Abstract
Hypoxia-ischemia (HI) occurs when blood and/or oxygen delivery to the brain is compromised. HI injuries can occur in infants born prematurely (<37 weeks gestational age) or at very low birth weight (<1500 grams), as well as in term infants with birth complications. In both preterm and term HI populations, brain injury is associated with subsequent behavioral deficits. Neonatal HI injury can be modeled in rodents (e.g., the Rice-Vannucci method, via cautery of right carotid followed by hypoxia). When this injury is induced early in life (between postnatal day (P)1–5), neuropathologies typical of human preterm HI are modeled. When injury is induced later (P7–12), neuropathologies typical of those seen in HI term infants are modeled. The current study sought to characterize the similarities/differences between outcomes following early (P3) and late (P7) HI injury in rats. Male rats with HI injury on P3 or P7, as well as sham controls, were tested on a variety of behavioral tasks in both juvenile and adult periods. Results showed that P7 HI rats displayed deficits on motor learning, rapid auditory processing (RAP), and other learning/memory tasks, as well as a reduction in volume in various neuroanatomical structures. P3 HI animals showed only transient deficits on RAP tasks in the juvenile period (but not in adulthood), yet robust deficits on a visual attention task in adulthood. P3 HI animals did not show any significant reductions in brain volume that we could detect. These data suggest that: 1) behavioral deficits following neonatal HI are task-specific depending on timing of injury; 2) P3 HI rats showed transient deficits on RAP tasks; 3) the more pervasive behavioral deficits seen following P7 HI injury were associated with substantial global tissue loss; and 4) persistent deficits in attention in P3 HI subjects might be linked to neural connectivity disturbances rather than a global loss of brain volume, given that no such pathology was found. These combined findings can be applied to our understanding of differing long-term outcomes following neonatal HI injury in premature versus term infants.
1.1 Introduction
Brain injury due to hypoxia-ischemia (HI) can lead to major behavioral and neuroanatomical morbidity in both premature (born < 37 week gestational age) and very low birth weight (VLBW; born < 1500 grams) infants [1–4], as well as term infants suffering from birth complications [5]. Such injuries in preterm infants tend to be focal (e.g., local vascular or ischemic insult in specific regions [3]), and commonly result in white matter/fiber tract damage [3]. Conversely, HI incidents at term (e.g., following prolonged cord compression) affect the entire brain, and typically lead to gray matter damage in the cortex, hippocampus, basal ganglia, and/or thalamus [6–7]. These neuropathological differences probably reflect, at least in part, differential vulnerability of specific brain regions at the time of injury.
Both preterm and term HI populations show a wide variety of subsequent deficits in behavioral and cognitive domains, including language processes [8–14], memory [15– 24], visual attention [25–26], and motor abilities [27–31]. With specific regard to language abilities -- although many different processes are involved in language development, one proposed mechanism that might lead to later language deficits is an underlying impairment in rapid auditory processing (RAP). RAP refers to the ability to discriminate differences between rapidly presented auditory cues, such as are found in formant transitions in speech (e.g., /ba/ versus /da/), or stop constants with short duration cues. RAP deficits are typically not restricted to the verbal domain, and can also be seen using non-verbal acoustic stimuli (thus making tasks amenable to pre-lingual infants). In fact, studies have demonstrated that preterm infants with severe periventricular leukomalacia (PVL) lesions (diagnosed at birth) showed deficits on RAP tasks in childhood [32]. Furthermore, deficits in RAP early in life correlated with and were predictive of later language impairments in children at risk for language disorders (as well as in typically developing controls [33–34]).
Given the wide range of behavioral deficits following neonatal HI injury in premature and full term infants, animal models of induced neonatal HI (e.g., see [35] for details of Rice-Vannuci method) have been used to provide insight into variables modulating long-term behavioral and anatomical outcomes. And, as in HI injured infants, neonatal HI injured rodents show variety of behavioral deficits and neuroanatomical pathology. For example, in regards to RAP, one study found that male rats with HI injury induced on P7 showed deficits on RAP tasks in both juvenile and adult periods. These findings could possibly reflect outcomes related to language deficits in human neonatal HI populations [36–40]. These animals also showed gray matter damage, particularly in the cortex and hippocampus, along with enlarged ventricular size as compared to sham animals (per post mortem analysis [37–40]). In addition to RAP deficits, P7 HI rats from this study also showed deficits on a variety of learning and memory tasks. Other studies have also demonstrated poorer performance for P7 HI injured rats on Morris Water Maze (MWM; a measure of spatial learning/memory, [41– 44]). P7 HI injured animals also showed deficits on a long-term reference memory and a short term working memory task [45], and reductions in hippocampal volume in post mortem histological analysis [40, 44]. Animals that received HI injury at 4 weeks old also revealed subsequent deficits on fine motor tasks, and fMRI showed less activation of the sensory motor cortex in response to left and right forepaw stimulation [46]. Animals with a P7 HI injury showed deficits in righting reflex, gait reflex, muscle power, motor coordination, and limb placing compared to shams [47–48]. A loss of volume in the cortex, thalamus, and striatum was also reported [48].
Fewer studies have been conducted using younger animals (<P7) in the HI rodent model, but in one study, P3 HI injured animals were found to show disorganized barrel patterns in the somatosensory cortex, and a week after injury, showed no somatosensory evoked potentials on the ipsi-lateral side of injury [49]. In another study using induced bilateral artery occlusion on P4, sensorimotor deficits were seen, as well as deficits on righting reflex, wire hanging, and locomotor activity [50]. Subjects also showed a decrease in mature oligodendrocytes and impaired myelination [50]. Additionally, animals with a P1 HI injury showed spontaneous hyperactivity and short-term memory deficits compared to shams [51]. Other investigators using a fixed interval extinction test (which detects ADHD-like symptoms such as hyperactivity) reported that animals exposed to hypoxia from P1–3 showed ADHD-type hyperactivity behaviors when compared to sham animals [52]. Finally, a study from our lab revealed transient RAP deficits in rats that had received HI injury on P1 (which were absent by adulthood), and no deficits in MWM [41].
Although there are a wide variety of animal studies demonstrating the effects of neonatal HI injury (both in premature and term injured models (separately)), there are few studies that have directly compared “premature” (postnatal day (P) 1–3) versus “full-term” (P7–10) HI injury via direct analysis of behavioral deficits and neuropathology. In one such study, investigators did find a difference in the neuro-inflammatory profile following early and late neonatal HI injury in rats. Specifically, when HI injury was induced on P1, the resulting inflammatory profile was “limited” (i.e., absent or down-regulated), and if seen, was primarily in white matter [53]. In contrast, animals that had HI injury induced on P12 showed a much stronger neuro-inflammatory profile, with mediators such as IL-1β increasing eight-fold. This dramatic increase in inflammatory response was associated with blood brain barrier leakage and massive neurophil infiltration [53]. The only other study we know of using this type of direct comparison between a preterm and term model is a prior study from our lab (referenced above), which examined the effects of HI on both P1 and P7 HI in rats. As noted, P1 HI rats showed deficits on a RAP task only juvenile periods, but not in adulthood. Interestingly, however, in this study P7 HI injured animals showed persistent RAP deficits across both juvenile and adult periods [41]. Furthermore, P1 HI injured animals showed no observed neuroanatomical alterations as compared to shams, whereas P7 HI subjects showed significant decrements in right cortical, hippocampal, and corpus callosum volume [41].
The current study sought to explore in greater detail the differences between a P3 HI and P7 HI injured rodent model on a variety of behavioral (RAP, learning/memory, motor, and visual attention), tasks as well as neuroanatomical pathology. These time points were chosen according to the website translating developmental time across mammalian species [54] where P7–8 in the rat is roughly equivalent to gestational age 36– 38 weeks in the human. For the premature model we chose P3 rather than P1 HI because it is sill within the window comparable to human prematurity, yet surgical outcomes are improved. Furthermore, comparable induced injuries were used (despite evidence that P3 pups may be more resistant to hypoxia [54]), because the use of two different injury protocols would render a comparison of outcomes in the two groups uninterpretable. We hypothesized (based on prior findings) that P7 HI injured animals would continue to show persistent deficits on behavioral tasks across the lifespan, as well as substantial neuroanatomical pathology, but that the P3 HI injured animals would show only “moderate” and possibly transient behavioral deficits as compared to P7 HI animals. We also predicted a preservation of neuroanatomical volumes in P3 as compared to P7 HI animals.
1.2 Methods
Pregnant time-mated female Wistar rats were ordered from Charles River Laboratories and were shipped to the University of Connecticut on embryonic day (E) 8 to minimize prenatal stress effects on the feti. Upon birth (P1), pups were culled into litters of 10 (8 males and 2 females). Only males were used in the current study because of known sex-specific differences in the neonatal HI model that are evident at both the behavioral and neuroanatomical levels [55]. In brief, deleterious effects of HI are consistently found to be more robust in males.
HI injury was induced on either P3 or P7, and this was randomly assigned within each litter. HI selected pups were anesthetized with isoflurane (2.5%) and a longitudinal mid-line incision was made in the neck. The right common carotid artery was located, separated from surrounding tissue, and completely cauterized. The incision was then sutured and animals were given a footpad injection indicating their specific treatment condition. Sham animals (also assigned within litter) were treated in the same manner, however there was no manipulation of the carotid artery. Approximately two hours after surgery (allowing for recovery and for the pups to feed), HI animals were placed in an airtight chamber (a heating pad and warming lamp were used to maintain normal body temperature) containing 8% humidified oxygen (balanced with nitrogen) for 120 minutes. Sham animals were placed in a similar container and exposed to normal room air. All treatment conditions were balanced across litters. All pups were then returned to the dam, and remained there until weaning. At P21, pups were weaned from their mother and double housed with like-treated animals. On P55, animals were single housed for the duration of testing. The final numbers in each group were: P3 HI, n=14; P7 HI, n=14; P3 sham, n=6; P7 sham, n=6. Based on previous investigations in our laboratory, it was assumed that data from P3 and P7 shams would be comparable and could be pooled for data analysis.
1.2.1 Behavioral Testing
1.2.1.1 Motor Coordination and Learning (P28–P32)
Rota-Rod
To assess motor coordination and learning in the juvenile period, animals were tested on a Rota-Rod task where they were required to remain stable on a rotating drum. Animals were placed on the rotating drum, which was set to accelerate from 4 rotations per minute (rpm) to 44 rpm over the span of 5 minutes. Subjects were given two trials per day for 5 days, and the latency for the subject to fall from the rotating drum was recorded for each trial (in seconds). Average latency was used for analysis. This rota-rod test is an accepted method to assess sensorimotor function in animal models of stroke and related disorders (57).
