% and 22%, respectively, compared with 2% and 9%, respectively, for patients aged <60 years (p=<0.0001).
Discussion
The proportion of HL patients age ≥60 years in population studies has ranged between 15%–35% (Stark et al 2002; Roy et al 2000; Levis et al 1994: Yarnold et al 1982; Enblad et al 1991), however, the ratio of older patients in HL clinical trials has been lower (i.e., < 5% of participants) (Mir et al 1993; Roy et al 2000; Engert et al 2005). Thus, data describing characteristics and outcomes for older patients with HL have been derived primarily from registry and retrospective population-based series. In these series, older age has been a consistent significant adverse prognostic factor for survival in HL (Engert, et al 2005, Erdkamp, et al 1992, Guinee, et al 1991, Mir, et al 1993, Roy, et al 2000, Stark, et al 2002). The associated chemotherapy regimens in these reports have been heterogeneous, while the last prospective studies of older HL patients that examined ABVD were reported nearly 20 years ago (Levis, et al 1994, Mir, et al 1993). Furthermore, to our knowledge, there are no existing data studying Stanford V in older patients with HL. In the randomized trial E2496 that compared ABVD and Stanford V therapy, we identified a high incidence of BLT in older patients treated with ABVD (i.e., 43%), while the tolerability appeared otherwise similar between these regimens. Furthermore, response rates and survival were similar. We found, however, that TRM was significantly increased in older compared with younger HL patients, as was FFS and OS. In interpreting these observations, several factors should be considered.
The last prospective study that reported results using ABVD in advanced-stage older HL patients was the Cancer and Leukemia Group B (CALGB) 8251 study(Mir, et al 1993). In that analysis, the 5-year OS for patients aged ≥60 years was 31% vs 63% for patients aged 40–59 years, and 79% for age <40 years (p<0.0001). Further, the median disease-free survival rates for ages 16–45 years was 8.9 years, 3.5 years for 46–55 years, 1.5 years for 56–65 years, and 0.7 years for >65 years (p<0.0001). Levis et al (1994) analysed the outcome of 65 patients aged ≥65 years who had received a ‘registry-recommended’ protocol of ABVD, MOPP (mechlorethamine, vincristine, procarbazine, prednisone) or ABVD/MOPP therapy. The 8-year EFS and OS was 41% and 46%, respectively, both significantly worse compared with patients aged <65 years. An important factor associated with the inferior survival of older subjects in that study was the 23% acute TRM rate associated with ABVD-based therapy.
Treatment-related toxicity is a significant concern for older patients, particularly the risk of infection, pulmonary, and cardiac toxicity. Among older HL patients in the HD-9 randomized study of the German Hodgkin Study Group (GHSG), a TRM rate of 9% for COPP (cyclophosphamide, oncovin, procarbazine, prednisone)-ABVD combination therapy and 21% for BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone)-baseline therapy was noted (Ballova, et al 2005). In a sub-group of patients treated off trial (on a registration study) in the SHIELD analysis, Proctor et al (2012) noted a TRM of 18% for advanced-stage older HL patients who were treated with ABVD; this compared with a TRM of 4% for patients on the prospective VEPMB trial (TRM due to BLT of 1%) (Proctor, et al 2012). In a retrospective study, the GHSG showed that severe toxicity (grade 4) was significantly more common in older vs younger patients (Engert, et al 2005). The frequency of non-haematological grade 3–4 toxicities on E2496 was not different among older patients compared with those aged <60 years, however haematological toxicities were increased and the TRM of older patients was increased 35-fold compared with HL subjects aged <60 years. A contributing factor to TRM was BLT.
The incidence of BLT in the literature is variable, up to 46% in some reports (Coiffier, et al 2002, Sleijfer 2001). Among all of the older HL patients in E2496, the incidence of BLT was 24%, although most cases occurred in patients who received ABVD (BLT rate with ABVD 43%). Reported risk factors for BLT include older age, renal insufficiency, baseline lung function, pulmonary radiation, tobacco, and granulocyte colony-stimulating factor (G-CSF) (Azambuja, et al 2005, Sleijfer 2001). We could not identify any predictive factors for BLT including detailed analysis of pre-treatment lung and cardiac function. It is important to note that most older patients in E2496 received G-CSF (according to protocol); thus it is not known how much this contributed to the development of BLT. It should also be highlighted that the common terminology criteria AE (CTCAE) did not reliably capture the diagnosis of BLT; as detailed in Table IV, the initial diagnostic coding was heterogeneous in depicting BLT (e.g., dyspnea, cough, hypoxia, pneumonitis, etc). Most diagnoses of BLT were based on further detailed physician workup.
