Abstract
Metal cations represent key elements of RNA structure and function. In the Neurospora VS ribozyme, metal cations play diverse roles; they are important for substrate recognition, formation of the active site, and shifting the pKa’s of two key nucleobases that contribute to the general acid–base mechanism. Recently, we determined the NMR structure of the A730 loop of the VS ribozyme active site (SLVI) that contributes the general acid (A756) in the enzymatic mechanism of the cleavage reaction. Our studies showed that magnesium (Mg2+) ions are essential to stabilize the formation of the S-turn motif within the A730 loop that exposes the A756 nucleobase for catalysis. In this article, we extend these NMR investigations by precisely mapping the Mg2+-ion binding sites using manganese-induced paramagnetic relaxation enhancement and cadmium-induced chemical-shift perturbation of phosphorothioate RNAs. These experiments identify five Mg2+-ion binding sites within SLVI. Four Mg2+ ions in SLVI are associated with known RNA structural motifs, including the G–U wobble pair and the GNRA tetraloop, and our studies reveal novel insights about Mg2+ ion binding to these RNA motifs. Interestingly, one Mg2+ ion is specifically associated with the S-turn motif, confirming its structural role in the folding of the A730 loop. This Mg2+ ion is likely important for formation of the active site and may play an indirect role in catalysis.
Metal cations play several essential functions in the biochemistry of RNA. Although Na+, K+, Mg2+, and Ca2+ ions are the predominant cations in cells, Mg2+ is considered to be the most important in RNA folding.1−4 Mg2+ can mediate helical packing,5 promote long-range interactions,2,6−9 organize multiway junction,10 stabilize RNA motifs,11−13 and reduce conformational fluctuations.8,9,14 Mg2+ is particularly adept at stabilizing tightly packed phosphate oxygens found in several RNA conformations because of its small ionic radius, high charge density, and well-defined octahedral geometry that accommodates six inner-sphere ligands. In addition, Mg2+ can either form outer-sphere interactions with ligands through its first-hydration shell or specific inner-sphere interactions by displacing a water molecule and interacting directly with a ligand.
Following the discovery of the catalytic properties of ribozymes, the role of Mg2+ ions in catalysis has been intensively investigated. Mg2+ ions can play either a direct or an indirect role in the enzymatic mechanism.3,15−17 Direct roles have been demonstrated including nucleophile activation,18,19 coordination of nonbridging oxygens of the scissile phosphate,20 promoting the optimal geometry of the reactive species,21 and stabilization of the leaving group.22 Potential indirect roles have been proposed involving long-range electrostatic stabilization16 and/or alteration of pKa’s to facilitate a general acid–base mechanism.16,23,24
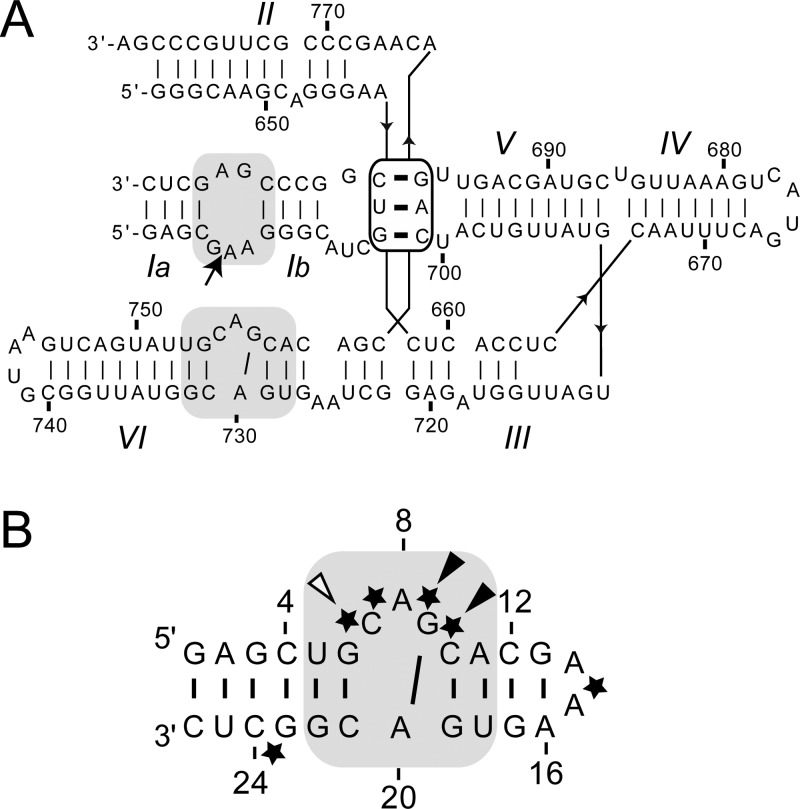
The Neurospora Varkud satellite (VS) ribozyme is a member of the small nucleolytic ribozyme family that also includes the hammerhead, hairpin, hepatitis delta virus (HDV), and glms ribozymes.25−30 The VS ribozyme self-cleaves at the phosphodiester bond between G620 and A621 to generate products with 5′-OH and 2′–3′-cyclic phosphate termini. Its secondary structure contains six helical domains: the stem-loop I (SLI) contains the cleavage site and is considered as the substrate domain, whereas stem-loops II–VI (SLII–SLVI) form the catalytic domain (Figure 1A).31 Substrate recognition is defined by a high-affinity kissing-loop interaction between the SLI and SLV terminal loops (Figure 1A) that induces a conformational change within the SLI substrate that is necessary for catalysis.6,32−34 The proposed general acid–base mechanism involves the close association of the SLI and SLVI internal loops to form the active site,35−39 where two nucleobases, G638 of SLI and A756 of the A730 loop, act as the general base and acid, respectively, in the cleavage reaction.24,40−46 Although the secondary and tertiary structures of the VS ribozyme are unique among the nucleolytic ribozymes, its cleavage mechanism is similar to that of the hairpin ribozyme in which the active site is also formed by the docking of two internal loops, and a nucleobase from each loop participates in the general acid–base mechanism.30
Figure 1.
Primary and secondary structures of the Neurospora VS ribozyme and the SLVI RNA. (A) trans-Cleaving VS ribozyme (containing wild-type residues 648–777) with an SLI substrate. The cleavage site is shown by an arrow. Both the cleavage site internal loop and the active-site internal loop are shaded in gray, and the I/V kissing-loop interaction is boxed. (B) SLVI RNA, which includes the A730 loop of the VS ribozyme (gray shading). Phosphate groups that display inhibitory effects on cleavage when substituted by phosphorothioates are indicated by an arrow, and the arrow is filled for those cases where the activity can be rescued by addition of Mn2+ ions.51 For this study, single phosphorothioate SLVI RNAs were prepared that contain either an Sp or Rp phosphorothioate at one of the phosphates indicated by black stars.
The VS ribozyme is dependent on the presence of divalent metal ions (Mg2+, Mn2+, or Ca2+) or high concentration of monovalent salt for its cleavage activity.47,48 Mg2+ ions have been found to be very important for several aspects of folding and function in the VS ribozyme. They are critical for substrate recognition, both for proper formation of the I/V kissing-loop interaction and for the conformational change in SLI.6,31,35 In addition, they are required for folding of the two three-way junctions49 and for formation of the active site.35,37 Metal cations do not play a direct role in the general acid–base mechanism but appear to play an indirect role by altering the pKa of the two key nucleobases.24,50
Phosphorothioate interference, chemical modifications, nucleotide analogue interference mapping, and NMR studies have been previously conducted in attempts to identify precisely the metal-binding sites in the VS ribozyme.12,40,51−54 In the phosphorothioate interference-rescue experiments, the effect of pro-Rp phosphorothioate substitutions in the VS ribozyme were monitored on the Mg2+-dependent cleavage, and several substitutions were shown to reduce the cleavage activity significantly.51 Interestingly, the phosphorothioate inhibition of cleavage for select residues of the SLI loop (U631), the SLV loop (C699), and the A730 loop (G757 and C758) could be rescued by thiophilic Mn2+ ions, suggesting that these phosphates are involved in inner-sphere metal coordination.51
Recently, we performed NMR studies with a small stem-loop containing the A730 internal loop, termed SLVI (Figure 1B), in order to gain structural insights into the formation of the VS ribozyme active site.50 We established that Mg2+ ions are required to stabilize the structure of the A730 loop, and we determined the NMR structure of SLVI in the presence of Mg2+ ions. The NMR structure reveals a cis-WC/WC G–A base pair and an S-turn motif in the A730 loop that protrude the A756 into an enlarged minor groove such that it becomes available to participate in catalysis. Here, we extend these investigations by defining the precise locations of associated Mg2+ ions by combining Mn2+-induced paramagnetic relaxation enhancement (PRE) with Cd2+-induced chemical-shift perturbation (CSP) of phosphorothioate RNAs. These data are used to refine the NMR structure of SLVI to include five Mg2+-ion binding sites. Four of the binding sites are associated with known RNA structural motifs, including the GNRA tetraloop and the G–U wobble pair. A comparison with similar structures from the PDB reveals new insights into the role of cation-binding sites in RNA structure. In agreement with the prior phosphorothioate interference-rescue experiments with the VS ribozyme,51 one Mg2+ ion is associated with the S-turn motif, revealing its role in the folding of the A730 loop as well as a possible indirect role in catalysis.
Experimental Procedures
Sample Preparation
Unlabeled, 15N-labeled, and 13C/15N-labeled SLVI RNAs (Figure 1B) were synthesized in vitro using T7 RNA polymerase (prepared in-house) and purified as described previously.55 The SLVI RNAs containing phosphorothioate modifications at nonbridging phosphate oxygens (phosphorothioate RNAs; Figure 1B) were purchased as diastereomeric mixtures (Thermo Fisher Scientific, MA). These mixtures were first purified by denaturing polyacrylamide gel electrophoresis (20% acrylamide/bisacrylamide 19:1 and 7 M urea). The Rp and Sp isomers were subsequently separated by reverse-phase HPLC on a μBondapak C-18 column (10 μm and 7.8 × 300 mm; Waters, MA) using a multilinear gradient of HPLC-A (0.1 M ammonium acetate) and HPLC-B (0.1 M ammonium acetate and 50% acetonitrile) buffers at 1 mL/min: (a) 0–10% HPLC-B over 180 mL, (b) 10–13% HPLC-B over 120 mL, (c) 13–100% HPLC-B over 5 mL, and (d) 100–0% over 5 mL. The precise determination of configuration was made on the basis of enzymatic digestions with snake venom phosphodiesterase and Nuclease P1.56
The purified RNA samples were exchanged in NMR buffer A (10 mM sodium cacodylate, pH 6.5, 50 mM KCl, and 0.05 mM NaN3 in 90% H2O/10% D2O) with Amicon Ultra-4 centrifugation filter devices (Millipore, MA). The RNAs were then heated at 95 °C for 2 min and then cooled in ice water for 5 min before changing to the final NMR buffer [NMR buffer A with either 5 mM MgCl2 99.995% (Sigma-Aldrich, MO) or a 5 mM mixture of varying concentrations of MgCl2 99.995% and CdCl2 99.999% (Sigma-Aldrich, MO)]. For NMR studies in D2O, the samples were obtained by multiple cycles of lyophilization and resuspension in 99.996% D2O.