1.2.1.2 Auditory Discrimination Testing (P34–38; P60)
Startle Reduction
The startle reduction paradigm utilizes the subject’s acoustic startle reflex (ASR). An ASR is a relatively large motor response to a startle eliciting stimulus (SES; or as implemented here, a 105 dB white noise burst). When the SES is coupled with a benign acoustic stimulus presented just prior to the burst, the animal exhibits what is commonly known as pre-pulse inhibition. This procedure provides a measure of the animal’s ability to detect the cue based on the magnitude of the startle attenuation elicited by the prepulse cue [58]. The magnitude of the animal’s startle response on cued versus uncued trials (as a function of cue properties) can thus provide an index of detection of the pre-SES cue (and thus discrimination thresholds).
Apparatus
During auditory testing each subject was placed on a Med Associates PHM-225B load cell platform in a black polypropylene cage in a quiet testing room. Output voltage from each platform was sent through the PHM-250-60 linear load cell amplifier and into a Biopac MP 100A-CE Acquisition system. This system was connected to a Power Macintosh G3 computer, which records the amplitude of each subject’s startle reflex using Acknowledge Software. Auditory stimuli were generated on a Pentium III Dell PC with custom programmed software and a Tucker Davis Technologies (RP2) real time processor, amplified by a Niles SI-1260 System Integrations Amplifier. The sound programs were delivered through 10 calibrated Cambridge Soundworks MC 100 loudspeakers placed 53 cm above the platforms, and adjusted to provide acoustic comparability at each station (as measured by dB SLP).
Single Tone PPI Procedures
The single tone task consisted of 103 cued or uncued trials. Trials were presented in random order, and animals were tested for two sessions (once in the juvenile period (P33) and once in adulthood (P57)). The single tone procedure was used to assess any underlying hearing or pre-pulse inhibition (gating) deficits that might confound further testing results. Uncued trials consisted of a silent background followed by the SES. On cued trials, a 75-dB, 7-ms, 2300-Hz tone was presented 50 ms before the SES.
Silent Gap (SG) 0–100 PPI Procedures
The SG 0–100 procedure consisted of 299 cued or uncued trials per session. All animals were tested on one session/day for 4 consecutive days in the juvenile period (4 days), and only one day in adulthood (1 day). For all trials, a continuous background broadband white noise (75 dB) was played. On uncued trials, the SES was embedded in the background white noise. On cued trials, a gap in the white noise varying in duration from 2–100 ms was presented 50 ms before the SES. Attenuation score (Att) for each subject and for each gap were used for analysis.
1.2.1.3 Learning and Memory Testing
Prior to maze testing, animals were assessed on a one-day water escape task to screen for general visual or motor deficits that might confound subsequent results. The water escape task involved the use of an oval tub (40.5 in × 21.5 in) filled with room temperature water, and a visible escape platform at one end. Animals were placed in the water at the opposite end of the tub from the platform, and were timed until they swam and climbed onto the platform. Latency to escape was recorded for each animal. All maze testing took place in adulthood.
Morris Water Maze (MWM; P83–87)
Spatial learning/memory was evaluated using the MWM [59–60]. Maze testing took place for 5 consecutive days in a 48-inch diameter hard black plastic tub, with a six-inch diameter escape platform submerged right below the water line (so that is was not visible to the subjects). On each trial, the escape platform was located in the same quadrant of the tub, and the tub was surrounded by various extra-maze cues in the room (painted shapes on the wall, lights, the experimenter). There were no intra-maze cues, forcing subjects to use a spatial strategy. Each testing day consisted of four trials in which the animal’s start position (north, south, east, west) was varied. The start position never repeated in the same day, and the order varied randomly between testing days. The animal’s trajectory was recorded with a Sony Digital 8 video camera, which was connected to a Dell Dimensions E21 computer. SMART Version 2.5 tracking software was used to record the animal’s latency (measured in sec) to reach the platform, as well as average swim-speed (measured in cm/sec). For each trial, the animal was given 45s to locate and climb onto the submerged platform. If the animal failed to reach the platform it was gently guided to the platform and allowed to sit for 5s before being removed. Total latency to reach the platform (across all four trials per day) was used for analysis.
Non-Spatial Water Maze (NSM; P91–95)
NSM testing took place in the same 48-inch diameter hard plastic tub, with the same six-inch diameter invisible escape platform. However in this paradigm, cues directing the animal to the escape platform were located intra-maze rather than in the outside room (and in fact the platform was moved to dissociate it from extra-maze cues) [60]. The four different cues consisted of a background with black and white vertical lines, horizontal lines, diagonal lines to the left, or diagonal lines to the right. Each cue was attached in a different quadrant in the maze via velcro. The order of the intra-maze cues was changed randomly at the beginning of each testing day. On each trial, the escape platform was located in a different quadrant adjacent to a single positively associated intra-maze cue (always the black and white vertical lines). Thus, the internal maze cues were shifted relative to the room across trials, but the target cue indexing the platform remained constant. The start position of the animal for each trial also remained constant. The animal’s trajectory was again recorded via video camera, and latencies/velocities were ascertained with SMART version 2.5 computer software. For each trial, the animal was given 45s to locate and climb onto the platform. If the animal did not reach the platform in the allotted amount of time they were gently guided to it and allowed to sit on the platform for 5s before being removed. Total latency to reach the platform (across all four trials per day) was used for analysis.
1.2.1.4 Visual Attention (P100–140)
A commonly used task to assess visual attention abilities in rodents is a 5-choice serial reaction time (5-CSRT) task, where animals are trained to respond to a light stimulus with a nose poke for a food reward when the correct association is made [61]. This paradigm could also be applied to assess possible visual attention deficits in neonatal HI injured rodent models. Animals were tested on a 5-choice serial reaction time task in order to assess visual attention. For a week prior to testing, animals were placed on a restricted diet (5 grams/100 grams of body weight per day). The testing chamber consisted of 5-square holes on a curved wall of the operant box, and a pellet dispenser located on the opposite wall. While in the testing chamber, animals were required to attend to all five holes and correctly identify the illuminated hole with a nose poke. If correctly identified, the animal was rewarded with a sugar pellet (45 mg, BioServe).
Animals were gradually trained on progressively harder tasks, and training began with a day of habituation training followed by a day of nose-poke training. Visual attention training tasks included (listed in order of difficulty): 60s-stimulus duration (light on); 30s-stimulus duration; 10s-stimulus duration; and 5s-stimulus duration. All animals were moved on to the next training task together when all group averages reached 70% correct responses [62]. For all training tasks, the inter-trial interval was held constant at 5s, but the position of the light for each trial varied. Following the completion of training, animals were tested for 5 days on a variable inter-trial interval (VITI) task. In the VITI task, the position of the illuminated hole and the inter-trial interval (duration) varied randomly, with a fixed stimulus duration of 10s (light on). This was considered the most difficult phase because the lack of a fixed interval made the illumination (targets) more unpredictable. Response latencies and percent correct responses were again measured (recorded) and analyzed.
1.2.2 Histological Preparation
Upon the completion of behavioral testing, all animals were transcardially perfused. Animals were weighed and deeply anesthetized with an intra-peritoneal (i.p.) injection of ketamine (100 mg/kg) and xylazine (15 mg/kg). Animals were perfused with .9% saline followed by 10% buffered formalin. Brain were removed from the skull and post-fixed in 10% buffered formalin.
At least 24 hours after fixation, brains were placed in 30% sucrose (mixed with distilled water) solution for 24 hours for cryo-protection prior to slicing. Brain tissue was sliced at 60 µm on a cryostat. Every third slice was saved and mounted on a slide in sequential coronal sections through the whole brain. Cresyl Violet staining procedures were used to prepare brain tissue for volumetric analysis [63].
1.2.3 Volumetric/Area Analysis
Using StereoInvestigator software, volumes of the right and left cortex, hippocampus, lateral ventricles, and striatum (the caudate and nucleus accumbens) were collected. Volumes were calculated using Cavalieri’s Estimator [64]. Additionally, the mid-sagittal area of the corpus callosum was reconstructed using the same StereoInvestigator software. Every other mounted section was counted, resulting in 16–18 representative sections throughout the brain. All measurements were performed blind to Treatment group.
1.2.4 Statistical Analysis
All statistical analyses were performed using SPSS 15.0 software and an alpha criterion of 0.05. Two-tailed analyses were used unless otherwise stated. Preliminary analyses compared scores for the two sham groups (P3 and P7 sham) on a variety of measures, and no significant differences were seen. Therefore, these groups were pooled for all analyses to create a sham group of n=12. For each task, the three groups (P3 and P7 shams being groups together as previously described) were compared using analysis of variance (ANOVAs) with multiple levels of Treatment. Additionally, based on specific a priori hypotheses, planned comparisons were performed (as a function of Treatment) between paired groups. The following specific comparisons were made: P3 HI versus sham, P7 HI versus sham, and P3 HI versus P7 HI.
Repeated measures ANOVA were used to analyze behavioral data for each task. For Rota-Rod, variables included Treatment (3 levels) and Day (5 levels). For the RAP task, variables included Treatment (3 levels), Day (4 levels for juvenile testing; Day was not a variable for adult silent gap since only one day was performed), and Gap (9 levels). For maze testing (both MWM and NSM) variables included Treatment (3 levels) and Day (5 levels). For the visual attention task, variables included Treatment (3 levels) and Day (6 levels for the 5s stimulus duration task and 4 levels for the VITI task). Univariate ANOVAs were used to analyze histology, specifically to compare right hemisphere and left hemisphere volumes between groups. For the corpus callosum, total area is reported as measured using StereoInvestigator software. Graphs presented in the results section show average scores for each Treatment condition. It should be noted that on the RAP task, lower Att score indicate better performance, and higher scores indicate poorer performance, as in the Rota Rod task where longer latencies reflect better motor skills. On the MWM & NSM, lower scores are better (reflecting faster location of the platform).
