Abstract
Telomerase reverse transcriptase (TERT), the catalytic subunit of the enzyme telomerase, is robustly expressed in cancer cells. TERT enables cells to avoid chromosome shortening during repeated replication by maintaining telomere length. However, several lines of evidence indicate that many cancer cells exhibit shorter telomere length than normal tissues, implying an additional function of TERT in tumor formation and progression. Here, we report a telomerase activity-independent function of TERT that induces cancer stemness in glioma cells. Overexpression of TERT712, a telomerase activity-deficient form of TERT, in U87MG cells promoted cell self-renewal in vitro, and induced EGFR expression and formation of gliomas exhibiting cellular heterogeneity in vivo. In patients with glioblastoma multiforme, TERT expression showed a high correlation with EGFR expression, which is closely linked to the stemness gene signature. Induction of differentiation and TERT-knockdown in glioma stem cells led to a marked reduction in EGFR expression, cancer stemness, and anti-cancer drug resistance. Together, our findings indicate that TERT plays a crucial role in tumor progression by promoting cancer stemness through expression of EGFR.
Keywords: bFGF, EGFR, glioblastoma multiforme, glioma stem cells, telomerase
INTRODUCTION
Glioblastoma multiforme (GBM), one of the most incurable malignancies with extremely short median survival after diagnosis, shows intensive infiltration toward surrounding normal tissue and a high recurrence rate (Holland, 2001). Several genetic changes are commonly observed in GBM, including loss of INK4A, p53, and PTEN, and gain of function of the EGFR and PDGF signaling pathways. In particular, amplification or activating mutations of EGFR (EGFRvIII) have been detected in 30 to 50% of GBMs (Cancer Genome Atlas Research Network, 2008). One of the most important properties of GBM is the heterogeneity of cells in the tumor tissue, which includes all brain cell types - neurons, astrocytes, and oligodendrocytes (Vescovi et al., 2006). This cellular heterogeneity may be explained by the presence of a small population of cells in glioma tumor tissue (cancer stem cells) that possess the developmenttal capacity to generate differentiated subpopulations with diverse lineages (Lee et al., 2006; Park and Rich, 2009; Vescovi et al., 2006). In addition to the potential for differentiation, cancer stem cells display a robust drug resistance, in part by transporting drugs out of the cells using specialized ATP binding cassette (ABC) transporters; thus, cancer stem cells are the major cause of tumor recurrence after chemotherapy (Hirschmann- Jax et al., 2004).
Recent studies have shown that in vitro establishment and self-renewing growth of cancer stem cells, including glioma stem cells, requires defined growth factors such as EGF and bFGF (Lee et al., 2006); in particular, EGF has been shown to play a crucial role in mitogenic regulation of brain cancer stem cells.
Several lines of evidence indicate that telomerase activity and TERT expression are associated with clinical aggressiveness in many types of malignancies (Lin et al., 2006; Tabori et al., 2006); therefore, TERT is considered a critical therapeutic target (Shay and Wright, 2006). In this respect, our previous study has demonstrated that induction of TERT could accelerate tumorigenesis in normal fibroblast and is indispensible for tumorigenesis in advanced cancer cells (Jin et al., 2010). However, a growing body of evidence shows that telomeres are shorter in malignant tumor cells such as colorectal cancer, hepatoma, and gastric carcinoma compared with the surrounding stromal cells, independent of telomerase activity (Engelhardt et al., 1997; Furugori et al., 2000; Ohashi et al., 1998). Furthermore, recent data have also demonstrated that the tumorigenic effect of telomerase is independent of telomere length (Stewart et al., 2002). These findings suggest that TERT may play additional roles in tumor progression; however, little is known about the mechanisms underlying tumorigenesis driven by telomere elongation-independent functions of TERT. In this study, we investigated the role of a telomerase activity-deficient form of TERT in tumor progression and aggressiveness.
MATERIALS AND METHODS
Cell culture and gene transduction
U87MG glioma cells were maintained in DMEM supplemented with 10% FBS, and glioma stem cells were cultured as described previously (Jeon et al., 2008; Lee et al., 2006). Cells were transfected with plasmid vectors encoding a variety of genes using Lipofectamine 2000 (Invitrogen, USA).