The outcomes for older patients in E2496 were significantly inferior compared with younger patients. Inadequate treatment delivery of chemotherapy has been shown to be an adverse prognostic factor in HL (Landgren, et al 2003, Levis, et al 1994, Yarnold, et al 1982). There were no apparent differences in frequency of dose reductions in E2496 among older and younger patients, however a formal comparison of dose intensity could not be performed. Several series have proposed that HL in older patients is biologically different compared with younger HL patient populations (Klimm, et al 2007). Prior reports in older HL patients have shown an increased frequency of mixed cellularity HL subtype (Engert, et al 2005, Levis, et al 1994, Mir, et al 1993) and poorer PS (Engert, et al 2005) compared with younger HL populations, while others have noted less frequent bulky mediastinal disease in older HL patients (Levis, et al 1994). We confirmed the findings of increased mixed cellularity and poorer PS in the older population, however, there were no differences detected in risk of disease progression when competing risk analyses were utilized. Including competing risk analysis was critical as a straightforward Kaplan-Meier method would result in incorrect and biased estimates of the risk of progression (Gray 1988, Kim 2007). The bias arises because the Kaplan-Meier method assumes that all events are independent, and thus, censors all events other than the event of interest (Kim 2007). Progression and death without progression are not independent because patients who experienced death before progression cannot be at further risk of progression of disease.
Altogether, ABVD and Stanford V produced comparable survivals in advanced-stage older patients with HL, despite a significantly increased risk of BLT with ABVD. Further, despite a TRM rate of 9%, the survival rates for older HL patients in E2496 compared favourably with the VEPEMB regimen from the recent SHIELD study (Proctor et al 2012) (3-year PFS and OS for E2496: 56% and 70%, respectively; SHIELD: 58% and 66%, respectively). And compared with historical controls (Enblad, et al 1991, Levis, et al 1994, Mir, et al 1993), survival of older HL patients in the modern era indeed appears improved over the past several decades. However, despite this apparent progress, older subjects continue to have a markedly inferior survival compared with younger HL patients. The age-related survival disparity we observed appears to be related mostly to non-progression causes including markedly higher TRM experienced by older subjects. This underscores the continued critical need for new therapeutic approaches for older HL patients, especially regimens that maintain efficacy, but with improved tolerability. It will be important to study the integration of novel therapeutic agents, such as brentuximab vedotin (NCT01476410 and NCT01716806) and lenalidomide (NCT01056679), into the first-line treatment of older patients with HL.
Acknowledgments
E2496 was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA21115, CA23318, CA66636, CA17145, CA77440, CA11083, CA32102, CA46441, CA46282, CA38926, CA77202, CA21076, CA31946, CA13650 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. This study was listed on clinicaltrials.gov as NCT00003389.
We acknowledge the ECOG core-coordinating centre for their work.
Footnotes
Conflict of interest disclosure: none (all authors).
Author Contributions: A.M.E. designed research, performed research, analysed data, and wrote the paper. F.H. designed research, analysed data, and wrote the paper. L.I.G. designed research, performed research, and wrote the paper. R.I.F. designed research and wrote the paper. N.L.B. designed research and wrote the paper. J.C. designed research. R.D.G. designed research and performed research. H.W. designed research, performed research, and wrote the paper. M.G. designed research and performed research. B.D.C designed research and wrote the paper. P.J.S. designed research. R.A. designed research, performed research, and wrote the paper. T.P.M. designed research and wrote the paper. R.T.H. designed research and performed research. B.S.K. designed research, performed research, analysed data, and wrote the paper. S.J.H. designed research, performed research, analysed data, and wrote the paper.