1.3 Results
1.3.1 Rota-Rod (P28–32)
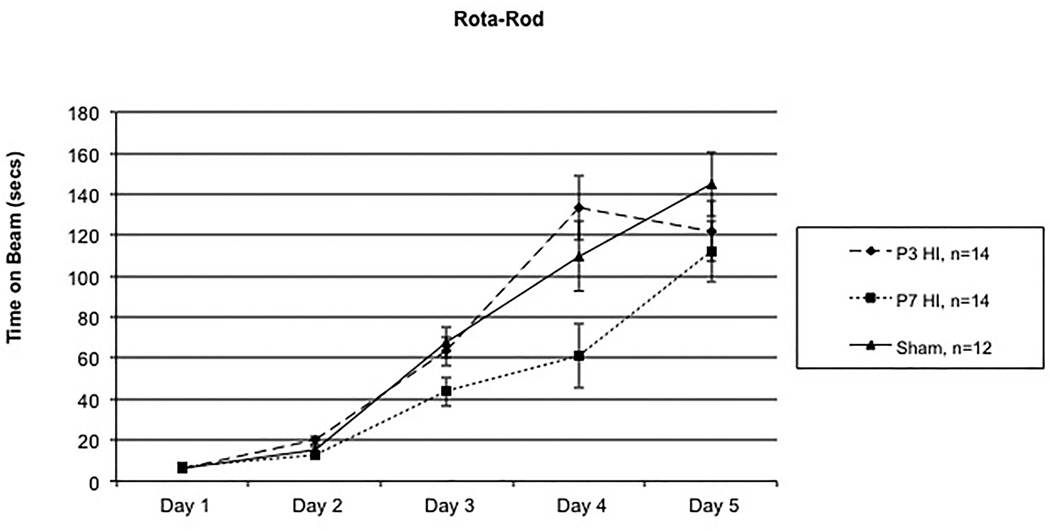
A 5 (Day) × 3 (Treatment) repeated measures ANOVA revealed a significant overall Treatment effect [F(2,37) = 5.247, p<.05]. Additionally, a significant Day × Treatment interaction was found [F(8,148) = 2.718, p<.05] (Figure 1), likely reflecting delayed learning in P7 HI subjects on days 3 and 4. Follow-up analyses revealed no significant Treatment effect between P3 HI and shams [F(1,24) = .007, p>.05]. There was, however, a significant Treatment effect found between P7 HI and shams [F(1,24) = 12.776, p<.01], with P7 HI performing worse. Independent samples t-tests revealed Treatment differences specifically on Day 3 [t(24) = 2.461, p<.05] and Day 4 [t(24) = 3.199, p<.05], with P7 HI performing worse than shams. Finally, a 5 (Day) × 2 (Treatment) repeated measures ANOVA revealed a significant Treatment effect between P3 HI and P7 HI [F(1,26) = 7.261, p<.05], again with P7 HI performing worse. Follow-up independent t-tests revealed differences on Day 2 [t(26)=2.334, p<.05], Day 3 [t(26)=2.132, p<.05] and Day 4 [t(26) = 2.985, p<.05].
Figure 1.
A 5 (Day) × 3(Treatment) repeated measures ANOVA revealed a significant overall Treatment effect [F(2,37) = 5.247, p<.05] along with a significant Day × Treatment interaction [F(8,148) = 2,718, p<.05]. Specifically, significant overall Treatment effects were seen between P7 HI and shams [F(1,24) – 12.776, p<.01] with specific differences on Day 3 [t(24) = 2.461, p<.05] and Day 4 [t(24) = 3.199, p<.05] with P7 HI performing worse. A significant Treatment effect was also seen between P3 HI and P7 HI [F(1,26) = 7.261, p<.05] with specific differences on Day 2 [t(26) = 2.334, p<.05], Day 3 [t(26) = 2.132, p<.05], and Day 4 [t(26) = 2.985, p<.05] with P7 HI performing worse. There were no difference between P3 HI and shams.
1.3.2 Rapid Auditory Processing
Normal Single Tone (P30 & P58)
On the normal single tone task, there was a significant Treatment effect found in both juveniles [F(2,37) = 3.753, p<.05] and adults [F(2,39) = 3.812, p<.05]. Therefore, all further auditory analyses used the normal single tone Att score as a covariate (to remove any confounds due to this difference).
Silent Gap 0–100 Juvenile (P34–38)
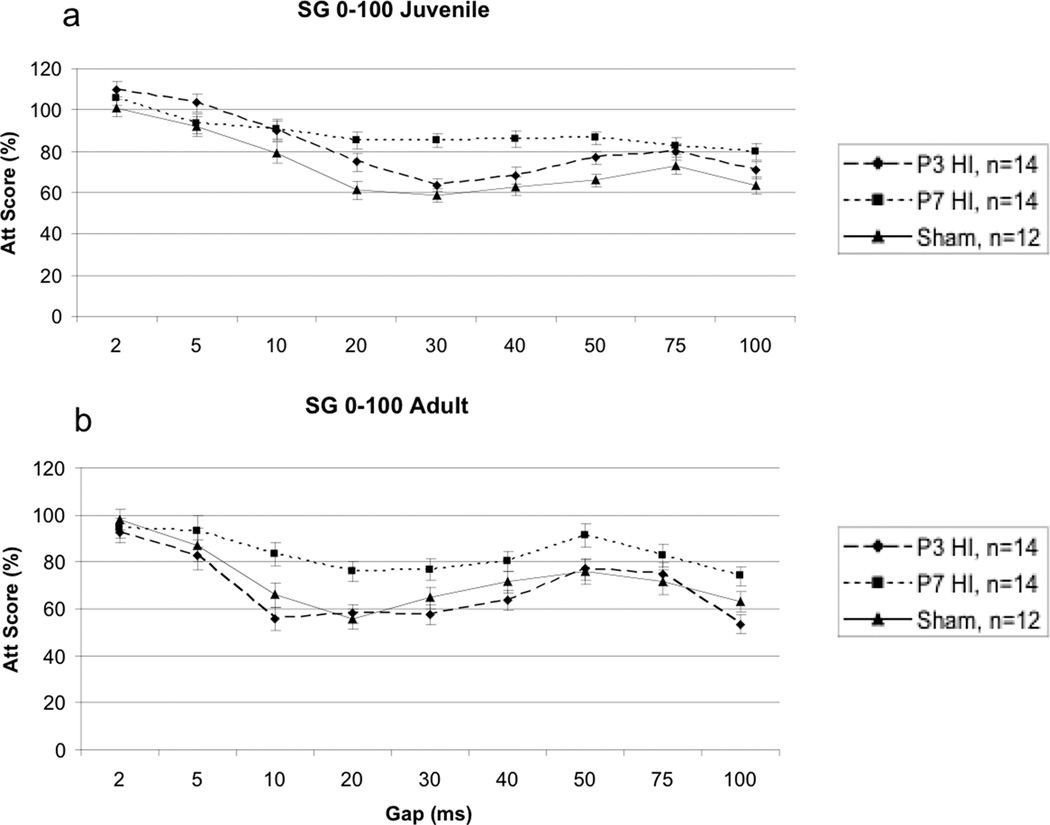
On the SG 0–100 task, a 4 (Day) × 9 (Gap) × 3 (Treatment) repeated measures ANOVA revealed a significant overall Treatment effect [F(2,14) = 7.482, p<.05]1 (Figure 2a). One-tailed tests were used in some of the subsequently analyses, based on prior reports of directionality in results for specific treatments on this task [41]. Follow up repeated measures ANOVA also revealed a significant difference between P3 HI and shams [F(1,9) = 3.935, p<.05, one-tailed], with P3 HI performing worse than shams. Additionally, a highly significant difference was seen between P7 HI and shams [F(1,9) = 14.785, p<.003] with P7 HI performing worse. There was no significant difference between P3 HI and P7 HI groups [F(1,9) = 4.405, p>.05].
Figure 2.
A - A 4 (Day) × (Gap) × 3 (Treatment) repeated measures ANOVA revealed a significant overall Treatment effect [F(2,14) = 7.482, p<.05} in the juvenile period. Specific differences were found between P3 HI and shams [F(1,9) = 3.935, p<.05] with P3 HI performing worse, and between P7 HI and shams [F(1,9) = 14.785, p<.05] with P7 HI performing worse. There was no difference between P3 HI and P7 HI. B – A 9 (Gap) × 3 (Treatment) repeated measures ANOVA revealed a significant overall difference in Treatment [F(2,36) = 5.799, p<.05]. There was a specific difference between P7 HI and shams, [F(1,23) = 3.699, p<.05, one tailed] and between P3 HI and P7 HI groups [F(1,25) = 10.085, p<.05] with P7 HI performing worse. There was no difference between P3 HI and shams.
Silent Gap 0–100 Adulthood (P60)
A 9 (Gap) × 3 (Treatment) repeated measures ANOVA revealed an overall Treatment effect [F(2,36) = 5.799, p<.05] (Figure 2b). Interestingly, in adulthood, there was no longer a significant difference seen between P3 HI and shams [F(1,23) = .201, p>.05]. A 9 (Gap) × 2 (Treatment) repeated measures ANOVA did however show a significant difference between P7 HI and shams [F(1,23) = 3.699, p<.05, one tailed), and between P3 HI and P7 HI groups [F(1,25) = 10.085, p<.05], with P7 HI performing worse compared to both groups.
1.3.3 Learning/Memory
Water Escape (P80)
A Univariate ANOVA revealed no differences between groups on the water escape task [F(2,37) = 2.024, p>.05], indicating a lack of underlying differences in ability to locate the visible escape platform, or in swim speed.