NMR Spectroscopy
NMR experiments were conducted at either 15 or 25 °C on Varian UnityINOVA 500 and 600 MHz spectrometers equipped with a pulse-field gradient unit and an actively shielded z-gradient probe (either a 1H/13C/15N triple resonance probe or a 1H{15N–31P} indirect detection probe). The NMR spectra were processed using the NMRPipe/NMRDraw package57 and analyzed with NMRView.581H, 13C, and 15N chemical shifts were referenced to an external standard of 2,2-dimethyl-2-silapentane-5-sulfonic acid (DSS)59 at 0.00 ppm, and 31P chemical shifts were referenced to an external standard of 85% phosphoric acid at 0.00 ppm.
Metal-Ion-Binding Studies
Manganese (Mn2+) titrations were performed by adding small volumes of a concentrated solution (0.5 M) of 99.99% MnCl2 (Sigma-Aldrich, MO) directly to the RNA sample to achieve final concentrations of 5, 10, 20, 40, and 80 μM MnCl2. The first Mn2+ titration was carried out with a 0.9 mM 13C/15N-labeled SLVI sample in NMR buffer A with 5 mM MgCl2 and 100% D2O. The paramagnetic effect was monitored by collecting 2D 1H–13C CT-HSQC60,61 and 2D 1H–13C HMQC62 spectra at 25 °C. A second Mn2+ titration was carried out with a 1.0 mM 15N-labeled SLVI sample in NMR buffer A with 5 mM MgCl2 and 90%:10% H2O/D2O. The paramagnetic effect was monitored by 1D 1H watergate63 and imino- and amino-optimized 2D 1H–15N HSQC64 spectra at 15 °C. The third Mn2+ titration was carried out with a 1.0 mM 15N-labeled SLVI sample in NMR buffer A with 5 mM MgCl2 and 100% D2O. The paramagnetic effect was monitored by 2D long-range 1H–15N HMQC65 spectra at 25 °C optimized for detection of adenine N1/N3 atoms and purine N7/N9 atoms (JHN = 21 Hz).
Cadmium (Cd2+) titrations were conducted by exchanging the RNA samples with Amicon filters to NMR buffer A containing varying concentrations of CdCl2 and MgCl2. The total divalent-metal-ion concentration was kept constant at 5 mM under all conditions. A first Cd2+ titration was conducted with a 0.2 mM 13C/15N-labeled SLVI RNA sample in 100% D2O and CdCl2 concentrations of 0.0625, 0.125, 0.25, 0.5, 1.0, 1.5, 2.5, and 4.0 mM. Chemical-shift changes (1H and 13C) were monitored for each CdCl2 concentration from 2D 1H–13C CT-HSQC60,61 spectra recorded at 25 °C. Additional Cd2+ titrations were conducted for SLVI RNAs with phosphorothioate modifications (0.15–0.4 mM) in 90%:10% H2O/D2O and CdCl2 concentrations of 0.0625, 0.125, and 0.25 mM. Chemical-shift changes (1H and 31P) were monitored for each CdCl2 concentration from 1D 1H watergate63 and 1D 31P spectra recorded at 25 °C.
UV Spectroscopy and Determination of Tm Values
Thermal-stability studies of SLVI RNAs were conducted with a Cary 300 UV–vis spectrophotometer equipped with a Peltier temperature-control accessory. All samples contained 5 μM SLVI RNA in NMR buffer A supplemented with divalent metal ions (either 5 mM MgCl2 or 4.75 mM MgCl2/0.25 mM CdCl2). Control samples were also prepared that contained only the selected buffers. Samples were heated from 25 to 98 °C at a rate of 1 °C/min, and absorbance data were collected at 260 nm after each 1 °C temperature increment. Subtraction of the control samples from the RNA samples was processed automatically using a dual-beam mode. Melting temperatures (Tm) were determined from the second derivative of the absorbance versus temperature curve, as previously described.12 Reported Tm are averaged values derived from two or more individual UV-denaturation profiles.
Native Gel Electrophoresis
Nondenaturing polyacrylamide gel electrophoresis was carried out on 7.5% polyacrylamide gels, acrylamide/bisacrylamide (37.5:1) in Tris-borate buffer (50 mM Tris-borate, pH 8.0) supplemented with 20 mM magnesium acetate. The gels were prerun for 30 min at 200 V, loaded with RNA samples (2 μg of RNA at a concentration of 20 μM), and then run for 2 h at 250 V and 4 °C. The gels were stained with Stains All (Sigma-Aldrich, MO).
Structural Modeling of Mg(H2O)n2+-Binding Sites
Three-dimensional structures of SLVI bound to Mg(H2O)n2+ complexes were determined by complementing previously defined restraints50 with distance restraints to position the five Mg(H2O)n2+ complexes. These new restraints were derived from Mn2+-induced paramagnetic relaxation enhancement (PRE) using the ratio of signal intensity (I0/IMn) determined from 2D spectra collected at 0 μM MnCl2 (I0) and 10 μM MnCl2 (IMn). For each signal in these spectra with I0/IMn ≥ 8-fold and 4 ≤ I0/IMn < 8-fold, distance restraints were set to 1.8–7.0 and 1.8–8.0 Å, respectively, between a defined Mg2+ center and the nuclei giving rise to the observed signal (Table S1 of Supporting Information). When the identity of the Mg2+ center could not be defined, ambiguous metal restraints were defined with bounds of 1.8–10.0 Å (Table S1 of Supporting Information). Restraints were also derived from Cd2+-induced 31P CSP of the sulfur-bound phosphorus from phosphorothioate SLVI RNAs [ΔP = (δMg – δCd)] calculated from 1D 31P spectra collected in 5 mM MgCl2 (δMg) and 0.25 mM CdCl2/4.75 mM MgCl2 (δCd). Phosphorothioates with ΔP ≥ 1.0 ppm were considered as inner-sphere ligands, and a distance constraint of 1.8–2.2 Å was defined between a Mg2+ center and the modified nonbridging phosphate oxygen. Each phosphorothioate with 0.2 < ΔP < 1.0 ppm was considered as an outer-sphere ligand, and a distance restraint of 1.8–7.0 Å was defined between a Mg2+ center and the modified nonbridging phosphate oxygen. On the basis of a clustering analysis of the nuclei that were most affected by Mn2+-induced PRE (I0/IMn ≥ 8-fold) and Cd2+-induced CSP of phosphorothioates (ΔP > 0.2 ppm), we inferred five distinct Mg2+-binding sites for SLVI, termed sites 1–5, including two pentahydrated Mg2+-ion complexes [Mg(H2O)52+; sites 1 and 3] and three fully hydrated Mg2+-ion complexes [Mg(H2O)62+; sites 2, 4, and 5].
Three-dimensional structures of SLVI with bound Mg(H2O)n2+ complexes were calculated by restraining molecular dynamics and simulated annealing with X-PLOR-NIH version 2.1.966 by adapting the two-stage protocol previously used for structure determination of free SLVI.50 At stage one, an initial set of structures was calculated from RNA structures with randomized backbone angles to which Mg(H2O)n2+ coordinates and parameters derived from HIC-Up were added.67 Several rounds of calculations were performed at this stage to allow stepwise incorporation of restraints to metal-ion complexes. At the end of stage one, 50 structures were obtained that satisfy all distance and dihedral experimental restraints (no distance violation of >0.2 Å and no torsion angle violation of >5°), including all of those involving the five metal complexes. At stage two, these structures were refined with the same set of restraints but were supplemented with RDC restraints.50 A final set of 500 structures was calculated, from which the 20 lowest-energy structures that satisfied the experimental restraints (no distance violation >0.2 Å, no torsion angle violation >5°, and no RDC violation >5 Hz) were selected for analysis. These 20 lowest-energy structures were used to calculate an average structure that was minimized against NOE and dihedral restraints. All structures were visualized and analyzed with PyMOL Molecular Graphics System, Version 1.3 Schrödinger, LLC.
Pattern Search of RNA Metal-Binding Motifs in the Protein Data Bank (PDB)
Metal-binding motifs similar to those found in SLVI were searched in all available X-ray, cryo-EM, and NMR structures of the PDB using WebFR3D.68 The search patterns were defined according to their sequential and structural context in SLVI using the FR3D symbolic search algorithm. For each search, the results were extracted from the FR3D html file (raw hits) and filtered to remove the PDB files that did not contain metal ions. For each resulting hit (hit with a cation), a heavy-atom superposition of the nucleotides defining the search pattern was obtained between the PDB coordinates of the hit and the SLVI average structure, and this superposition was used to extract a pairwise heavy-atom rmsd. In cases where residues used for the superposition differ, the base of these residues was omitted for the superposition and rmsd calculation. The superposition was also used to extract the distance between the divalent metal ions of the two structures. For each FR3D search, those structures with metal distances smaller than 4.5 Å were termed positive hits and kept for further analysis.
Results
Probing Divalent Metal-Ion-Binding Sites in SLVI through Mn2+-Induced Paramagnetic Relaxation Enhancement (PRE)
To identify metal-ion-binding sites in the SLVI RNA (Figure 1B), we performed several Mn2+ titrations and monitored them by NMR spectroscopy. Mn2+ is a paramagnetic metal ion that specifically enhances the relaxation of nuclei located within a short distance range (∼10 Å).69 The PRE is proportional to r–6 and can be used to derive distance (r) restraints between the Mn2+ and the observed nuclei.69 Thus, Mn2+-induced PRE represents a common approach for identifying divalent metal-ion-binding sites in RNA.12,70−76
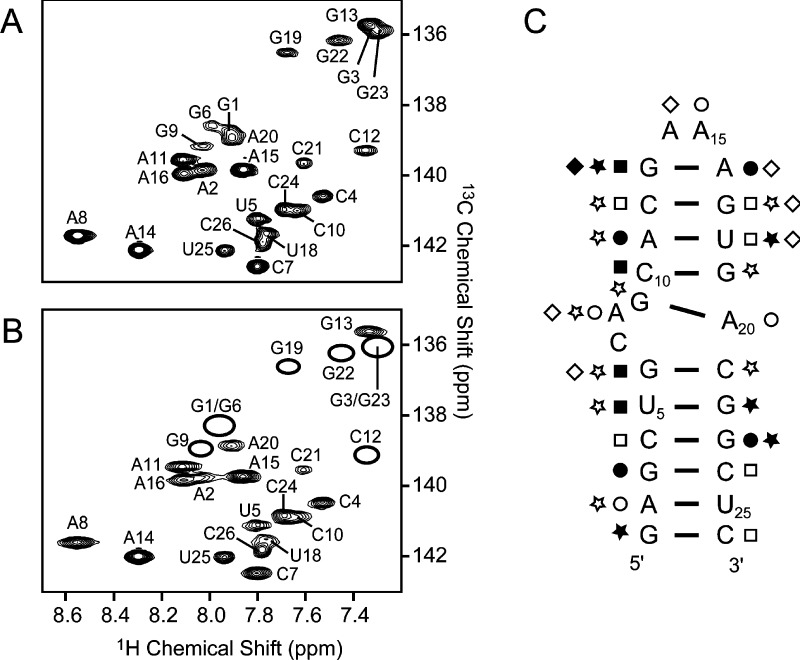
Most of the previously assigned 1H, 13C, and 15N signals in SLVI were probed using 2D 1H–13C and 1H–15N correlation experiments recorded during the titration of 0–80 μM MnCl2 to an SLVI RNA folded in 5 mM MgCl2. The high concentration of Mg2+ is necessary for proper RNA folding, whereas a ∼1000-fold lower concentration of Mn2+ is sufficient for specific binding of the paramagnetic ion in the fast-exchange regime.70,71,77 At 10 μM MnCl2, the relaxation of several nuclei in SLVI is specifically affected, as shown for aromatic signals of the 2D 1H–13C CT-HSQC spectrum (Figure 2A,B) that either become significantly less intense (G13) or completely disappear (G1, G3, G6, G9, C12, G19, G22, and G23). For each well-resolved signal in this and other 2D spectra, the signal intensity was measured in the absence (I0) and presence of 10 μM Mn2+ (IMn) to calculate a disappearance ratio (I0/IMn). Interestingly, the signals that undergo the strongest decrease in intensity (I0/IMn ≥ 8) correspond to 1H, 13C, and 15N nuclei that are dispersed throughout the RNA (Figure 2C and Table S1 of the Supporting Information). Thus, the results from Mn2+-induced PRE are compatible with several divalent-metal-binding sites in SLVI. The precise localization of these binding sites is, however, not possible at this stage because specific phosphates involved in metal binding could not be identified from Mn2+-induced PRE as a result of the severe 31P spectral overlap and the lack of specific 31P assignment.