References
- Azambuja E, Fleck JF, Batista RG, Menna Barreto SS. Bleomycin lung toxicity: who are the patients with increased risk? Pulm Pharmacol Ther. 2005;18:363–366. doi: 10.1016/j.pupt.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Ballova V, Ruffer JU, Haverkamp H, Pfistner B, Muller-Hermelink HK, Duhmke E, Worst P, Wilhelmy M, Naumann R, Hentrich M, Eich HT, Josting A, Loffler M, Diehl V, Engert A. A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin's disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly) Ann Oncol. 2005;16:124–131. doi: 10.1093/annonc/mdi023. [DOI] [PubMed] [Google Scholar]
- Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood. 2008;111:2977–2983. doi: 10.1182/blood-2007-10-115493. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Enblad G, Glimelius B, Sundstrom C. Treatment outcome in Hodgkin's disease in patients above the age of 60: a population-based study. Ann Oncol. 1991;2:297–302. doi: 10.1093/oxfordjournals.annonc.a057939. [DOI] [PubMed] [Google Scholar]
- Enblad G, Sandvej K, Sundstrom C, Pallesen G, Glimelius B. Epstein-Barr virus distribution in Hodgkin's disease in an unselected Swedish population. Acta Oncol. 1999;38:425–429. doi: 10.1080/028418699431942. [DOI] [PubMed] [Google Scholar]
- Engert A, Ballova V, Haverkamp H, Pfistner B, Josting A, Duhmke E, Muller-Hermelink K, Diehl V. Hodgkin's Lymphoma in Elderly Patients: A Comprehensive Retrospective Analysis From the German Hodgkin's Study Group. J Clin Oncol. 2005;23:5052–5060. doi: 10.1200/JCO.2005.11.080. [DOI] [PubMed] [Google Scholar]
- Erdkamp FL, Breed WP, Bosch LJ, Wijnen JT, Blijham GB. Hodgkin disease in the elderly. A registry-based analysis. Cancer. 1992;70:830–834. doi: 10.1002/1097-0142(19920815)70:4<830::aid-cncr2820700418>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Evens AM, Cilley J, Ortiz T, Gounder M, Hou N, Rademaker A, Miyata S, Catsaros K, Augustyniak C, Bennett CL, Tallman MS, Variakojis D, Winter JN, Gordon LI. G-CSF is not necessary to maintain over 99% dose-intensity with ABVD in the treatment of Hodgkin lymphoma: low toxicity and excellent outcomes in a 10-year analysis. Br J Haematol. 2007;137:545–552. doi: 10.1111/j.1365-2141.2007.06598.x. [DOI] [PubMed] [Google Scholar]
- Evens AM, Sweetenham JW, Horning SJ. Hodgkin lymphoma in older patients: an uncommon disease in need of study. Oncology (Williston Park) 2008;22:1369–1379. [PubMed] [Google Scholar]
- Evens AM, Helenowski I, Ramsdale E, Nabhan C, Karmali R, Hanson B, Parsons B, Smith S, Larsen A, McKoy JM, Jovanovic B, Gregory S, Gordon LI, Smith SM. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119:692–695. doi: 10.1182/blood-2011-09-378414. [DOI] [PubMed] [Google Scholar]
- Gandhi MK, Tellam JT, Khanna R. Epstein-Barr virus-associated Hodgkin's lymphoma. Br J Haematol. 2004;125:267–281. doi: 10.1111/j.1365-2141.2004.04902.x. [DOI] [PubMed] [Google Scholar]
- Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Stiff PJ, Cheson BD, Gospodarowicz M, Advani R, Kahl BS, Friedberg JW, Blum KA, Habermann TM, Tuscano JM, Hoppe RT, Horning SJ. Randomized Phase III Trial of ABVD Versus Stanford V With or Without Radiation Therapy in Locally Extensive and Advanced-Stage Hodgkin Lymphoma: An Intergroup Study Coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2012 doi: 10.1200/JCO.2012.43.4803. Epub ahead of print, November 26th, 2012. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1140–1154. [Google Scholar]
- Guinee VF, Giacco GG, Durand M, van den Blink JW, Gustavsson A, McVie JG, Zewuster R, Dische S, Fahey T, Lane W. The prognosis of Hodgkin's disease in older adults. J Clin Oncol. 1991;9:947–953. doi: 10.1200/JCO.1991.9.6.947. [DOI] [PubMed] [Google Scholar]