Morris Water Maze (P83–87)
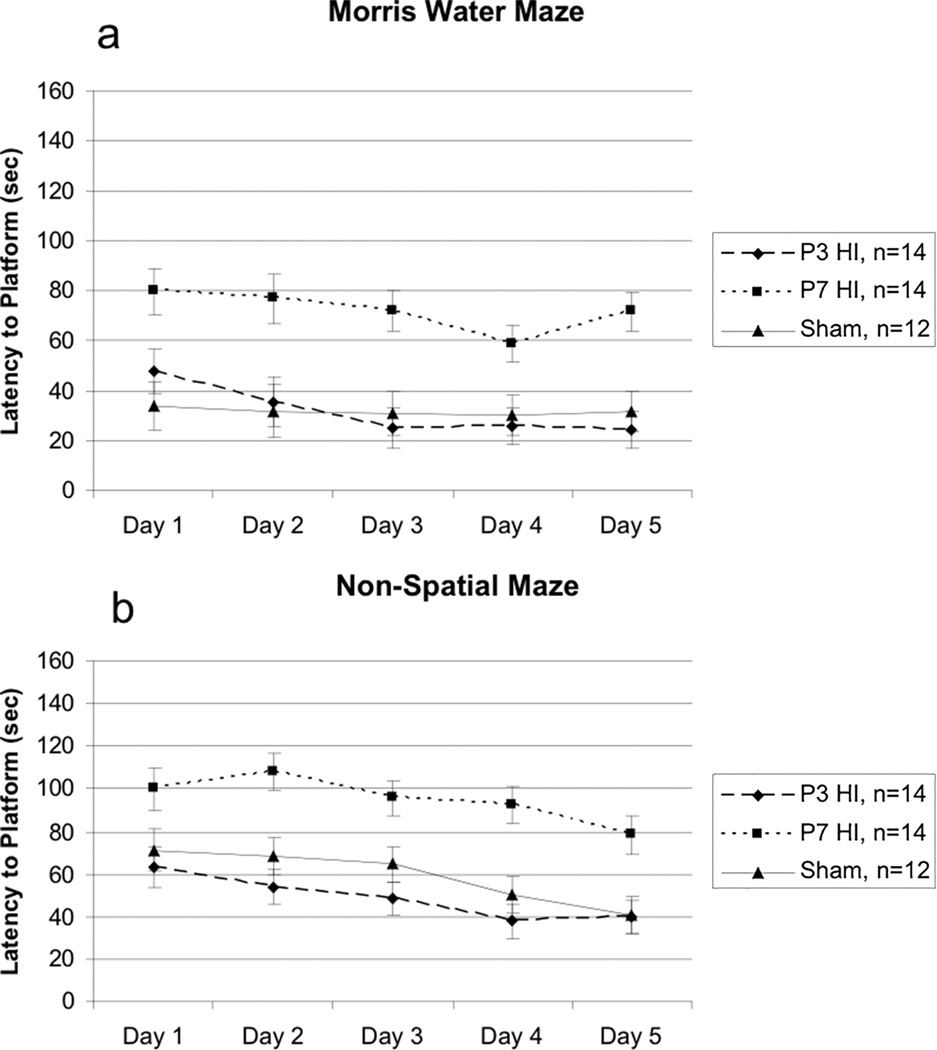
For the MWM a 5 (Day) × 3 (Treatment) repeated measures ANOVA confirmed a lack of significant differences in swim-speed [F(2,37) = .502, p>.05] between the three groups, confirming an absence of underlying swimming deficits in either HI group that might confound results. For total latency to the platform, a 5 (Day) × 3 (Treatment) repeated measures ANOVA revealed a significant Treatment effect [F(2,37) = 9.959, p<.05], as well as a significant Day effect [F(4,148) = 4.687, p<.001] indicating overall learning of the task (Figure 3a). Follow up repeated measures ANOVA revealed no differences between P3 HI and shams [F(1,24) = .00003, p>.05]. There was, however, a significant difference found between P7 HI and shams [F(1,24) = 10.098, p<.01] with P7 performing worse. We also found a difference between P3 HI and P7 HI groups [F(1,26) = 10.985, p<.05], again with P7 HI performing worse. Finally, to test for learning across days, paired samples t-tests were performed between Day 1 and 5 scores within each group. In the P3 HI group, there was a significant difference between Day 1 and Day 5 (t(13)=4.041, p<.01) indicating improvement (learning). There was no difference found in the P7 HI group (t(13) = .736, p>.05), nor in the sham group (t(11) = .579, p>.05) although sham scores were in the expected direction.
Figure 3.
A – A 5 (Day) × 3 (Treatment) repeated measures ANOVA revealed a significant overall Treatment effect [F(2,37) = 9.959, p<.05]. Specific differences were seen between P7 HI and shams [F(1,24) = 0.098, p<.01] and P3 HI and P7 HI [F(1,26) = 10.985, p<.05] with P7 HI performing worse. There was no difference between P3 HI and shams. Furthermore, a paired samples t-test showed a significant difference between Day 1 and Day 5 of testing in P3 HI animals (t(13) = 4.041, p<.001). There was no significant difference between days in P7 HI animals (t(13) = .736, p>.05) or sham animals (t(11) = .579, p>.05). B – A repeated measures ANOVA revealed a significant overall Treatment effect [F(2,37) = 12.048, p<.01). Specific differences were seen between P7 HI and shams [F(1,24) = 9.232, p<.01] and between P3 HI and P7 HI [F(1,26) = 16.835, p<.01] with P7 HI performing worse. There was no difference between P3 HI and shams. Additionally, a paired samples t-test showed a significant difference between Day 1 and Day 5 of testing in P3 HI animals (t(13) = 2.216, p<.05) and in sham animals (t(13) = 3.486, p<.01). There was no difference in P7 HI animals (t(13) = 1.824, p>.05).
Non-Spatial Maze (P91–95)
For the NSM a 5 (Day) × 3 (Treatment), repeated measures ANOVA again revealed no differences in swim speed between groups [F(2,37) = 2.223, p<.05]. For total latency to reach the platform, a 5 (Day) × 3 (Treatment) repeated measures ANOVA revealed a significant overall Treatment effect [F(2,37) = 12.048, p<.01] and a significant Day effect [F(4,148) = 10.251, p<.001] (Figure 3b), again indicating overall learning. Follow up repeated measures ANOVAs revealed no difference between P3 HI and shams [F(1,24) = 3.385, p>.05]. However, a 5 (Day) × 2 (Treatment) repeated measures ANOVA revealed a significant difference between P7 HI and shams [F(1,24) = 9.232, p<.01] with P7 HI performing worse than shams. There was also a significant Treatment effect between P3 HI and P7 HI [F(1,26) = 16.835, p<.01], again with P7 HI performing worse. As above, to test for learning effects, paired samples t-tests were performed between Day 1 and 5 scores within each group. In the P3 HI group there was a significant difference between Day 1 and 5 (t(13) = 2.216, p<.05), as well as in the sham group (t(11) = 3.486, p<.005), indicating learning in both groups. However, there was no significant difference between Day 1 vs 5 in the P7 HI group (t(13) = 1.824, p>.05).
1.3.4 Visual Attention (P100–140):2
5-second stimulus duration
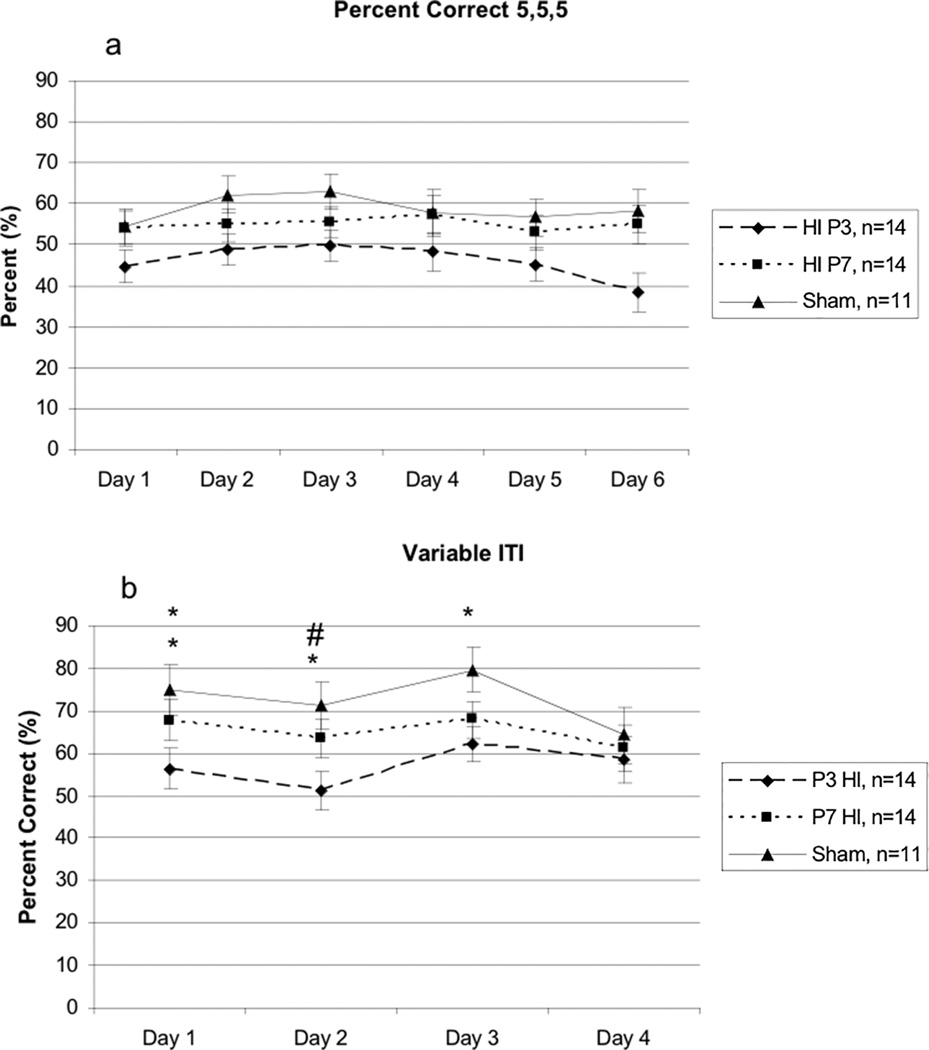
On the 5-second stimulus duration task, a 6 (Day) × 3 (Treatment) repeated measures ANOVA revealed a significant overall effect of Treatment [F(2,36) = 3.122, p<.05] (Figure 4a). Follow up repeated measures ANOVAs revealed a significant difference between P3 HI and shams [F(1,23) = 5.845, p<.05] with P3 HI performing worse. There was no significant difference between P7 HI and shams [F(1,22) = .570, p>.05] or between P3 HI and P7 HI [F(1,25) = 2.64, p>.05] although measures were in the expected direction (P7 HI animals performing worse). There was also no significant difference in average latency to make a correct response [F(2,36) = 2.596, p>.05].
Figure 4.