Figure 2.
Probing divalent-metal-ion-binding sites in SLVI through Mn2+-induced PRE. The effect of Mn2+ ions on the relaxation of SLVI nuclei is shown for aromatic (C6–H6 and C8–H8) signals by comparing the 2D 1H–13C HSQC spectra of SLVI RNA collected in (A) the absence and (B) the presence of 10 μM MnCl2. Peaks that disappeared below the noise level are denoted with empty ovals. (C) Summary of the effect of Mn2+ ions on the relaxation of SLVI nuclei mapped on the secondary structure of SLVI. Filled and open symbols represent large (I0/IMn ≥ 8-fold) and medium (4 ≤ I0/IMn < 8-fold) PRE. Different symbols are used to distinguish PRE of imino and amino N–H signals (squares), N7, N9, and N3 signals (circles), aromatic C–H signals (stars), and ribose C–H signals (diamonds).
Identification of Phosphate–Metal Interactions by Cd2+-Induced 31P Chemical-Shift Perturbation (CSP) of Phosphorothioate RNAs
A phosphorothioate RNA contains a modification that substitutes either the pro-Rp or the pro-Sp nonbonded phosphate oxygen (pro-Rp/Sp OP) by a sulfur atom and significantly alters the chemical shift of the associated 31P signal such that it can be easily identified in a 1D 31P NMR spectrum.78−80 Because phosphorothioates prefer thiophilic metal ions, like Cd2+, over Mg2+ ions, formation of phosphorothioate–metal complexes can be monitored by recording 1D 31P NMR spectra during Cd2+ titration.79,80 Chemical-shift perturbations (CSP) of the sulfur-bound phosphorus have been associated with coordination of Cd2+ ions, with large upfield shifts being characteristic of inner-sphere coordination.79,80
To investigate the contribution of phosphates to metal coordination, several SLVI RNAs containing single phosphorothioate modifications were prepared (Figure 1B). Phosphorothioate modifications of C7, A8, G9, and C10 are of particular interest to examine metal binding in the S-turn of SLVI. In addition, modification of A15 provides a positive control because an inner-sphere metal contact has been previously reported at this position of a GAAA tetraloop,79 and modification of C24 provides a negative control because this residue is not predicted to interact with a metal ion. The phosphorothioate RNAs were prepared by chemical synthesis as mixtures of pro-Rp and pro-Sp isomers, and reverse-phase HPLC was used to separate the individual isomers. SLVI RNAs with a 5′-phosphorothioate at a specific residue N are denoted as either N-Rp or N-Sp. With the exception of the C24-Rp/Sp mixture, all of the individual isomers were separated from their mixtures in high purity.
The Cd2+-titration conditions were carefully defined to prevent Cd2+-induced structural changes in SLVI and/or RNA aggregation that may result from an excess of Cd2+ ions.81,82 We first collected 2D 1H–13C CT-HSQC spectra of a 0.2 mM 13C/15N-labeled SLVI RNA sample in the presence of increasing Cd2+ concentrations. Only small changes in chemical shifts were observed between 0 and 0.25 mM of Cd2+, suggesting that the SLVI structure remains essentially intact. However, significant CSP are observed at Cd2+ concentrations ≥1.0 mM (Figure S1 of the Supporting Information), which indicates that the SLVI structure is significantly modified by high Cd2+/RNA ratios. In addition, high Cd2+ concentrations cause smearing of SLVI on native gels (at 4 mM Cd2+; not shown) and a change in the shape of the UV melting curves (at 1.5 mM Cd2+; not shown), in agreement with aggregation of SLVI under these conditions.
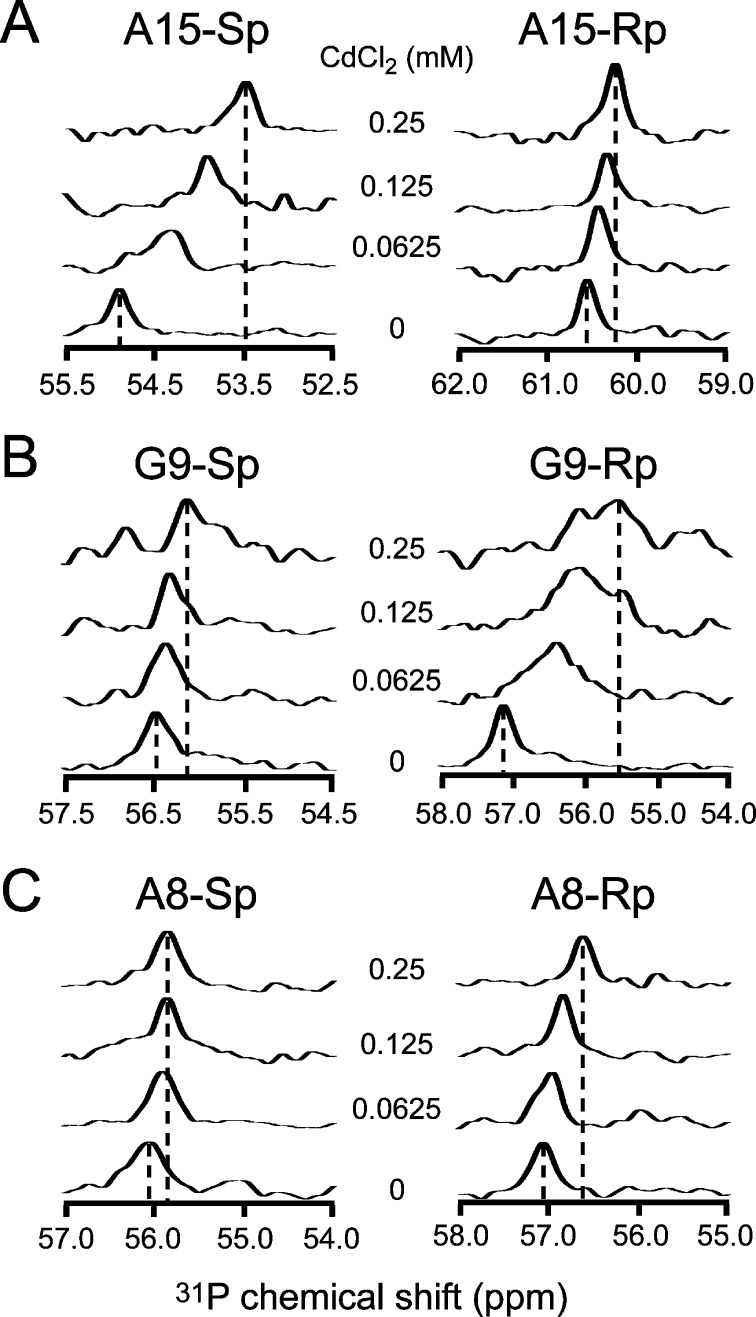
To preserve the structural integrity of SLVI, Cd2+ titrations of phosphorothioate RNAs were thus performed at low Cd2+/RNA ratios. These titrations were monitored by 1D 31P spectra (Figure 3), and CSP of the sulfur-bound phosphorus was determined for each phosphorothioate RNA using ΔP = (δMg – δCd), where δMg = chemical shift (δ) at 5 mM MgCl2 and δCd = δ at 4.75 mM MgCl2/0.25 mM CdCl2 (Table 1). A large CSP (ΔP ≥ 1.0 ppm) was detected for the A15-Sp phosphorothioate (ΔP = 1.43 ppm; Figure 3A and Table 1), as was previously observed for an equivalent phosphorothioate of a GAAA tetraloop involved in an inner-sphere metal coordination.79 Similarly, the large CSP associated with the G9-Rp phosphorothioate (ΔP = 1.62 ppm; Figure 3B and Table 1) provides evidence for inner-sphere metal coordination at the nonbridging G9pro-Rp phosphate oxygen. Smaller but still significant CSP (0.2 < ΔP < 1.0 ppm) for seven other phosphorothioates (C7-Rp/Sp, A8-Rp/Sp, G9-Sp, C10-Rp, and A15-Rp; Figure 3, Figure S2 of the Supporting Information, and Table 1) are compatible with outer-sphere metal coordination. Finally, the C10-Sp and C24-Rp/Sp phosphorothioates display small CSP (ΔP < 0.2 ppm; Figure S2 of the Supporting Information and Table 1), as is expected for those not involved in divalent metal coordination.
Figure 3.
Effect of Cd2+ ions on the 1D 31P NMR spectrum of selected SLVI RNAs containing single phosphorothioate modifications. The 1D 31P spectra of (A) A15-Sp and A15-Rp, (B) G9-Sp and G9-Rp, and (C) A8-Sp and A8-Rp were collected in buffer containing CdCl2/MgCl2 concentrations of 0.0:5.0, 0.0625:4.9375, 0.125:4.875, and 0.25:4.75 mM. Only the most downfield region of the spectra containing the signal from the phosphorothioate is shown.
Table 1. 31P NMR CSP (ΔP) Following Cd2+ Titrations of Phosphorothioate SLVI RNAs.
| phosphorothioate RNA | ΔP (ppm)a |
|---|---|
| C7-Rp | 0.31 |
| C7–Sp | 0.35 |
| A8-Rp | 0.41 |
| A8-Sp | 0.23 |
| G9-Rp | 1.62 |
| G9-Sp | 0.39 |
| C10-Rp | 0.32 |
| C10–Sp | 0.09 |
| A15-Rp | 0.35 |
| A15-Sp | 1.43 |
| C24-Rp/Spb | 0.05/0.01 |
ΔP = (δMg – δCd), where δMg = δ at 5 mM MgCl2 and δCd = δ at 4.75 mM MgCl2/0.25 mM CdCl2.