- Keegan TH, Glaser SL, Clarke CA, Gulley ML, Craig FE, Digiuseppe JA, Dorfman RF, Mann RB, Ambinder RF. Epstein-Barr virus as a marker of survival after Hodgkin's lymphoma: a population-based study. J Clin Oncol. 2005;23:7604–7613. doi: 10.1200/JCO.2005.02.6310. [DOI] [PubMed] [Google Scholar]
- Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- Klimm B, Diehl V, Engert A. Hodgkin's lymphoma in the elderly: a different disease in patients over 60. Oncology (Williston Park) 2007;21:982–990. discussion 990, 996, 998 passim. [PubMed] [Google Scholar]
- Landgren O, Algernon C, Axdorph U, Nilsson B, Wedelin C, Porwit-MacDonald A, Grimfors G, Bjorkholm M. Hodgkin's lymphoma in the elderly with special reference to type and intensity of chemotherapy in relation to prognosis. Haematologica. 2003;88:438–444. [PubMed] [Google Scholar]
- Levis A, Depaoli L, Urgesi A, Bertini M, Orsucci L, Vitolo U, Buchi G, Gallamini A, Gavarotti P, Novarino A, Scalabrini DR, Mazza U, Pileri A, Sannazzari GL, Resegotti L. Probability of cure in elderly Hodgkin's disease patients. Haematologica. 1994;79:46–54. [PubMed] [Google Scholar]
- Levis A, Depaoli L, Bertini M, Botto B, Ciravegna G, Freilone R, Gallamini A, Gavarotti P, Ricardi U, Scalabrini DR, Salomone A, Salvi F, Vitolo U, Pileri A, Sannazzari GL, Resegotti L. Results of a low aggressivity chemotherapy regimen (CVP/CEB) in elderly Hodgkin's disease patients. Haematologica. 1996;81:450–456. [PubMed] [Google Scholar]
- Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. J Clin Oncol. 2005;23:7614–7620. doi: 10.1200/JCO.2005.02.7243. [DOI] [PubMed] [Google Scholar]
- Mir R, Anderson J, Strauchen J, Nissen NI, Cooper MR, Rafla S, Canellos GP, Bloomfield CD, Gottlieb AJ, Peterson B, et al. Hodgkin disease in patients 60 years of age or older. Histologic and clinical features of advanced-stage disease. The Cancer and Leukemia Group B. Cancer. 1993;71:1857–1866. doi: 10.1002/1097-0142(19930301)71:5<1857::aid-cncr2820710524>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Proctor SJ, Wilkinson J, Jones G, Watson GC, Lucraft HH, Mainou-Fowler T, Culligan D, Galloway MJ, Wood KM, McNally RJ, James PW, Goodlad JR. Evaluation of treatment outcome in 175 patients with Hodgkin lymphoma aged 60 years or over: the SHIELD study. Blood. 2012;119:6005–6015. doi: 10.1182/blood-2011-12-396556. [DOI] [PubMed] [Google Scholar]
- Roy P, Vaughan Hudson G, Vaughan Hudson B, Esteve J, Swerdlow AJ. Long-term survival in Hodgkin's disease patients. A comparison of relative survival in patients in trials and those recorded in population-based cancer registries. Eur J Cancer. 2000;36:384–389. doi: 10.1016/s0959-8049(99)00267-1. [DOI] [PubMed] [Google Scholar]
- Sleijfer S. Bleomycin-Induced Pneumonitis. Chest. 2001;120:617–624. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- Stark GL, Wood KM, Jack F, Angus B, Proctor SJ, Taylor PR. Hodgkin's disease in the elderly: a population-based study. Br J Haematol. 2002;119:432–440. doi: 10.1046/j.1365-2141.2002.03815.x. [DOI] [PubMed] [Google Scholar]
- Weekes CD, Vose JM, Lynch JC, Weisenburger DD, Bierman PJ, Greiner T, Bociek G, Enke C, Bast M, Chan WC, Armitage JO. Hodgkin's Disease in the Elderly: Improved Treatment Outcome With a Doxorubicin-Containing Regimen. J Clin Oncol. 2002;20:1087–1093. doi: 10.1200/JCO.2002.20.4.1087. [DOI] [PubMed] [Google Scholar]
- Yarnold JR, Jelliffe AM, Hudson GV. Factors affecting relapse following chemotherapy for Hodgkin's disease. Clin Radiol. 1982;33:627–629. doi: 10.1016/s0009-9260(82)80386-3. [DOI] [PubMed] [Google Scholar]