A – A 6 (Day) × 3 (Treatment) repeated measures ANOVA revealed a significant overall Treatment effect [F(2,36) = 3.122, p<.05]. Specific differences were seen between P3 HI and shams, [F(1,23) = 5.845, p<.05] with P3 HI performing worse. There were no differences seen between P3 HI and P7 HI or between P7 HI and shams. B – A 4 (Day) × 3 (Treatment) repeated measures ANOVA revealed a trend for an overall Treatment effect [F(2,29) = 2.569, p=.09] and a significant Day × Treatment interaction [F(6,87) = 2.287, p<.05]. One Way ANOVAs revealed a significant effect of Day 1 [F(2,36) = 4.428, p<.05], of Day 2 [F(2,36) = 4.186, p<.05], and of Day 3 [F(2,36) = 3.325, p<.05]. There was no significant difference on Day 4 [F(2,36) = .673, p>.05]. For Day one, a significant difference was seen between P3 HI and sham groups (t(23) = 2.562, p<.05) and between P3 HI and P7 HI groups (t(26) = 2.199, p<.05). There was no difference between P7 HI and shams (t(23) = .753, p>.05). For Day 2, there was a significant difference between P3 HI and sham groups (t(23) = 3.01, p<.01) and a trend between P7 HI and P3 HI groups (t(26) = 1.112, p=.08) There was no difference between P7 HI and shams (t(23) = 1.78, p>.05). For Day 3, there was a significant difference between P3 HI and sham groups (t(23) = 2.431, p<.05). There were no differences between P3 HI and P7 HI groups (t(26) = 1.538, p>.05) or between P7 HI and sham groups (t(23) = 1.142, p>.05).
Variable Inter-Trial Interval (VITI)
A 4 (Day) × 3 (Treatment) repeated measures ANOVA revealed a trend towards an overall Treatment effect [F(2,29) = 2.569, p=.09]3. Additionally, there was a significant Day × Treatment interaction [F(6,87) = 2.287, p<.05] (Figure 4b), suggesting that over days, higher performing Treatment groups improved more dramatically than poorer performing groups. A follow-up one way ANOVA revealed a significant Treatment effect on Day 1 [F(2,36) = 4.428, p<.05], Day 2 [F(2,36) = 4.186, p<.05], and Day 3 [F(2,36) = 3.325, p<.05]. There was no significant effect on Day 4 [F(2,36) = .673, p>.05]. Follow-up independent samples t-test analysis showed that on Day one of testing, there was a significant difference between P3 HI and sham groups (t(23) = 2.562, p<.05), and between P3 HI and P7 HI groups (t(26) = 2.199, p<.05), with P3 animals performing worse than both groups. There was no difference between P7 HI and shams (t(23) = .753, p>.05), although scores were in the correct direction (P7 HI worse than shams). For Day 2, there was a significant difference between P3 HI and sham groups (t(23) = 3.01, p<.01) and a trend between P7 HI and P3 HI groups (t(26) = 1.112, p=.08) with P3 HI animals performing worse. There was no difference between P7 HI and shams (t(23) = 1.78, p>.05) though scores were in the expected direction (P7 HI performing worse). Finally for Day 3, there was a significant difference between P3 HI and sham groups (t(23) = 2.431, p<.05) with P3 HI animals performing worse. There were no differences between P3 HI and P7 HI groups (t(26) = 1.538, p>.05) or between P7 HI and sham groups (t(23) = 1.142, p>.05) although scores were in the expected direction. There was also no significant group differences in average latency to make a correct response [F(2,36) = 2.135, p>.05].
1.3.5 Histology
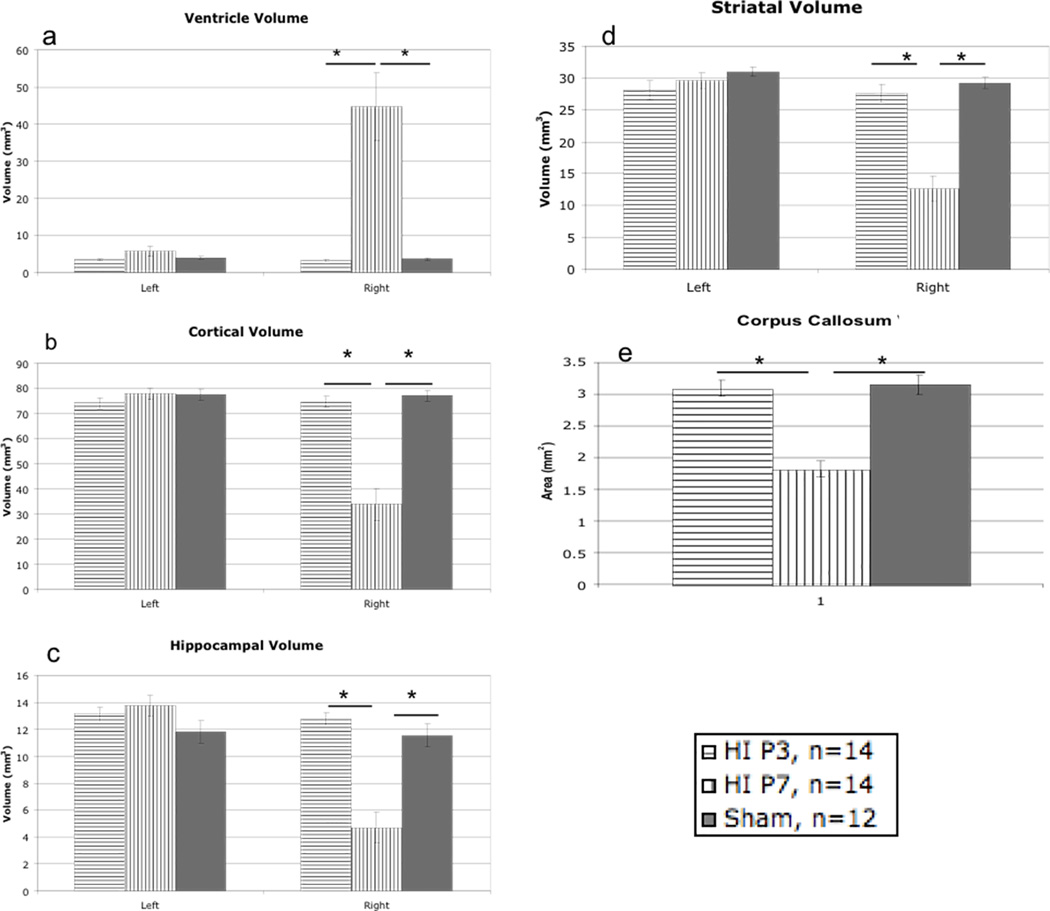
Ventricular Volume
A Univariate ANOVA revealed a significant Treatment effect for right ventricular volume [F(2,39) = 18.77, p<.001], but no Treatment differences for left ventricular volume [F(2,39) = 2.20, p>.05] (Figure 5a). Follow-up independent sample t-tests revealed a significant difference specifically between P3 HI and P7 HI [t(26) = −4.529, p<.001] and between P7 HI and shams [t(24) = −4.137, p<.001], with P7 HI animals showing a higher rate of right ventriculomegaly. There was no significant difference between P3 HI and shams in right ventricular volume.
Figure 5.
A – A Univariate ANOVA revealed a significant overall Treatment effect for right ventricular volume [F(2,39) = 18.77, p<.001] with specific differences between P7 HI and shams [t(24) = −4.137, p<.001] and between P3 HI and P7 HI [t(26) = −4.529, p<.001] with P7 HI showing higher right ventricle volume. There was no difference between P3 HI and shams, or in left ventricle volume. B – A Univariate ANOVA revealed a significant overall Treatment effect right for cortical volume [F(2,39) = 33.9, p<.001] with specific differences between P7 HI and shams [t(24) = 6.063, p<.001] and between P3 HI and P7 HI [t(26) = 6.089, p<.001] with P7 HI showing smaller right cortical volume. There was no difference between P3 HI and shams, or in left cortical volume. C - A Univariate ANOVA revealed a significant overall Treatment effect for right hippocampal volume [F(2,39) = 25.58, p<.001] with specific differences between P7 HI and shams [t(24) = 4.675, p<.001] and between P3 HI and P7 HI [t(26) = 6.531, p<.001] with P7 HI showing smaller right hippocampal volume. There was no difference between P3 HI and shams, or in left hippocampal volume. D - A Univariate ANOVA revealed a significant overall Treatment effect for right striatal volume [F(2,39) = 34.08, p<.001] with specific differences between P7 HI and shams [t(24) = 7.084, p<.001] and between P3 HI and P7 HI [t(26) = 6.021, p<.001] with P7 HI showing smaller right striatal volume. There was no difference between P3 HI and shams, or in left striatal volume. E - A Univariate ANOVA revealed a significant overall Treatment effect for corpus callosum area [F(2,39) = 32.364, p<.001] with specific differences between P7 HI and shams [t(24) = 6.372, p<.001] and between P3 HI and P7 HI [t(26) = 6.357, p<.001] with P7 HI showing smaller callosum area. There was no difference between P3 HI and shams.
Cortical Volume
A Univariate ANOVA revealed a significant Treatment effect for right cortical volume [F(2,39) = 33.9, p<.001] but no effect on left cortical volume [F(2,39) = .923, p>.05] (Figure 5b). Follow-up independent sample t-test revealed a significant difference between P3 HI and P7 HI [t(26) = 6.089, p<.001], and between P7 HI and shams [t(24) = 6.063, p<.001], with P7 HI animals showing significantly smaller right cortical volume than both other groups. There was no difference between P3 HI and shams.
Hippocampal Volume
A Univariate ANOVA revealed a significant Treatment effect for right hippocampal volume [F(2,39) = 25.58, p<.001] but no effect on left hippocampal volume [F(2,39) = 1.83, p>.05] (Figure 5c). Follow-up independent samples t-tests revealed a significant difference between P3 HI and P7 HI [t(26) = 6.531, p<.001], and between P7 HI and shams [t(24) = 4.675, p<.001], with P7 HI showing significantly smaller right hippocampal volume than both other groups. There was no difference seen between P3 HI and shams.
Striatal Volume
A Univariate ANOVA revealed a significant Treatment effect in right striatal volume [F(2,39) = 34.08, p<.001], but no significant effect on left striatal volume [F(2,39) = 1.19, p>.05] (Figure 5d). Follow-up independent sample t-tests revealed a significant difference between P3 HI and P7 HI [t(26) = 6.021, p<.001], and between P7 HI and shams [t(24) = 7.084, p<.001], with P7 HI showing smaller right striatal volumes as compared to both other groups. There was no difference between P3 HI and shams.
Corpus Callosum Area
A Univariate ANOVA revealed a significant Treatment effect on corpus callosum area [F(2,39) = 32.364, p<.001] (Figure 5e). Follow-up independent sample t-tests revealed a significant difference between P3 HI and P7 HI [(t) = 6.357, p<.001], as well as a difference between P7 HI and shams [t(24) = 6.372, p<.001], with P7 HI animals showing smaller area compared to both groups. Again, there was no difference between P3 HI and shams.