This sample is a racemic mixture of Rp and Sp phosphorothioates.
Previous reports have indicated that phosphorothioate modifications may affect RNA structure by inducing distortions in the sugar–phosphate backbone;83,84 thus, control experiments were performed to verify that they have a minimal effect on the structure of SLVI. First, 1D 1H imino NMR spectra were collected as part of the Cd2+ titration of phosphorothioate SLVI RNAs, and these spectra are all very similar to that of the unmodified SLVI (Figure S3 of the Supporting Information), suggesting that the phosphorothioate modifications do not significantly affect the global structure of SLVI. In addition, melting temperatures (Tm) were determined for each phosphorothioate RNA by UV–vis spectroscopy (Table 2). Generally, the observed Tm values of the phosphorothioate SLVI RNAs (Table 2) are very similar to that of the unmodified SLVI (74.6 ± 0.1 °C), further supporting that these modifications do not alter the structural integrity of the RNA. However, significant differences in Tm values are noted for two phosphorothioate RNAs. The Tm value for the A15-Rp phosphorothioate (82.5 ± 0.1 °C) is 8 °C higher than that of the unmodified SLVI RNA, similar to what was previously observed for an equivalent residue in a GAAA hairpin loop.83 In contrast, the Tm value for the C7-Rp phosphorothioate RNA (64.6 ± 1.1 °C) is 10 °C lower than that of the unmodified SLVI RNA, indicating that this particular modification destabilizes SLVI. Given the position of the C7 5′-PO4– in the S-turn,50 the destabilizing effect of the C7-Rp phosphorothioate modification could be due to a perturbed divalent metal coordination involving the modified atom. To investigate this possibility, Tm values were obtained in the presence of 0.25 mM Cd2+/4.75 mM Mg2+ (Table 2). Interestingly, although this Cd2+-containing buffer does not affect the stability of the unmodified SLVI RNA (Tm = 74.6 ± 0.2), it restores the stability of the C7-Rp phosphorothioate RNA (Tm = 73.5 ± 0.2), implying that the modified position is involved in metal coordination. Consequently, these controls support the validity of the Cd2+-titration results, at least in terms of inferring specific phosphate–metal interactions in SLVI.
Table 2. Tm Values for the Unmodified and Phosphorothioate SLVI RNAs.
| RNA | Tm (°C) |
|---|---|
| 5 mM MgCl2 | |
| SLVI | 74.6 ± 0.1 |
| C7-Rp | 64.6 ± 1.1 |
| C7–Sp | 74.4 ± 0.1 |
| A8-Rp | 74.3 ± 0.2 |
| A8-Sp | 74.5 ± 0.1 |
| G9-Rp | 74.2 ± 0.1 |
| G9-Sp | 73.8 ± 0.1 |
| C10-Rp | 73.7 ± 0.3 |
| C10–Sp | 73.4 ± 0.1 |
| A15-Rp | 82.5 ± 0.1 |
| A15-Sp | 75.0 ± 0.1 |
| C24-Rp/Spa | 74.0 ± 0.2 |
| 4.75 mM MgCl2 + 0.25 mM CdCl2 | |
| SLVI | 74.6 ± 0.2 |
| C7-Rp | 73.5 ± 0.2 |
This SLVI RNA is a racemic mixture of Rp and Sp phosphorothioates.
Structural Modeling of SLVI with Mg(H2O)n2+ Complexes
Three-dimensional NMR structures of SLVI bound to Mg(H2O)n2+ complexes (SLVIMg) were determined as previously reported for free SLVI (SLVIfree),50 with the addition of Mg2+-RNA distance restraints derived from the Mn2+-induced PRE (Table S1 of the Supporting Information) and the Cd2+-induced CSP of phosphorothioate RNAs (Table 1). Five Mg(H2O)n2+ binding sites (sites 1–5) were defined by circumscribing into separate clusters atoms that were most affected by the Mn2+ and Cd2+ titrations. Metal complexes at sites 2, 4, and 5 were modeled as hexahydrated Mg2+ complexes [Mg(H2O)62+], whereas those at sites 1 and 3 were modeled as pentahydrated Mg2+ complexes [Mg(H2O)52+], given the evidence for inner-sphere metal coordination of the 5′-PO4– of A15 and G9 (Table 1). The resulting 20 lowest-energy structures of SLVIMg (Table 3) are compatible with all of the experimental restraints, indicating that the new Mg2+–RNA restraints are fully consistent with distance, dihedral, and RDC restraints defined for structure determination of the SLVIfree RNA.50 The NMR structure of SLVIMg is very well-defined with an overall heavy-atom rmsd of 0.33 ± 0.14 Å (Table 3). Thus, the ensemble of SLVIMg structures is well-represented by the minimized average structure (Figure 4). By comparison, the NMR structure of SLVIfree was previously determined with an overall heavy-atom rmsd of 0.67 ± 0.17 Å,50 indicating that the new Mg2+–RNA restraints help to define better the SLVI structure.
Table 3. Structural Statistics of the SLVI RNA with Mg(H2O)n2+ Complexes.
| distance restraints | 1086 | |
| number of NOE-derived distance restraints | 965 | |
| internucleotide | 591 | |
| intranucleotide | 355 | |
| ambiguous | 19 | |
| hydrogen-bond restraints | 52 | |
| number of Mg2+–RNA distance restraints | ||
| from Mn2+-induced PRE | 60 | |
| from Cd2+-induced CSP (ΔP) | 9 | |
| dihedral angle restraints | 88 | |
| residual dipolar coupling restraints | 30 | |
| total number of restraints | 1204 | |
| rmsd from experimental restraints | ||
| NOE (Å) (none >0.2) | 0.0123 ± 0.004 | |
| dihedral (deg) (none >5°) | 0.11 ± 0.01 | |
| residual dipolar couplings (none >5 Hz) | 0.23 ± 0.01 | |
| rmsd from idealized geometry | ||
| bonds (Å) | 0.00547 ± 0.00002 | |
| angles (deg) | 1.1741 ± 0.0007 | |
| impropers (deg) | 0.441 ± 0.004 | |
| heavy-atom rmsd (Å)a | ||
| overall (residues 2–25) | 0.33 ± 0.14 | |
| 5′–3′ stem (residues 2–5 and 22–25) | 0.18 ± 0.08 | |
| hairpin stem (residues 11–18) | 0.15 ± 0.07 | |
| A730 loop (residues 6–10 and 19–21) | 0.23 ± 0.08 | |
| metal site 1 (residues 13–16) | 0.087 ± 0.025 | |
| metal site 2 (residues 10–12 and 17–19) | 0.14 ± 0.06 | |
| metal site 3 (residues 7–9) | 0.23 ± 0.06 | |
| metal site 4 (residues 3–6 and 21–24) | 0.20 ± 0.11 | |
| metal site 5 (residues 1–4 and 23–26) | 0.17 ± 0.06 | |
Heavy-atom rmsd to the minimized averaged structure.
Figure 4.
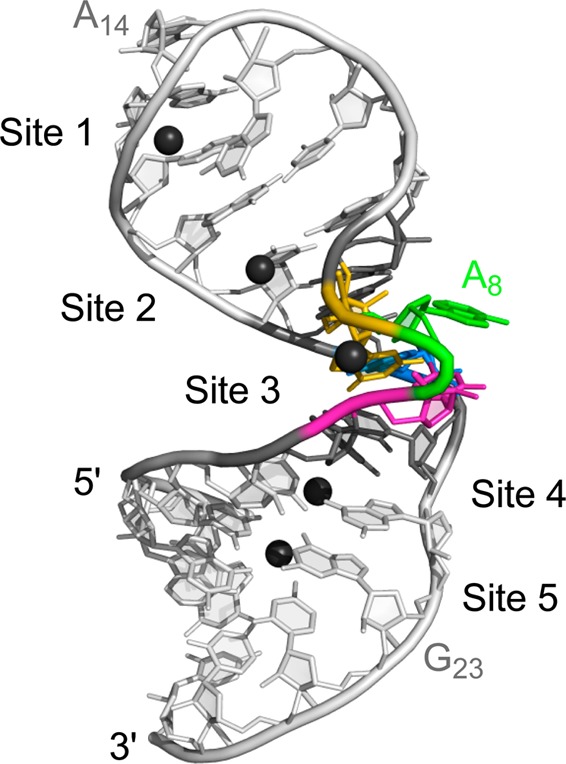
Lowest-energy structure of the SLVI RNA determined in the presence of Mg(H2O)n2+ complexes. The five Mg2+ ions associated with SLVI are shown (black), but their bound water molecules were omitted for clarity. For SLVI, only the heavy atoms are shown, and the ribbon replacing the phosphorus and the nonbonded oxygen atoms is used to show the backbone. SLVI nucleotides are color-coded: the loop-closing base pairs (G6-C21 and C10-G19) are dark gray, C7 (C755) is magenta, A8 (A756) is green, G9 (G757) is gold, and A20 (A730) is blue.
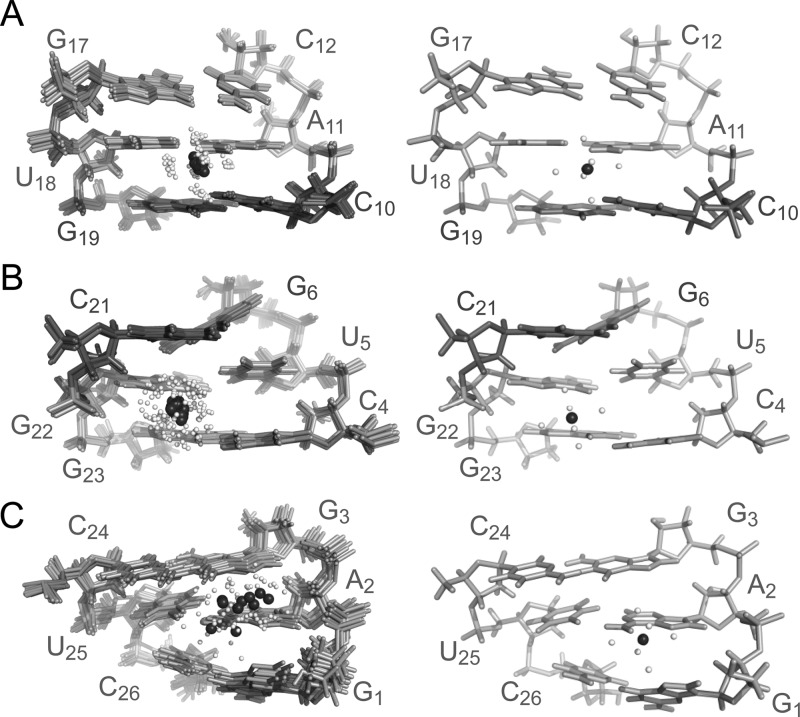
The structure of SLVIMg reveals five well-defined Mg2+-binding sites. The three outer-sphere metal-binding sites, sites 2, 4, and 5, are all located in the major groove of SLVI (Figure 5 and Table 4). Site 2 is located in the hairpin stem near the C10 5′-PO4– of the S-turn (Figure 5A), where G17 O6, U18 O4, and G19 O6 act as potential outer-sphere ligands and C10 5′-PO4– likely contributes to electrostatic stabilization. Site 4 is associated with the U5–G22 wobble base within the 5′–3′ stem (Figure 5B), with U5 O4, G22 O6/N7/5′-pro-Rp OP, and G23 O6/N7 acting as potential outer-sphere ligands and G22 5′-PO4– ensuring electrostatic stabilization. Interestingly, the Mg(H2O)62+ complex at site 4 also interacts with the base of C21, forming a cation−π interaction. The Mg(H2O)62+ complex at site 5 is found at the extremity of the 5′–3′ stem (Figure 5C), close enough to G1 O5′/N7/O6, A2 N7/5′-pro-Rp OP, and G3 O6/N7 for outer-sphere coordination and to the 5′-PO4– of A2 and G3 for electrostatic stabilization. For site 5, the position of the Mg(H2O)62+ complex is not as well-defined as for sites 2 and 4 by the NMR data (Figure 5), indicating that metal coordination may be undergoing dynamic exchange of ligands.
Figure 5.
Mg(H2O)62+-binding sites associated with the (A) C10 5′-PO4– (site 2), (B) G22–U5 wobble (site 4), and (C) 5′ end (site 5) of SLVI RNA. In panels A–C, a superposition of the 10 lowest-energy structures (left panel) and the lowest-energy structure (right panel) are shown. The Mg2+ ions are shown in black, with their bound water molecules in white.
Table 4. Description of Mg(H2O)n2+-Binding Sites in SLVI.
| site | inner-sphere ligandsa | outer-sphere ligandsb | electrostatic interactionsc | cation−π interactionsd |
|---|---|---|---|---|
| site 1 (n = 5) | A15 5′-pro-Sp OP | A15 O5′ | A15 5′-PO4– | G13 |
| A15 5′-pro-Rp OP | A16 5′-PO4– | |||
| A16 5′-pro-Rp OP | ||||
| site 2 (n = 6) | G17 O6 | C10 5′-PO4– | ||
| U18 O4 | ||||
| G19 O6 | ||||
| site 3 (n = 5) | G9 5′- pro-Rp OP | C7 O3′ | ||
| C7 O5′ | ||||
| C7 5′-pro-Rp OP | C7 5′-PO4– | |||
| A8 O5′ | A8 5′-PO4– | |||
| A8 5′-pro-Rp OP | G9 5′-PO4– | |||
| G9 O5′ | ||||
| site 4 (n = 6) | U5 O4 | G22 5′-PO4– | C21 | |
| G22 O6 | ||||
| G22 N7 | ||||
| G22 5′-pro-Rp OP | ||||
| G23 O6 | ||||
| G23 N7 | ||||
| site 5 (n = 6) | G1 O5′ | |||
| G1 N7 | ||||
| G1 O6 | ||||
| A2 N7 | A2 5′-PO4– | |||
| A2 5′-pro-Rp OP | G3 5′-PO4– | |||
| G3 O6 | ||||
| G3 N7 |
Inner-sphere ligands were determined from Cd2+-induced CSP (ΔP > 1.0 ppm).
Outer-sphere ligands were determined by selecting all hydrogen-bond acceptors within 4.0 Å of a Mg(H2O)n2+ oxygen that were in a favorable orientation for outer-sphere interaction in at least 5 of the 20 lowest-energy structures.
Electrostatic interactions were determined by selecting 5′-PO4– within 8.0 Å of a Mg2+ ion.
Cation−π interactions were determined for Mg2+ ions within 5.2 Å and at an angle smaller than 52° from the ring centroid.85
The two inner-sphere metal-binding sites, sites 1 and 3 (Figure 6), are formed by unique structural elements within SLVI that contain electronegative pockets rich in phosphate oxygens, with one of the phosphates involved in inner-sphere metal coordination (A15 5′-pro-Sp OP at site 1 and G9 5′- pro-Rp OP at site 3; Table 4). Site 1 within the G13A14A15A16 tetraloop also utilizes three potential outer-sphere ligands identified as A15 O5′/5′-pro-Rp OP and A16 5′-pro-Rp OP (Table 4). Remarkably, the Mg(H2O)52+ at site 1 is positioned on one face of the G13 base, forming a cation−π interaction (Figure 6A).85 Site 3 also involves several potential outer-sphere ligands identified as C7 O3′/O5′/5′-pro-Rp OP, A8 O5′/5′-pro-Rp OP, and G9 O5′ (Table 4). Moreover, site 3 is unique in that the 5′-PO4– of C7, A8, and G9 within the S-turn form an electronegative cluster resembling a three-prong mount that holds the Mg(H2O)52+ complex (Figure 6B).
Figure 6.
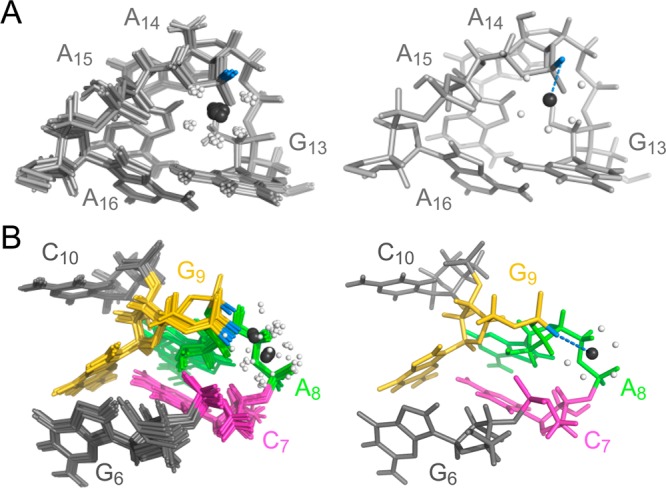
Mg(H2O)52+-binding sites associated with the (A) GAAA hairpin loop (site 1) and (B) S-turn (site 3) of SLVI RNA. In both panels A and B, a superposition of the 10 lowest-energy structures (left panel) and the lowest-energy structure (right panel) are shown. Blue dotted lines represent inner-sphere metal binding involving the A15pro-Sp OP in panel A and the G9pro-Rp OP in panel B. The Mg2+ ions are shown in black, with their bound water molecules in white.
Search for Metal-Binding Motifs in the PDB
To complement our NMR study of metal-binding sites in SLVI, we used the WebFR3D symbolic search algorithm68 to find similar metal-binding sites in the PDB. The search patterns correspond to sites 1–5 (Figure 4), as summarized in Table S2 of the Supporting Information. Positive hits from this search fit the search pattern and also contain a metal ion that is within 4.5 Å of that found for the same motif in SLVI.
For the GNRA motif in SLVI (site 1), the search pattern was defined by the consecutive CGNRAG sequence (N is an undefined nucleotide and R is a purine), with the first and last nucleotides forming a Watson–Crick base pair. The search resulted in three positive hits with pairwise heavy-atom rmsd values and metal distances to the SLVI average structure of 1.0–1.3 and 0.7–4.1 Å, respectively (Figure 7A). All of these motifs adopt a GNRA tetraloop fold with a metal ion in the minor groove interacting with the 5′-PO4– corresponding to that of A15 in SLVI, although this interaction does not involve inner-sphere coordination as observed for SLVI. Remarkably, in all cases, the metal ion forms a cation−π interaction with the conserved G nucleobase of the motif.
Figure 7.
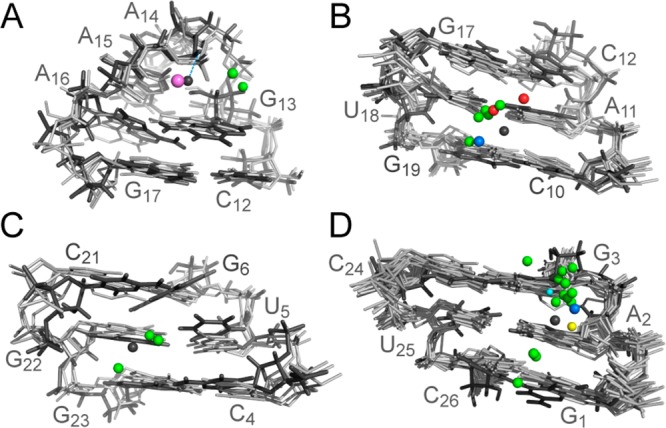
Superposition of metal-binding sites in SLVI with PDB structures containing similar metal-binding sites identified using WebFR3D. The metal-binding sites in SLVI are associated with the (A) GNRA hairpin loop (site 1), (B) C10 5′-PO4– (site 2), (C) G22–U5 wobble (site 4), and (D) 5′ end (site 5). The structure and Mg2+ ions of SLVI are shown in black, whereas the other structures (PDB entries in Table S2 of Supporting Information) are shown in gray, with their associated metal ion color coded according to the type of metal (Mg2+, green; Na+, purple; Co3+, red; Ca2+, blue; Ni2+, yellow; and Ir3+; light blue).
For site 2, the search pattern corresponds to a 3 bp helical domain that matches the exact sequence of the first three base pairs of the hairpin stem (C10-C12/G17-G19). The search resulted in eight positive hits, with pairwise heavy-atom rmsd values and metal distances to the SLVI average structure of 0.6–1.1 and 1.6–3.4 Å, respectively (Figure 7B). From the superposition of the eight positive hits to the average SLVI structure, it is clear that the associated metal ions always lie in the major groove, although they occupy several different locations within this ensemble of structures.
For the S-turn motif (site 3), two search patterns were defined from the A730 loop sequence (Table S2 of the Supporting Information); however, only one raw hit (i.e., without metal) was obtained corresponding to the previously determined SLVI structure.50
For the G–U base pair in SLVI (site 4), the search pattern was defined as a helical domain with three Watson–Crick base pairs corresponding to nucleotides C4-G6/C21-G23 in SLVI. This search resulted in three positive hits with pairwise heavy-atom rmsd values and metal distances to the SLVI average structure of 1.0–1.9 and 1.5–2.0 Å, respectively (Figure 7C). In one case (PDB code 1FJG), the divalent metal ion forms a cation−π interaction with a cytidine.85
For the purine stretch at the 5′ end of the helix (site 5), the search pattern corresponds to a 3 bp helical domain that matches the exact sequence of the three first base pairs in SLVI (G1-G3/C24-C26). The search resulted in 16 positive hits with pairwise heavy-atom rmsd values and metal distances to the SLVI average structure of 0.4–1.1 and 1.7–4.0 Å, respectively (Figure 7D). From the superposition of the 16 positive hits to the average SLVI structure, it is clear that the associated metal ions lie in the major groove, although they occupy a variety of different locations in these structures.