1.4 Discussion
In the current study, we have replicated prior evidence of behavioral deficits in P7 HI rats [39], as well as reports that animals with an “early” (preterm equivalent) HI injury show a recovery of function on an RAP task compared to animals that received a “late” (term equivalent) injury [41]. Specifically, animals that had HI injury induced on P3 showed deficits on the RAP task in juvenile periods but not in adulthood, whereas P7 HI injured animals showed persistent deficits throughout early life and adulthood on the RAP task. Furthermore, P7 HI injured animals showed deficits on learning/memory tasks in adulthood, as well as a motor learning task in the juvenile period. These findings also replicate previous data showing P7 HI deficits on a variety of learning and memory tasks in male rats [39, 41–45], as well as on sensorimotor tasks [47–48]. Additionally, we report that P7 HI animals showed a reduction in right cortical, hippocampal, and striatal volume along with a reduction in corpus callosum area, as compared to P3 HI and shams. P7 HI animals also showed increases in right ventricular size. Moreover, we report novel findings that, although P3 HI animals show only transient RAP deficits and no learning/memory or motor learning deficits, they do show highly robust deficits on a visual attention task as compared to P7 HI rats.
1.4.1 Motor Learning deficits
With regards to our motor learning task, P7 HI animals exhibited a clear deficit. Specifically, on Days 2, 3, and 4 of testing, P7 HI injured animals spent a significantly shorter time on the rotating beam as compared to both P3 HI and sham animals. However, there were no differences seen on Days 1 or 5 of testing between the three groups. This indicates that P7 HI injured animals were able to eventually learn the task, but took significantly longer to do so compared to both P3 HI and shams. This finding is important, as many experiments use only one day of rotarod testing to assess coordination deficits, and our findings suggest that although P7 HI animals are equivalent to shams in regard to baseline (naïve) coordination, they do show a delay in motor learning (performance improvement) when the task is administered over multiple trials. In clinical literature, infants diagnosed with HIE also show motor impairments, and these impairments are believed to reflect to basal ganglia, cortical and/or cerebellum damage [7, 29–30]. Specifically, HIE children (diagnosed with encephalopathy following term insult) showed significant reductions in basal ganglia and thalamic structures, as well as in the internal capsule, as measured by MRI. Moreover, these reductions were associated with deficits on neuromotor tasks [7, 29–30]. The current study revealed a similar pattern of brain injury in the P7 HI injured group, with evidence of significant reductions in striatal and cortical volume as well as a reduction in corpus callosum area. This extends previous research showing sensorimotor deficits in P7 HI injured animals along with cortical, thalamic, and striatal injury [47]. Interestingly, P3 HI injured animals did not show any deficits on the rotarod task, nor any significant reductions in brain volume, even though premature birth in human infants is often associated with motor deficits such as cerebral palsy [27]. It may be that due to the relatively small degree of myelination and white matter in the small rodent as compared to larger HI models (e.g., sheep, pigs), it is much harder to induce a white matter injury and associated motor deficits comparable to those seen in preterm human neonates [65].
1.4.2 Rapid Auditory Processing Deficits
With regards to rapid auditory processing deficits, the current study replicated previous work showing a recovery of function following early (P1–P3) neonatal HI injury [41]. That is, while P7 HI injured animals showed persistent deficits on the SG 0–100 RAP task in juvenile and adult periods, P3 HI injured animals only showed deficits during juvenile RAP testing - - not in adulthood. Taken together, these data show that animals with very early HI injuries (simulating preterm) are more able to recover from early RAP deficits compared to HI injuries induced later in life (near term). This suggests that neural-reorganization and plasticity following early HI injury may lead to more optimal brain re-organization and functional recovery, as well as suggesting that neural systems critical to RAP may be less vulnerable to damage following early HI insult. Conversely, P7 HI animals showed widespread brain injury, including volumetric reductions in the right cortex, hippocampus, striatum, and increases in right ventricular volume in parallel with persistent behavioral deficits. Importantly, although the neural substrates underlying rapid auditory processing are not as well delineated (as for example, the role of the hippocampus in spatial memory), there is some evidence that anatomic alterations to the medial geniculate nucleus may index RAP deficits (in both humans and animal models [66–67]). Thus, it is critical to note that in a concurrent publication, we report reductions in cell size in the MGN of P7 HI but not P3 HI rats as measured from subjects in the current study (see [67] for further discussion of these findings).
1.4.3 Learning/Memory deficits
In addition, P7 HI injured animals showed significant memory and learning deficits on both the spatial and non-spatial learning/memory tasks in adulthood as compared to P3 HI injured and sham animals. P7 HI animals also revealed a lack of improvement in escape latencies over five days (i.e., did not display any learning effects), whereas P3 HI subjects (on both the MWM and NSM) and shams (on the NSM) did so. Furthermore, P7 HI injured animals also showed significant reductions in right striatal and right hippocampal volume. These anatomic indices may be important given the extensive data showing that the hippocampus mediates both spatial navigation and memory [68–69]. It is possible that reductions in right hippocampal volume in the P7 HI injured animals contribute to deficits seen on the spatial memory (Morris) maze. In the same way, dorsal striatal structures, (such as nucleus accumbens) have been suggested to mediate non-spatial navigation and learning [69–70]. Thus anatomic disruptions of striatum might account in part for the non-spatial memory deficits seen in P7 HI rats. In the clinical literature, term HIE infants with disruptions to the hippocampus and associated projections to cortex also showed disrupted memory function and spatial processing [71].
1.4.4 Visual Attention deficits
Interestingly, in stark contrast to behavioral patterns on all other tasks, P3 HI animals showed robust deficits on the visual attention task as compared to both sham and P7 HI injured animals. There are no other data that we are aware of demonstrating visual attention deficits following preterm neonatal HI injury in rodent models, nor comparing behavioral outcome as a function of timing of injury. Notably, there is one report using a model where animals were exposed to hypoxia between P1–3, and these subjects did show attention-deficit hyperactivity disorder (ADHD) like symptoms (specifically hyperactivity) on a fixed interval extinction test [52], although measures of attention similar to those reported here were not obtained. Overall, combined data certainly support the general conclusion that HI injuries may represent a particular risk for ADD/ADHD in the preterm population. Indeed, clinical data shows that children born prematurely are clearly at increased risk for ADD/ADHD [25, 72], and attention problems are believed to result from underlying deficits in processing speed, working memory, and/or poor visuospatial abilities [73–74]. It has been proposed that damage to sub-plate neurons in premature infants with HI injury may results in disruptions to fronto-striato-thalamic circuitry, which could, in turn, result in functional deficits in attention, modulation of activity, and executive functioning [71]. In fact, data show that adolescents born prematurely exhibit deficits on pre-attentive and attention tasks [75], with subjects showing dysfunction of the frontal cortex as measured by EEG [75]. Also of note is another study in which animals with induced HI injury on P3 showed anxiety-like behaviors on an elevated T-maze [76]. We did not measure anxiety-like behaviors in the current study, and this represents an important venue for future research.
Moreover, in the current study, P3 HI animals did not show any significant volume loss in specific brain regions such as the striatum, although attention deficits might reflect specific cellular or circuitry dysfunction without global tissue loss (our anatomic criteria for striatal measurements may not have captured specific and focal alterations). Interestingly, in other reports, animals with HI injury induced on P3 showed decreases in enzymatic activity (Na+/K+ ATPase) [77], as well as increases in necrotic cells and axonal disruption with prolonged astrocytic and microglial activation in deep cortical areas [78]. Studies also show reductions in cortical size with altered patterns of myelination 18 days following such injury [79]. This specific neuropathology might contribute to alterations in connectivity following P3 HI that could contribute to attention deficits.
1.4.5 Timing differences in Neurodevelopment for P3 versus P7
In order to interpret the differing pattern of behavioral deficits following early versus late HI injury, it is important to review what is known about the neuroanatomical areas of susceptibility based on the timing of injury. That is, the variety of behavioral deficits seen in the P7 HI injured animals might be explained by the timing in development of affected regions such as the cortex, hippocampus, and striatum. Research has demonstrated that at birth, rodent cortical development is in an “elementary” stage, and it is not until 6–12 days after birth that the six typical cortical layers become apparent [80]. Additionally, rapid dendritic branching occurs around P12, and the density of axons increases markedly between P6–18 [80]. In the striatum, cell death regulated by apoptosis (measured by TUNNEL) is apparent during the first week of postnatal life, specifically between P3 and P7 [81]. Finally, maturation of hippocampal cells does not occur until P15–18 in the neonatal rodent [82]. The timing of these developmental processes may contribute to the heightened vulnerability of these regions following late (P7+) HI injury in the rodent. Additionally, the development of different cortical catecholamine and other receptor subtypes (e.g., AMPA and NMDA) might also contribute to vulnerability to brain injury in cortical regions following late injury. Specifically, increases in the NMDA receptor do not occur in the hippocampus and cortex until late in prenatal development in rodents (P7–P12), and these increases continue through postnatal development in the human infant [83]. This is a key temporal event since the NMDA receptor has been implicated in the detrimental glutotoxicity effects of the apoptotic cascade following HI injury [84]. This may in turn contribute to the lack of extensive tissue loss in P3 HI injured rats as compared to P7 HI rats seen in the current study. Furthermore, severity of lesion size as well as sensitivity to brain injury is seen with increasing age in rodent models of HI [54, 85–86], and studies have demonstrated a progressive increase in the overall frequency of cerebral lesion size with increasing age, as well as a shift towards vulnerability of various brain regions in later injured (P7) animals (i.e., animals with HI injury induced on P2–3 showed more resistance to resulting injury compared to P7 HI injured animals [86]).
Interestingly, P3 HI injured animals showed robust deficits on the visual attention task despite a lack of volumetric alterations in key brain structures. This dissociation is not altogether incongruent, since behavioral changes may also result from subtle changes in neural circuitry. For example, a recent study demonstrated widespread decreases in connectivity strength of intracerebral connections during development in premature subjects [87]. To the best of our knowledge, there are few studies specifically investigating visual attention and neural connectivity in premature children. However other cognitive behaviors (such as language) have been associated with altered connectivity in adolescents born prematurely and with no diagnosis of IVH-PVH. Here, using both fRMI and DTI, preterm subjects were found to employ neural systems for auditory language that were different from term controls - - specifically, right hemisphere activation that differed from typical left hemisphere language processing [88–90]. Preterm subjects also showed alterations in resting state connections in executive control networks as measured by fMRI [30]. Although little is known about vulnerability of neural connectivity patterns to deleterious changes following preterm birth, anomalies here could reflect disruptions in formation or pruning of neural synaptic connections [88]. Future studies will perform additional analyses to attempt to ascertain what circuitry changes may underlie these attention deficits, but this will likely require an entirely new study using tissue preparation and staining methods pertinent to the cellular measures of interest.