Discussion
Powerful NMR Approach for Localization of Divalent Metal-Binding Sites in RNA
The present work highlights the strength of combining Mn2+-induced PRE experiments and Cd2+-induced CSP of phosphorothioate RNAs to localize divalent-cation-binding sites in RNA. Mn2+-induced PRE experiments have been widely used to probe RNA structures and to define metal-binding sites9,12,71,74,76 and can provide a large number of RNA–metal restraints, especially for 1H, 13C, and 15N atoms of 13C/15N-labeled RNAs. However, Cd2+-induced CSP of phosphorothioate RNAs identifies specific metal–phosphate interactions and provides information on the coordination state of the associated metal ions.79,80,86−88 Thus, these two approaches are complementary, and together they provide a large number of nonredundant experimental restraints to define precisely metal-ion binding in the SLVI RNA. An important consideration in using this dual approach is ensuring that the different metal ions and chemically modified RNAs employed do not affect the structural integrity of the RNA. Importantly, the two metal ions used, Mn2+ and Cd2+, both support the catalytic activity of the VS ribozyme and thus likely the correct folding at the active site.47,48,51 In addition, experimental controls were performed to ensure reliable measurement of distance restraints between the RNA and the associated divalent metals. Overall, the distance restraints derived from these data help to define better the structure of SLVI and to allow the localization of five well-defined divalent metal-binding sites in the major groove of the RNA. One of the metal-binding sites is specific to the S-turn of the A730 loop of SLVI, whereas the other four are commonly found in other RNAs. Thus, the NMR structure of SLVI with bound divalent cations provides valuable insights into both our general understanding of RNA–metal interactions and the cation requirement for formation of the VS ribozyme active site.
New Insights about Known Cation-Binding Sites in RNA
According to our FR3D search, four of the five metal-binding sites in SLVI have been previously observed in similar RNA structures. Two of these sites (sites 2 and 5) bind a hexahydrated Mg2+ ion within the major groove of A-form helical stems formed by standard Watson–Crick base pairs. The site located at the 5′ end of SLVI (site 5) is formed by three consecutives purines. This site offers a favorable environment for cations because of the presence of several electronegative groups associated with the 5′-GAG-3′ sequence. This metal-binding site is not as well-defined by the NMR data as other sites in SLVI, suggesting that more than one coordination mode are possible and that diffuse binding may occur along the purine stretch. Using FR3D, we identified 16 similar metal-binding sites in purine patches with the same sequence and structure context. The location of the metal is also not well-defined within these metal-binding sites, further supporting that the major groove of consecutive purines represents a common metal-binding site in double-stranded RNA that allows diffuse binding over the Hoogsteen edge of these purines.71,74,89 In contrast, the binding of a Mg2+ ion within the hairpin stem at site 2 is better defined, with the base keto-oxygens of the 5′-GUG-3′ sequence and the pro-Rp OP of the C10 5′-PO4– as potential outer-sphere ligands. Using FR3D, we identified eight similar sites in the PDB, and the location of the metal ion is more clearly defined within these available structures than for the 5′-GAG-3′ site, indicating fewer modes of metal coordination at the 5′-GUG-3′ site. These results also point out sequence-dependent variations in cation binding within the major groove of A-form RNA helices.
G–U wobble pairs are well-known metal-binding sites that form depending on the sequence and structure context.5,71,73,90−93 In SLVI, a metal ion is associated with the G22–U5 base pair in the 5′–3′ stem near the A730 loop (site 4). The Hoogsteen edges of G22 and G23 are mainly involved in the interaction with the hexahydrated Mg2+ ion, in agreement with previous studies. In the structure of SLVI, a cation−π interaction is also observed between the Mg2+ ion and the C21 nucleobase.85 To our knowledge, this interaction has not been previously associated with a G–U wobble pair. Using FR3D, we found three similar structures in the PDB. Interestingly, in one of these three structures (PDB entry 1FJG),94 the bound metal ion also forms a cation−π interaction with a cytidine. In this case, however, the metal ion is located further from the center of the base and has a larger angle with the centroid of the nucleobase, suggesting a weaker cation−π interaction. In SLVI, the nearby A730 loop may render C21 more accessible for a cation−π interaction with the Mg2+ ion. However, given that the G–U pair is found in a different context in SLVI versus the full-length VS ribozyme, it is not clear if Mg2+ binding at this site is relevant to the activity of the ribozyme.
The GNRA tetraloop has been extensively characterized as a metal-binding site.72,79,95−97 In the context of the present study, the GAAA loop of SLVI was used as a positive control because an inner-sphere divalent-metal interaction involving the 5′-PO4– of A15 was previously identified in an equivalent position of a GAAA hairpin on the basis of Cd2+ titration of phosphorothioates.79 These NMR results were essentially reproduced here for the GNRA loop of SLVI. In addition, we identified a cation−π interaction in the SLVI structure between the Mg2+ ion and the pyrimidine ring of the first G nucleobase of the GNRA loop. The importance of this cation−π interaction in the GNRA fold has not been previously established, at least to our knowledge; therefore, it is interesting to examine cation binding to other GNRA loop structures in the PDB. Using FR3D, we identified three other similar GNRA loop structures associated with a cation. Remarkably, although the precise location of the cation differs between these structures, it always interacts with the R 5′-PO4– and forms a cation−π interaction with the conserved G nucleobase.
Cation-Binding Site in the A730 Loop Is Important for the Activity of the VS Ribozyme
From previous NMR studies, we established that the presence of Mg2+ ions is required for the formation of a stable A730 loop. It is now clear that binding of a specific Mg2+ ion at the S-turn (site 3) is central to the folding of the A730 loop.50 This S-turn brings three phosphate groups in proximity to one another [5′-PO4– of C7 (C755), A8 (A756), and G9 (G757)] with particularly short OP–OP distances between G9 and C7 and between G9 and A8 (4.1 and 5.1 Å, respectively, in the average structure). This tight packing of repulsive electronegative charges is dependent on the multidentate chelation of a Mg2+ ion, which involves an inner-shell ligand [5′-pro-Rp OP of G9 (G757)] and several potential outer-sphere ligands. Although our FR3D search indicates that there is no precedent for a S-turn structure in a similar loop context, the S-turn motif is found in other structural contexts, both with or without a divalent cation.74,98−102 With the A730 loop being relatively small, this clearly imposes topological and electrostatic constraints on the phosphate backbone at the S-turn that prevents stable folding in the absence of metal ions.
In previous work with the VS ribozyme, phosphorothioate interference and manganese-rescue experiments suggested that the pro-Rp OP of G757 (G9) and C758 (C10) are involved in direct metal binding and that the phosphate group of C755 (C7) makes an outer-sphere contact to a metal ion or participates in hydrogen bonding.40,51 The NMR studies of SLVI reveal an outer-sphere metal contact for C755 (C7) pro-Rp OP and an inner-sphere metal contact for G757 (G9) pro-Rp OP, in agreement with the biochemical studies.51 For C758 (C10), the NMR studies reveal an outer-sphere contact to a Mg2+ ion but not the predicted inner-sphere contact.51 Within SLVI, the C758 5′-PO4– may partially contribute to stabilization of the S-turn via an outer-sphere interaction with a Mg2+ ion in the hairpin stem. However, within the context of the VS ribozyme, this metal interaction may be modified because of nearby elements of secondary and tertiary structures, including formation of the active site between stem-loops I and VI. In summary, our NMR studies of SLVI generally agree with phosphorothioate interference of VS ribozyme cleavage, which indicates that the Mg2+-dependent S-turn in the A730 loop is essential for catalysis by the VS ribozyme.50
The Mg2+ ion at the S-turn plays a structural role in catalysis by the VS ribozyme by allowing the folding of the S-turn in the A730 loop and enabling A756 to be extruded in the minor groove to participate as a general acid in catalysis.50 In the hairpin ribozyme, which catalyzes phosphodiester-bond cleavage by a similar mechanism,30 the proposed general acid in the reaction (A38) also protrudes from an S-turn structure that is stabilized by at least two divalent cations.103,104 Although these cations do not play a direct role in the cleavage mechanism, they may contribute either through electrostatic stabilization or by modulating the pKa of functional residues.16,23 In the VS ribozyme, it is also unlikely that the Mg2+ ion at the S-turn plays a direct role in catalysis, given that its binding site is remote from the scissile phosphate in a model of the VS ribozyme active site.50 Nevertheless, given that this Mg2+ ion is not too distant from the catalytic site, we can not rule out the possibility that it contributes to the chemistry of the reaction. It is known that the pKa value of the A756 and the G638 nucleobases are shifted toward neutrality to facilitate the cleavage reaction40,41,43−46 and that the pKa of these nucleobases is modulated by the identity of the metal cation.24 Thus, the Mg2+ ion in the A730 loop clearly plays a structural role in defining the VS ribozyme active site, but it also likely contributes indirectly to the general acid–base chemistry.
Acknowledgments
We are very grateful to Luke Ward and Victoria De Rose for useful discussions and to Nicolas Girard for help with motif search in WebFR3D. We also thank Patricia Bouchard and James G. Omichinski for their critical reading of the manuscript.
Glossary
Abbreviations Used
- 2D
two dimensional
- 3D
three dimensional
- bp
base pair
- Cd2+
cadmium
- CSP
chemical-shift perturbation
- CT
constant time
- δ
chemical shift
- ΔP
31P CSP of the phosphorothioate
- HSQC
heteronuclear single quantum coherence
- Mg2+
magnesium
- Mn2+
manganese
- NOE
nuclear Overhauser effect
- NOESY
NOE spectroscopy
- OP
phosphate oxygen
- PO4–
phosphate
- PRE
paramagnetic relaxation enhancement
- PDB
protein data bank
- rmsd
root-mean-square deviation
- Tm
melting temperature
Supporting Information Available
Effect of Cd2+ on the 2D 1H–13C HSQC spectrum of SLVI RNA; effect of Cd2+ on the 1D 31P NMR spectrum and 1H imino NMR spectrum of SLVI RNAs containing a single phosphorothioate modification; distance restraints derived from Mn2+-induced PRE; and results from the WebFR3D motif search. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession Codes
The NMR chemical shifts, structural restraints, and structural coordinates of SLVI with bound Mg(H2O)n2+ complexes have been deposited through the RCSB Protein Data Bank and given BMRB entry code 19692 and PDB entry code 2MIS.
This work was supported by a Canadian Institutes of Health Research (CIHR) grant (MOP-86502) as well as graduate scholarships (E.B.) from CIHR and the Université de Montréal. P. L. holds a Canada Research Chair in Structural Biology and Engineering of RNA.
The authors declare no competing financial interest.
Supplementary Material
References
- Feig A. L., and Uhlenbeck O. C. (1999) The role of metal ions in RNA biochemistry, In The RNA World (Gesteland R. F., Cech T. R., and Atkins J. F., Eds.) 2nd ed., Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- Hsiao C., Tannenbaum E., VanDeusen H., Herkshkovitz E., Perng G., Tannenbaum A. R., and Williams L. D. (2009) Complexes of nucleic acids with group I and II cations, In Nucleic Acid-Metal Ion Interactions (Hud N. V., Ed.) RSC Publishing, Cambridge, UK. [Google Scholar]
- Frederiksen J. K., Fong R., and Piccirilli J. A. (2009) Metal ions in RNA catalysis, In Nucleic Acid-Metal Ion Interactions (Hud N. V., Ed.) RSC Publishing, Cambridge, UK. [Google Scholar]
- DeRose V. J. (2009) Characterization of nucleic-acid-metal ion binding by spectroscopic techniques, In Nucleic Acid-Metal Ion Interactions (Hud N. V., Ed.) p 447, RSC Publishing, Cambridge, UK. [Google Scholar]
- Cate J. H.; Doudna J. A. (1996) Metal-binding sites in the major groove of a large ribozyme domain. Structure 4, 1221–1229. [DOI] [PubMed] [Google Scholar]
- Rastogi T.; Beattie T. L.; Olive J. E.; Collins R. A. (1996) A long-range pseudoknot is required for activity of the Neurospora VS ribozyme. EMBO J. 15, 2820–2825. [PMC free article] [PubMed] [Google Scholar]
- Klein D. J.; Moore P. B.; Steitz T. A. (2004) The contribution of metal ions to the structural stability of the large ribosomal subunit. RNA 10, 1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmer M.; Dunham C. M.; Murphy F. V. t.; Weixlbaumer A.; Petry S.; Kelley A. C.; Weir J. R.; Ramakrishnan V. (2006) Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942. [DOI] [PubMed] [Google Scholar]
- Noeske J.; Schwalbe H.; Wohnert J. (2007) Metal-ion binding and metal-ion induced folding of the adenine-sensing riboswitch aptamer domain. Nucleic Acids Res. 35, 5262–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. (2000) Structures of helical junctions in nucleic acids. Q. Rev. Biophys. 33, 109–159. [DOI] [PubMed] [Google Scholar]
- Cabello-Villegas J.; Tworowska I.; Nikonowicz E. P. (2004) Metal ion stabilization of the U-turn of the A37 N6-dimethylallyl-modified anticodon stem-loop of Escherichia coli tRNAPhe. Biochemistry 43, 55–66. [DOI] [PubMed] [Google Scholar]
- Campbell D. O.; Bouchard P.; Desjardins G.; Legault P. (2006) NMR structure of Varkud satellite ribozyme stem-loop V in the presence of magnesium ions and localization of metal-binding sites. Biochemistry 45, 10591–10605. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. (2012) The structure and folding of kink turns in RNA. WIREs RNA 3, 797–805. [DOI] [PubMed] [Google Scholar]
- Casiano-Negroni A.; Sun X.; Al-Hashimi H. M. (2007) Probing Na(+)-induced changes in the HIV-1 TAR conformational dynamics using NMR residual dipolar couplings: New insights into the role of counterions and electrostatic interactions in adaptive recognition. Biochemistry 46, 6525–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor M. J. (2002) The role of metal ions in RNA catalysis. Curr. Opin. Struct. Biol. 12, 289–295. [DOI] [PubMed] [Google Scholar]
- Sigel R. K.; Pyle A. M. (2007) Alternative roles for metal ions in enzyme catalysis and the implications for ribozyme chemistry. Chem. Rev. 107, 97–113. [DOI] [PubMed] [Google Scholar]
- Chen J.; Ganguly A.; Miswan Z.; Hammes-Schiffer S.; Bevilacqua P. C.; Golden B. L. (2013) Identification of the catalytic Mg2+ ion in the hepatitis delta virus ribozyme. Biochemistry 52, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. M.; Fong R.; Piccirilli J. A. (2007) A second divalent metal ion in the group II intron reaction center. Chem. Biol. 14, 607–612. [DOI] [PubMed] [Google Scholar]
- Thaplyal P.; Ganguly A.; Golden B. L.; Hammes-Schiffer S.; Bevilacqua P. C. (2013) Thio effects and an unconventional metal ion rescue in the genomic hepatitis delta virus ribozyme. Biochemistry 52, 6499–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnberg T.; Lonnberg H. (2005) Chemical models for ribozyme action. Curr. Opin. Chem. Biol. 9, 665–673. [DOI] [PubMed] [Google Scholar]
- Herschlag D.; Jencks W. P. (1990) Catalysis of the hydrolysis of phosphorylated pyridines by Mg(OH)+: A possible model for enzymatic phosphoryl transfer. Biochemistry 29, 5172–5179. [DOI] [PubMed] [Google Scholar]
- Narlikar G. J.; Gopalakrishnan V.; McConnell T. S.; Usman N.; Herschlag D. (1995) Use of binding energy by an RNA enzyme for catalysis by positioning and substrate destabilization. Proc. Natl. Acad. Sci. U.S.A. 92, 3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert B. (2008) Ligand-pKa shifts through metals: Potential relevance to ribozyme chemistry. Chem. Biodiversity 5, 1455–1474. [DOI] [PubMed] [Google Scholar]
- Smith M. D.; Mehdizadeh R.; Olive J. E.; Collins R. A. (2008) The ionic environment determines ribozyme cleavage rate by modulation of nucleobase pKa. RNA 14, 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville B. J.; Collins R. A. (1990) A site-specific self-cleavage reaction performed by a novel RNA in Neurospora mitochondria. Cell 61, 685–696. [DOI] [PubMed] [Google Scholar]
- Collins R. A. (2002) The Neurospora Varkud satellite ribozyme. Biochem. Soc. Trans. 30, 1122–1126. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. (2004) The Varkud satellite ribozyme. RNA 10, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. J. (2008) The hairpin and Varkud satellite ribozymes, In Ribozymes and RNA Catalysis (Lilley D. M. J., and Eckstein F., Eds.), pp 66–91, RSC Publishing, Cambridge, UK. [Google Scholar]
- Cochrane J. C.; Strobel S. A. (2008) Catalytic strategies of self-cleaving ribozymes. Acc. Chem. Res. 41, 1027–1035. [DOI] [PubMed] [Google Scholar]
- Wilson T. J.; Lilley D. M. (2011) Do the hairpin and VS ribozymes share a common catalytic mechanism based on general acid-base catalysis? A critical assessment of available experimental data. RNA 17, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie T. L.; Olive J. E.; Collins R. A. (1995) A secondary-structure model for the self-cleaving region of Neurospora VS RNA. Proc. Natl. Acad. Sci. U.S.A. 92, 4686–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A.; Collins R. A. (2000) Rearrangement of a stable RNA secondary structure during VS ribozyme catalysis. Mol. Cell 5, 469–478. [DOI] [PubMed] [Google Scholar]
- Andersen A. A.; Collins R. A. (2001) Intramolecular secondary structure rearrangement by the kissing interaction of the Neurospora VS ribozyme. Proc. Natl. Acad. Sci U.S.A. 98, 7730–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard P., and Legault P. (2013) Structural insights into substrate recognition by the Neurospora Varkud satellite ribozyme: Importance of U-turns at the kissing-loop junction. Biochemistry [Online early access]. DOI: 10.1021/bi401491g. Published Online: Dec 10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley S. L.; Collins R. A. (2001) Rapid formation of a solvent-inaccessible core in the Neurospora Varkud satellite ribozyme. EMBO J. 20, 5461–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine D. A.; Wilson T. J.; Norman D. G.; Lilley D. M. (2001) The A730 loop is an important component of the active site of the VS ribozyme. J. Mol. Biol. 312, 663–674. [DOI] [PubMed] [Google Scholar]
- Hiley S. L.; Sood V. D.; Fan J.; Collins R. A. (2002) 4-Thio-U cross-linking identifies the active site of the VS ribozyme. EMBO J. 21, 4691–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine D. A.; Wilson T. J.; Zhao Z.-Y.; Lilley D. M. J. (2002) Functional group requirements in the probable active site of the VS ribozyme. J. Mol. Biol. 323, 23–34. [DOI] [PubMed] [Google Scholar]
- Sood V. D.; Collins R. A. (2002) Identification of the catalytic subdomain of the VS ribozyme and evidence for remarkable sequence tolerance in the active site loop. J. Mol. Biol. 320, 443–454. [DOI] [PubMed] [Google Scholar]
- Jones F. D.; Strobel S. A. (2003) Ionization of a critical adenosine residue in the Neurospora Varkud satellite ribozyme active site. Biochemistry 42, 4265–4276. [DOI] [PubMed] [Google Scholar]
- McLeod A. C.; Lilley D. M. (2004) Efficient, pH-dependent RNA ligation by the VS ribozyme in trans. Biochemistry 43, 1118–1125. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Y.; McLeod A.; Harusawa S.; Araki L.; Yamaguchi M.; Kurihara T.; Lilley D. M. (2005) Nucleobase participation in ribozyme catalysis. J. Am. Chem. Soc. 127, 5026–5027. [DOI] [PubMed] [Google Scholar]
- Smith M. D.; Collins R. A. (2007) Evidence for proton transfer in the rate-limiting step of a fast-cleaving Varkud satellite ribozyme. Proc. Natl. Acad. Sci. U.S.A. 104, 5818–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. J.; McLeod A. C.; Lilley D. M. (2007) A guanine nucleobase important for catalysis by the VS ribozyme. EMBO J. 26, 2489–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaikaran D.; Smith M. D.; Mehdizadeh R.; Olive J.; Collins R. A. (2008) An important role of G638 in the cis-cleavage reaction of the Neurospora VS ribozyme revealed by a novel nucleotide analog incorporation method. RNA 14, 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. J.; Li N. S.; Lu J.; Frederiksen J. K.; Piccirilli J. A.; Lilley D. M. (2010) Nucleobase-mediated general acid-base catalysis in the Varkud satellite ribozyme. Proc. Natl. Acad. Sci. U.S.A 107, 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. A.; Olive J. E. (1993) Reaction conditions and kinetics of self-cleavage of a ribozyme derived from Neurospora VS RNA. Biochemistry 32, 2795–2799. [DOI] [PubMed] [Google Scholar]
- Murray J. B.; Seyhan A. A.; Walter N. G.; Burke J. M.; Scott W. G. (1998) The hammerhead, hairpin and VS ribozymes are catalytically proficient in monovalent cations alone. Chem. Biol. 5, 587–595. [DOI] [PubMed] [Google Scholar]
- Lafontaine D. A.; Norman D. G.; Lilley D. M. (2001) Structure, folding and activity of the VS ribozyme: Importance of the 2-3-6 helical junction. EMBO J. 20, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins G.; Bonneau E.; Girard N.; Boisbouvier J.; Legault P. (2011) NMR structure of the A730 loop of the Neurospora VS ribozyme: Insights into the formation of the active site. Nucleic Acids Res. 39, 4427–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood V. D.; Beattie T. L.; Collins R. A. (1998) Identification of phosphate groups involved in metal binding and tertiary interactions in the core of the Neurospora VS ribozyme. J. Mol. Biol. 282, 741–750. [DOI] [PubMed] [Google Scholar]
- Tzokov S. B.; Murray I. A.; Grasby J. A. (2002) The role of magnesium ions and 2′-hydroxyl groups in the VS ribozyme-substrate interaction. J. Mol. Biol. 324, 215–226. [DOI] [PubMed] [Google Scholar]