1.5 Conclusions
In conclusion, the current study provides a direct comparison of anatomic and behavioral outcomes following comparable HI injury in a P3 (preterm equivalent) rodent model and a P7 (term equivalent) rodent model. Although clinical literature indicates elevated risk for cognitive/behavioral difficulties in both preterm HI and term HIE populations, it has been difficult to conduct any direct comparisons in outcomes due to the difficulties in matching other key variable across these diverse populations. The current study, however, suggests that when all other factors are held constant, HI injury at term likely has far greater lifelong consequences than HI injury in preterm infants. Nonetheless, the transient RAP deficits and robust attention deficits seen in P3 HI subjects indicate that some deleterious effects have occurred here as well, and our finding is consistent with clinical evidence of heightened risk for language and attention problems among preterm neonates [25, 72]. The combined findings stress the importance of comparing and contrasting HI injury in both preterm and term infants relative to follow-up morbidity, plasticity and long-term behavioral development. They also heighten the urgency to continue a search for neuroprotective interventions such as whole body/head cooling or erythropoietin in term HIE infants [91–92]. Finally, our findings also show subtle but significant early RAP deficits and robust attention deficits in a preterm HI model, emphasizing the importance of developing new interventions for this population as well. Future studies should examine the specific regions and neural processes that are vulnerable following early and late neonatal HI injury, as well as further exploring changes in brain connectivity and resulting behavioral deficits following preterm birth.
Highlights.
-
-
P3 HI injured rodents showed transient RAP and robust attention deficits
-
-
P7 HI injured rodents showed deficits on motor learning, RAP, and learning/memory
-
-
Histologically, postnatal day 7 HI injured rodents showed global brain tissue loss
-
-
P3 HI injured animals had no global tissue loss suggesting connectivity alterations
Acknowledgments
This research was supported by NIH grant HD049792. We would also like to acknowledge Dr. Michael Gorgieff for his contributions to the editing of this manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
It should be noted that on one day of testing, computer malfunctioning resulting in a loss of data for some animals, thus leading to differences in degrees of freedom for juvenile RAP.
For visual attention analysis, differences in degrees of freedom reflect one subject dropped from testing due to the time constraints during a testing day.
Differences in degrees of freedom in the inter-trial interval task were due to the fact that on one day of testing there was a computer malfunction resulting in a loss of data.
References
- 1.Annibale D, Hill J. Periventricular hemorrhage-intraventricular hemorrhage. 2008 Retrieved from www.emedicine.medscape.com.
- 2.Volpe J. Brain injury in the premature infant--from pathogenesis to prevention. Brain Dev. 1997;19:519–534. doi: 10.1016/s0387-7604(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 3.Volpe J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpe J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Vannucci R. Hypoxic-ischemic encephalopathy. Am J Perinatol. 2000;17:113–120. doi: 10.1055/s-2000-9293. [DOI] [PubMed] [Google Scholar]
- 6.Huang B, Castillo M. Hypoxic-ischemic brain injury: Imaging findings from birth to adulthood. Radiographics. 2008;28:417–439. doi: 10.1148/rg.282075066. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, Cowan FM. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76:2055–2061. doi: 10.1212/WNL.0b013e31821f442d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansson-Verkasalo E, Valkama M, Vainionpaa L, Paakko E, Ilkko E, Lehtihalmes M. Language development in very low birth weight preterm children: a follow-up study. Folia Phoniatr Logo. 2004;56:108–119. doi: 10.1159/000076062. [DOI] [PubMed] [Google Scholar]
- 9.Steinman KJ, Gorno-Tempini ML, Glidden DV, Kramer JH, Miller SP, Barkovich AJ, Ferriero DM. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–1030. doi: 10.1542/peds.2008-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortiz-Mantilla S, Choudhury N, Leevers H, Benasich AA. Understanding language and cognitive deficits in very low birth weight children. Dev Psychobiol. 2008;50:107–126. doi: 10.1002/dev.20278. [DOI] [PubMed] [Google Scholar]
- 11.Jansson-Verkasalo E, Korpilahti P, Jantti V, Valkama M, Vainionpaa L, Alku P, Suominen K, Naatanen R. Neurophysiologic correlates of deficient phonological representations and object naming in prematurely born children. Clin Neurophysiol. 2004;115:179–187. doi: 10.1016/s1388-2457(03)00319-5. [DOI] [PubMed] [Google Scholar]
- 12.Badawi N, Keogh JM, Dixon G, Kurinczuk JJ. Developmental outcomes of newborn encephalopathy in the term infant. Indian J Pediatr. 2001;68:527–530. doi: 10.1007/BF02723247. [DOI] [PubMed] [Google Scholar]
- 13.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 14.Lubsen J, Vohr B, Myers E, Hampson M, Lacadie C, Schneider KC, Ment LR. Microstructural and functional connectivity in the developing preterm brain. Semin Perinatol. 2011;35:34–43. doi: 10.1053/j.semperi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicari S, Caravale B, Carlesimo GA, Casadei AM, Allemand F. Spatial working memory deficits in children at ages 3–4 who were low birth weight, preterm infants. Neuropsychology. 2004;18:673–678. doi: 10.1037/0894-4105.18.4.673. [DOI] [PubMed] [Google Scholar]
- 16.Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- 17.Curtis WJ, Zhuang J, Townsend EL, Hu X, Nelson CA. Memory in early adolescents born prematurely: A functional magnetic resonance imaging investigation. Dev Neuropsychol. 2006;29:341–377. doi: 10.1207/s15326942dn2902_4. [DOI] [PubMed] [Google Scholar]
- 18.Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005;90:F380–F387. doi: 10.1136/adc.2004.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fazzi E, Bova S, Giovenzana A, Signorini S, Uggetti C, Bianchi P. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev Med Child Neurol. 2009;51:974–981. doi: 10.1111/j.1469-8749.2009.03272.x. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson J, Braddick O, Anker S, Nardini M, Birtles D, Rutherford MA, Mercuri E, Dyet LE, Edwards AD, Cowan FM. Cortical vision, MRI and developmental outcome in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2008;93:F292–F297. doi: 10.1136/adc.2007.116988. [DOI] [PubMed] [Google Scholar]
- 21.Gimenez M, Junque C, Narberhaus A, Caldu X, Salgado-Pineda P, Bargallo N, Segarra D, Botet F. Hippocampal gray matter reduction associates with memory deficits in adolescents with history of prematurity. Neuroimage. 2004;23:869–877. doi: 10.1016/j.neuroimage.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Luu TM, Ment L, Allan W, Schneider K, Vohr BR. Executive and memory function in adolescents born very preterm. Pediatrics. 2011;127:e639–e646. doi: 10.1542/peds.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briscoe J, Gathercole SE, Marlow N. Short-term memory and language outcomes after extreme prematurity at birth. J Speech Lang Hear Res. 1998;41:654–666. doi: 10.1044/jslhr.4103.654. [DOI] [PubMed] [Google Scholar]
- 25.Mercuri E, Atkinson J, Braddick O, Anker S, Cowan F, Rutherford M, Pennock J, Dubowitz L. Visual function in full-term infants with hypoxic-ischaemic encephalopathy. Neuropediatrics. 1997;28:155–161. doi: 10.1055/s-2007-973693. [DOI] [PubMed] [Google Scholar]
- 26.Mercuri E, Haataja L, Guzzwtta A, Anker S, Cowan F, Rutherford M, Andrew R, Braddick O, Cinoni G, Dubowitz L, Atkinson J. Visual function in term infants with hypoxic-ischaemic insults: Correlation with neurodevelopment at 2 years of age. Arch Dis Child Fetal Neonatal Ed. 1999;80:F99–F104. doi: 10.1136/fn.80.2.f99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Haastert IC, de Vries LS, Eijsermans MJ, Jongmans MJ, Helders PJ, Gorter JW. Gross motor functional abilities in preterm-born children with cerebral palsy due to periventricular leukomalacia. Dev Med Child Neurolo. 2004;50:684–689. doi: 10.1111/j.1469-8749.2008.03061.x. [DOI] [PubMed] [Google Scholar]
- 28.Kono Y, Mishina J, Yonemoto N, Kusuda S, Fujimura M. NICU Network, J: Neonatal correlates of adverse outcomes in very low-birthweight infants in the NICU network. Pediatr Int. 2011;53:930–935. doi: 10.1111/j.1442-200X.2011.03424.x. [DOI] [PubMed] [Google Scholar]
- 29.Mercuri E, Barnett AL. Neonatal brain MRI and motor outcome at school age in children with neonatal encephalopathy: A review of personal experience. Neural Plast. 2003;10:51–57. doi: 10.1155/NP.2003.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercuri E, Barnett A, Rutherford M, Guzzetta A, Haataja L, Cioni G, Cowan F, Dubowitz L. Neonatal cerebral infarction and neuromotor outcome at school age. Pediatrics. 2004;113:95–100. doi: 10.1542/peds.113.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Cho HK, Jang SH, Lee E, Kim SY, Kim S, Kwon YH, Son SM. Diffusion tensor imaging-demonstrated differences between hemiplegic and diplegic cerebral palsy with symmetric periventricular leukomalacia. AJNR Am J Neuroradiol. 2013;34:650–654. doi: 10.3174/ajnr.A3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Downie AL, Jakobson LS, Frisk V, Ushycky I. Auditory temporal processing deficits in children with periventricular brain injury. Brain and Lang. 2002;80:208–225. doi: 10.1006/brln.2001.2594. [DOI] [PubMed] [Google Scholar]
- 33.Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. (2002) [DOI] [PubMed] [Google Scholar]
- 34.Choudhury N, Leppanen PH, Leevers HJ, Benasich AA. Infant information processing and family history of specific language impairment: Converging evidence for RAP deficits from two paradigms. Dev Sci. 2007;10:213–236. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. DevNeurosci. 2005;27:81–86. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- 36.Clancy B, Darlington B, Finlay BL. Translating developmental time across mammalian species. Neurosci. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 37.Alexander ML, Hill CA, Rosenkrantz TS, Fitch RH. Evaluation of the therapeutic benefit of delayed administration of erythropoietin following early hypoxic-ischemic injury in rodents. Dev Neurosci. 2013;34:515–524. doi: 10.1159/000345645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29:381–388. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClure MM, Peiffer AM, Rosen GD, Fitch RH. Auditory processing deficits in rats with neonatal hypoxic-ischemic injury Int J Dev Neurosci. 2013;23:351–362. doi: 10.1016/j.ijdevneu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 40.McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Auditory processing deficits in unilaterally and bilaterally injured hypoxic-ischemic rats. Neuroreport. 2005;16:1309–1312. doi: 10.1097/01.wnr.0000175613.16183.6c. [DOI] [PubMed] [Google Scholar]