- Hoffmann B.; Mitchell G. T.; Gendron P.; Major F.; Andersen A. A.; Collins R. A.; Legault P. (2003) NMR structure of the active conformation of the Varkud satellite ribozyme cleavage site. Proc. Natl. Acad. Sci. U.S.A. 100, 7003–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacheva Y. S.; Tzokov S. B.; Murray I. A.; Grasby J. A. (2004) The role of phosphate groups in the VS ribozyme-substrate interaction. Nucleic Acids Res. 32, 6240–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. O.; Legault P. (2005) NMR structure of the Varkud satellite ribozyme stem-loop V RNA and magnesium-ion binding from chemical-shift mapping. Biochemistry 44, 4157–4170. [DOI] [PubMed] [Google Scholar]
- Slim G.; Gait M. J. (1991) Configurationally defined phosphorothioate-containing oligoribonucleotides in the study of the mechanism of cleavage of hammerhead ribozymes. Nucleic Acids Res. 19, 1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F.; Grzesiek S.; Vuister G. W.; Zhu G.; Pfeifer J.; Bax A. (1995) NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293. [DOI] [PubMed] [Google Scholar]
- Johnson B. A.; Blevins R. A. (1994) NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614. [DOI] [PubMed] [Google Scholar]
- Wishart D. S.; Bigam C. G.; Yao J.; Dyson H. J.; Oldfield E.; Markley J. L.; Sykes B. D. (1995) 1H, 13C, 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR 6, 135–140. [DOI] [PubMed] [Google Scholar]
- Vuister G. W.; Bax A. (1992) Resolution enhancement and spectral editing of uniformly 13C-enriched proteins by homonuclear broadband 13C decoupling. J. Magn. Reson. 98, 428–435. [Google Scholar]
- Santoro J.; King G. C. (1992) A constant-time 2D overbodenhausen experiment for inverse correlation of isotopically enriched species. J. Magn. Reson. 97, 202–207. [Google Scholar]
- Ikura M.; Kay L. E.; Tschudin R.; Bax A. (1990) Three-dimensional NOESY-HMQC spectroscopy of a 13C-labeled protein. J. Magn. Reson. 86, 204–209. [Google Scholar]
- Piotto M.; Saudek V.; Skleñár V. (1992) Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR 2, 661–665. [DOI] [PubMed] [Google Scholar]
- Kay L. E.; Keifer P.; Saarinen T. (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Am. Chem. Soc. 114, 10663–10665. [Google Scholar]
- Skleñár V.; Peterson R. D.; Rejante M. R.; Feigon J. (1994) Correlation of nucleotide base and sugar protons in a 15N-labeled HIV-1 RNA oligonucleotide by 1H–15N HSQC experiments. J. Biomol. NMR 4, 117–122. [DOI] [PubMed] [Google Scholar]
- Schwieters C. D.; Kuszewski J. J.; Tjandra N.; Clore G. M. (2003) The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 160, 66–74. [DOI] [PubMed] [Google Scholar]
- Kleywegt G. T.; Jones T. A. (1998) Databases in protein crystallography. Acta Crystallogr., Sect. D 54, 1119–11131. [DOI] [PubMed] [Google Scholar]
- Petrov A. I.; Zirbel C. L.; Leontis N. B. (2011) WebFR3D–a server for finding, aligning and analyzing recurrent RNA 3D motifs. Nucleic Acids Res. 39, W50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini I., and Lychinat C. (1986) NMR of Paramagnetic Molecules in Biological Systems, Benjamin/Cummings, Menlo Park, CA. [Google Scholar]
- Ott G.; Arnold L.; Limmer S. (1993) Proton NMR studies of manganese ion binding to tRNA-derived acceptor arm duplexes. Nucleic Acids Res. 21, 5859–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain F. H.; Varani G. (1995) Divalent metal ion binding to a conserved wobble pair defining the upstream site of cleavage of group I self-splicing introns. Nucleic Acids Res. 23, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. R.; Simorre J. P.; Hanson P. P.; Mokler V.; Bellon L.; Beigelman L.; Pardi A. (1999) Identification and characterization of a novel high affinity metal-binding site in the hammerhead ribozyme. RNA 5, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenarejo G.; Tinoco I. Jr. (1999) Structure and thermodynamics of metal binding in the P5 helix of a group I intron ribozyme. J. Mol. Biol. 290, 119–135. [DOI] [PubMed] [Google Scholar]
- Butcher S. E.; Allain F. H.-T.; Feigon J. (2000) Determination of metal ion binding sites within the hairpin ribozyme domains by NMR. Biochemistry 39, 2174–2182. [DOI] [PubMed] [Google Scholar]
- Zuleeg T.; Hartmann R. K.; Kreutzer R.; Limmer S. (2001) NMR spectroscopic evidence for Mn(2+)(Mg(2+)) binding to a precursor-tRNA microhelix near the potential RNase P cleavage site. J. Mol. Biol. 305, 181–189. [DOI] [PubMed] [Google Scholar]
- Davis J. H.; Foster T. R.; Tonelli M.; Butcher S. E. (2007) Role of metal ions in the tetraloop-receptor complex as analyzed by NMR. RNA 13, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot J.; Feigon J.; Kearns D. R. (1982) Interactions of DNA with divalent ions. I. 31P-NMR studies. Biopolymers 21, 181–201. [DOI] [PubMed] [Google Scholar]
- Bertini I.; Luchinat C.; Scozzafava A. (1978) A 31P NMR study of phosphate in presence of cobalt(II)- and copper(II)- substituted bovine carbonic anhydrase B. FEBS Lett. 93, 251–254. [DOI] [PubMed] [Google Scholar]
- Maderia M.; Horton T. E.; DeRose V. J. (2000) Metal interactions with a GAAA RNA tetraloop characterized by 31P NMR and phosphorothioate substitution. Biochemistry 39, 8193–8200. [DOI] [PubMed] [Google Scholar]
- Maderia M.; Hunsicker L. M.; DeRose V. J. (2000) Metal-phosphate interactions in the hammerhead ribozyme observed by 31P NMR and phosphorothioate substitutions. Biochemistry 39, 12113–12120. [DOI] [PubMed] [Google Scholar]
- Duguid J. G.; Bloomfield V. A. (1995) Aggregation of melted DNA by divalent metal ion-mediated cross-linking. Biophys. J. 69, 2642–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. G.; Bloomfield V. A.; Benevides J. M.; Thomas G. J. Jr. (1995) Raman spectroscopy of DNA-metal complexes. II. The thermal denaturation of DNA in the presence of Sr2+, Ba2+, Mg2+, Ca2+, Mn2+, Co2+, Ni2+, and Cd2+. Biophys. J. 69, 2623–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton T. E.; Maderia M.; DeRose V. J. (2000) Impact of phosphorothioate substitutions on the thermodynamic stability of an RNA GAAA tetraloop: An unexpected stabilization. Biochemistry 39, 8201–8207. [DOI] [PubMed] [Google Scholar]
- Smith J. S.; Nikonowicz E. P. (2000) Phosphorothioate substitution can substantially alter RNA conformation. Biochemistry 39, 5642–5652. [DOI] [PubMed] [Google Scholar]
- McFail-Isom L.; Shui X.; Williams L. D. (1998) Divalent cations stabilize unstacked conformations of DNA and RNA by interacting with base pi systems. Biochemistry 37, 17105–17111. [DOI] [PubMed] [Google Scholar]
- Huppler A.; Niksstad L. J.; Allman A. M.; Brow D. A.; Butcher S. E. (2002) Metal binding and base ionization in the U6 RNA intramolecular stem-loop structure. Nat. Struct. Biol. 9, 431–435. [DOI] [PubMed] [Google Scholar]
- Osborne E. M.; Ward W. L.; Ruehle M. Z.; DeRose V. J. (2009) The identity of the nucleophile substitution may influence metal interactions with the cleavage site of the minimal hammerhead ribozyme. Biochemistry 48, 10654–10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. L.; Derose V. J. (2012) Ground-state coordination of a catalytic metal to the scissile phosphate of a tertiary-stabilized Hammerhead ribozyme. RNA 18, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennifar E.; Walter P.; Dumas P. (2003) A crystallographic study of the binding of 13 metal ions to two related RNA duplexes. Nucleic Acids Res. 31, 2671–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft J. S.; Tinoco I. J. (1997) Solution structure of a metal-binding site in the major groove of RNA complexed with cobalt(III) hexammine. Curr. Biol. 5, 713–721. [DOI] [PubMed] [Google Scholar]
- Keel A. Y.; Rambo R. P.; Batey R. T.; Kieft J. S. (2007) A general strategy to solve the phase problem in RNA crystallography. Structure 15, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H.; Gong B.; Bevilacqua P. C.; Carey P. R.; Golden B. L. (2009) A catalytic metal ion interacts with the cleavage Site G.U wobble in the HDV ribozyme. Biochemistry 48, 1498–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H.; Yajima R.; Chadalavada D. M.; Chase E.; Bevilacqua P. C.; Golden B. L. (2010) A 1.9 Å crystal structure of the HDV ribozyme precleavage suggests both Lewis acid and general acid mechanisms contribute to phosphodiester cleavage. Biochemistry 49, 6508–6518. [DOI] [PubMed] [Google Scholar]
- Carter A. P.; Clemons W. M. Jr.; Brodersen D. E.; Wimberley B. T.; Morgan-Warren R.; Ramakrishnan V. (2000) Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407, 340–348. [DOI] [PubMed] [Google Scholar]
- Rüdisser S.; Tinoco I. J. (2000) Solution structure of cobalt(III) hexammine complexed to the GAAA tetraloop, and metal-ion binding to G-A mismatches. J. Mol. Biol. 295, 1211–1223. [DOI] [PubMed] [Google Scholar]
- Mundoma C.; Greenbaum N. L. (2002) Sequestering of Eu(III) by a GAAA RNA tetraloop. J. Am. Chem. Soc. 124, 3525–3532. [DOI] [PubMed] [Google Scholar]
- Seetharaman M.; Eldho N. V.; Padgett R. A.; Dayie K. T. (2006) Structure of a self-splicing group II intron catalytic effector domain 5: Parallels with spliceosomal U6 RNA. RNA 12, 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczak A. A.; Moore P. B.; Chang Y. L.; Wool I. G. (1993) The conformation of the sarcin/ricin loop from 28S ribosomal RNA. Proc. Natl. Acad. Sci. U.S.A. 90, 9581–9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly B.; Varani G.; Tinoco I. Jr. (1993) The conformation of loop E of eukaryotic 5S ribosomal RNA. Biochemistry 32, 1078–1087. [DOI] [PubMed] [Google Scholar]
- Correll C. C.; Munishkin A.; Chan Y. L.; Ren Z.; Wool I. G.; Steitz T. A. (1998) Crystal structure of the ribosomal RNA domain essential for binding elongation factors. Proc. Natl. Acad. Sci. U.S.A. 95, 13436–13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher S. E.; Allain F. H.; Feigon J. (1999) Solution structure of the loop B domain from the hairpin ribozyme. Nat. Struct. Biol. 6, 212–216. [DOI] [PubMed] [Google Scholar]
- Zimmermann G. R.; Wick C. L.; Shields T. P.; Jenison R. D.; Pardi A. (2000) Molecular interactions and metal binding in the theophylline-binding core of an RNA aptamer. RNA 6, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert P. B.; Ferré-D’Amaré A. R. (2001) Crystal structure of a haripin ribozyme-inhibitor complex with implications for catalysis. Nature 410, 780–786. [DOI] [PubMed] [Google Scholar]
- Alam S.; Grum-Tokars V.; Krucinska J.; Kundracik M. L.; Wedekind J. E. (2005) Conformational heterogeneity at position U37 of an all-RNA hairpin ribozyme with implications for metal binding and the catalytic structure of the S-turn. Biochemistry 44, 14396–14408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.