- 41.McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Rapid auditory processing and learning deficits in rats with P1 versus P7 neonatal hypoxic-ischemic injury. Behav Brain Res. 2006;172:114–121. doi: 10.1016/j.bbr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander M, Smith AL, Rosenkrantz TS, Fitch RH. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013;3:177–190. doi: 10.3390/brainsci3010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arteni NS, Salgueiro J, Torres I, Achaval M, Netto CA. Neonatal cerebral hypoxia- ischemia causes lateralized memory impairments in the adult rat. Brain Res. 2003;973:171–178. doi: 10.1016/s0006-8993(03)02436-3. [DOI] [PubMed] [Google Scholar]
- 44.McClure MM, Threlkeld SW, Fitch RH. Auditory processing and learning/memory following erythropoietin administration in neonatally hypoxic-ischemic injured rats. Brain Res. 2007;1132:203–209. doi: 10.1016/j.brainres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda T, Mishima K, Yoshikawa T, Iwasaki K, Fujiwara M, Xia YX, Ikenoue T. Selective and long-term learning impairment following neonatal hypoxic-ischemic brain insult in rats. Behav Brain Res. 2001;118:17–25. doi: 10.1016/s0166-4328(00)00287-4. [DOI] [PubMed] [Google Scholar]
- 46.Tuor UI, Hudzik TJ, Malisza K, Sydserff S, Kozlowski P, Del Bigio MR. Long-term deficits following cerebral hypoxia-ischemia in four-week-old rats: Correspondence between behavioral, histological, and magnetic resonance imaging assessments. Exp Neurol. 2001;167:272–281. doi: 10.1006/exnr.2000.7565. [DOI] [PubMed] [Google Scholar]
- 47.Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, Lengvari I. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157:157–165. doi: 10.1016/j.bbr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Pazaiti A, Soubasi V, Spandou E, Karkavelas G, Georgiou T, Karalis P, Guiba-Tziampiri O. Evaluation of long-lasting sensorimotor consequences following neonatal hypoxic-ischemic brain injury in rats: The neuroprotective role of MgSO4. Neonatology. 2009;95:33–40. doi: 10.1159/000151753. [DOI] [PubMed] [Google Scholar]
- 49.Quairiaux C, Sizonenko SV, Megevand P, Michel CM, Kiss JZ. Functional deficit and recovery of developing sensorimotor networks following neonatal hypoxic-ischemic injury in the rat. Cereb Cortex. 2010;20:2080–2091. doi: 10.1093/cercor/bhp281. [DOI] [PubMed] [Google Scholar]
- 50.Fan LW, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res. 2005;165:80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 51.Delcour M, Olivier P, Chambon C, Pansiot J, Russier M, Liberge M, Xin D, Gestreau C, Alescio-Lautler B, Gressens P, Verney C, Barbe MF, Baud O, Coq JO. Neuroanatomical, sensorimotor and cognitive deficits in adult rats with white matter injury following prenatal ischemia. Brain Pathol. 2012;22:1–16. doi: 10.1111/j.1750-3639.2011.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oorschot DE, Voss L, Covey MV, Bilkey DK, Saunders SE. ADHD-like hyperactivity, with no attention deficits, in adult rats after repeated hypoxia during the equivalent of extreme prematurity. J. Neurosci Methods. 2007;166:315–322. doi: 10.1016/j.jneumeth.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Brochu ME, Girard S, Lavoie K, Sebire G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J Neuroinflammation. 2011;8:55. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grafe MR. Developmental changes in the sensitivity of the neonatal rat brain to hypoxic/ischemic injury. Brain Res. 1994;653:161–166. doi: 10.1016/0006-8993(94)90385-9. [DOI] [PubMed] [Google Scholar]
- 55.Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia- ischemia in rodent models: Implications for sex-specific neuroprotection in clinical neonatal practice. Neurol Res Int. 2012;2012:867531. doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scafidi J, Fagel DM, Ment LR, Vaccarino FM. Modeling premature brain injury and recovery. Int J Dev Neurosci. 2009;27:863–871. doi: 10.1016/j.ijdevneu.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Trans Stroke Med. 2010;19:2–13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 2008;76:1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Hooge R, De Deyn PP. Applications of the morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 60.Hodges H. Maze procedures: the radial arm and water maze compared. Cog Brain Res. 1996:167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 61.Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- 62.Schneider T, Ilott N, Brolese G, Bizzarro L, Asherson PJ, Stolerman IP. Prenatal exposure to nicotine impairs performance on the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology. 2011;36:1114–1125. doi: 10.1038/npp.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davenport HA. Histological and histochemical techniques. WB Saunders. 1960 [Google Scholar]
- 64.Gunderson HJG, Jensen EB. The efficacy of systematic sampling in stereology and its predication. J Microscope. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 65.Silbereis JC, Huang EJ, Back SA, Rowitch DH. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Disease Models & Mechanisms. 2010;31:678–688. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- 67.Alexander M, Garbus H, Smith AL, Fitch RH. Cell size anomalies in the MGN in P3 and P7 HI injured rodents who showed rapid auditory processing deficits. In review [Google Scholar]
- 68.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 69.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 70.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Ann Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 71.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Lindstrom K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics. 2011;127(5):858–865. doi: 10.1542/peds.2010-1279. [DOI] [PubMed] [Google Scholar]
- 73.de Kieviet JF, van Elburg RM, Lafeber HN, Oosterlaan J. Attention problems of very preterm children compared with age-matched term controls at school-age. J Pediatr. 2012;161:824–829. doi: 10.1016/j.jpeds.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Mulder H, Pitchford NJ, Marlow N. Inattentive behaviour is associated with poor working memory and slow processing speed in very pre-term children in middle childhood. B J Educ Psycholo. 2011;81:147–160. doi: 10.1348/000709910X505527. [DOI] [PubMed] [Google Scholar]
- 75.Hall RW, Huitt TW, Thapa R, Williams DK, Anand KJ, Garcia-Rill E. Long-term deficits of preterm birth: Evidence for arousal and attentional disturbances. Clin Neurophysiol. 2008;119:1281–1291. doi: 10.1016/j.clinph.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanches EF, Arteni NS, Nicola F, Boisserand L, Willborn S, Netto CA. Early hypoxia-ischemia causes hemisphere and sex dependant cognitive impairment and histological damage. Neurosci. 2013:208–215. doi: 10.1016/j.neuroscience.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 77.Sanches EF, Arteni NS, Scherer EB, Kolling J, Nicola F, Willborn S, Wyse ATS, Netto CA. Are the consequences of neonatal hypoxia-ischemia dependant on animals’ sex and brain lateralization? Brain Res. 2013;1507:105–114. doi: 10.1016/j.brainres.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 78.Sizonenko SV, Kiss JZ, Inder T, Gluckman PD, Williams CE. Distincitive neuropathologic alterations in the deep layers of the parietal cortex after moderate ischemic-hypoxia injury in the P3 immature rat brain. Ped Res. 2005;57:865–872. doi: 10.1203/01.PDR.0000157673.36848.67. [DOI] [PubMed] [Google Scholar]
- 79.Sizonenko SV, Sirimanne E, Mayall Y, Gluckman PD, Inder T, Williams C. Selective cortical alterations after hypoxic-ischemic injury in the very immature rat brain. Ped Res. 2003;54:263–269. doi: 10.1203/01.PDR.0000072517.01207.87. [DOI] [PubMed] [Google Scholar]
- 80.Eayers JT, Goodhead B. Postnatal development of the cerebral cortex in the rat. J Anat. 1959;93:385–402. [PMC free article] [PubMed] [Google Scholar]
- 81.Maciejewsa B, Lipowska M, Kowianski P, Domaradzka-Pytel B, Morys J. Postnatal development of the rat striatum - a study using in situ DNA end labeling technique. Acta Neurobiol Exp. 1998;58:23–28. doi: 10.55782/ane-1998-1255. [DOI] [PubMed] [Google Scholar]
- 82.Bayer S, Altman J. Hippocampal development in the rat: Cytogenesis and morphogenesis examined with autography and low-level X-irradiation. J Comp Neurol. 1974;158:55–79. doi: 10.1002/cne.901580105. [DOI] [PubMed] [Google Scholar]
- 83.Rice D, Barone S. Critical periods of vulnerability in the developing nervous system: Evidence from human and animal models. Perspect. 2000;31:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Banasiak K, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Progress in Neurobiology. 2000;62:215–249. doi: 10.1016/s0301-0082(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 85.Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Dev Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- 86.Blumenfeld KS, Welsh FA, Harris VA, Pesenson MA. Regional expression of c-fos and heat shock protein-70 mRNA following hypoxia-ischemis in immature rat brian. J Cereb Blood Flow Meab. 1992;12:987–995. doi: 10.1038/jcbfm.1992.136. [DOI] [PubMed] [Google Scholar]
- 87.Pandit AS, Robinson E, Aljabar P, Ball G, Gouias IS, Wang Z, Hajnal JV, Rueckert D, Counsell SJ, Montana G, Edwards AD. Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht086. E pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz K, Maller-Kesselman J, Schneider K, Peterson B, Rajeevan N, Makuch R, Constable RT, Ment L. Alterations in neural connectivity in preterm children at school age. NeuroImage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, Katz KH, Schneider KC, Makuch RW, Constable RT, Ment LR. Functional connectivity to a right hemisphere language center in prematurely born adolescents. NeuroImage. 2010:1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mullen K, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR. Preterm birth results in alterations in neural connectivity at age 16 age. NeuroImage. 2011;54:2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Juul S. Neuroprotective role of erythropoietin in neonates. J Matern Fetal Neonatal Med. 2012;25:105–107. doi: 10.3109/14767058.2012.715025. [DOI] [PubMed] [Google Scholar]
- 92.Shankaran S, Laptook AR. Hypothermia as a treatment for birth asphyxia. Clin Obstet Gynecol. 2007;50:624–635. doi: 10.1097/GRF.0b013e31811eba5e. [DOI] [PubMed] [Google Scholar]