Neurosphere formation assay
GIC3 glioma stem cells (Joo et al., 2008) and U87MG cells were cultured in a suspension (without laminin-coating) or in adherent (with laminin- coating, Sigma) culture conditions using neurobasal medium (NBE; Invitrogen) enriched with modified N2, 1X B27, EGF (R&D Systems), and bFGF (R&D Systems). For neurosphere formation, 400 cells were plated in a 24-well plate (1 cell/mm2) and maintained in NBE medium for 7-10 days.
Plasmids, shRNA construction, and transfection
Cells were transfected with pCI-hTERT-Neo, pcDNA3.1-T712- Puro, pcDNA3.1-bFGF(FL)-myc-his, pcDNA3.1-bFGF(SF)-myc-his, and pcDNA3.1-EGFR (4 μg each) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours after transfection, cells were subjected to drug selection for 2 weeks.
Cells were infected with retrovirus expressing control Scramble- shRNA or hTERT-shRNA cloned into the constitutive RNAi expression vectors pSuperRetro-Puro or pMKO-Puro, respectively. The target sequences were tttcatcagcaagtttgga for human hTERT-shRNA, and agacggaggcttacagtctgg for Scramble-shRNA.
RNA and protein analysis
Total RNA was isolated from cells using TRIzol (Gibco BRL) according to the manufacturer’s instructions. For semi-quantitative RT-PCR, 3 μg DNase I-treated RNA was converted to cDNA with Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. For semi-quantitative and real-time reverse transcriptase-polymerase chain reaction (RT-PCR), 1 μl of the RT reaction was used to amplify TERT, bFGF, EGFR, CD133, CD15, Nestin, GFAP, S100B, Tuj1, 18S rRNA, and GAPDH fragments using the corresponding gene-specific primer sets (details available upon request). Real-time RT-PCR was conducted using the iCycler IQ (Bio- Rad) and IQ Supermix with SYBR-Green (Bio-Rad).
For Western blot analysis, whole cell extracts were prepared using RIPA lysis buffer [150 mM NaCl, 1% NP-40, 0.1% SDS, and 50 mM Tris (pH 7.4)] containing 1 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM NaF, 1 mM Na3VO4, and protease inhibitor (Roche). Proteins in the extracts were quantitated using the Bradford assay reagent (Bio-Rad) according to the manufacturer’s instructions. Protein (30-100 μg) was separated by a 4-12% gradient or 10% SDS-PAGE NuPAGE gel (Invitrogen) and transferred to a PVDF membrane (Millipore). Membranes were blocked with 5% non-fat milk and incubated with antibodies specific for EGFR (1005), bFGF (both from Santa Cruz Biotechnology), and α-tubulin (Sigma). Membranes were then incubated with horseradish peroxidase-conjugated anti-IgG secondary antibody (Pierce) and visualized with SuperSignal West Pico Chemiluminescent Substrate (Pierce).
In vivo tumor formation assay
Cells (2 × 106) were subcutaneously transplanted into nude mice (BALB/c nu/nu) and the mice were kept under observation for 8 weeks. All mouse experiments were approved by the Animal Care Committee at the College of Life Sciences and Biotechnology, Korea University, and were performed in accordance with government and institutional guidelines and regulations.
Immunofluorescence and immunohistochemistry assays
Mice harboring various U87MG-driven tumors were perfused with PBS and 4% paraformaldehyde. Dissected tumor tissues were paraffinized and sliced into 5-μm sections. Tumor sections were incubated with primary antibodies against EGFR, bFGF, Ki67 (BD Biosciences), CD133 (AC133, MACS), Nestin (Chemicon), S100β (Sigma), Tuj1 (Chemicon), O4 (Chemicon), and GFAP (DAKO) and examined using optical and confocal microscopy (Zeiss).
Bioinformatics expression profile analysis of patients with glioblastoma multiforme (GBM)
Raw data from the REMBRANDT (Repository of Molecular Brain Neoplasia Data) database of the National Cancer Institute (https://caintegrator.nci.nih.gov/rembrandt/, National Cancer Institute. 2005) were gathered and analyzed with the R Software Package. To minimize problems associated with high RNA degradation in GBM tissues, 33 datasets showing low RNA degradation (RNAdeg < 2.5) were chosen and normalized with the Robust Multiarray Averaging (RMA) method. Probe-set correlation was determined with Pearson Correlation Coefficient and depicted as a heatmap with Heatmap Builder 1.1 (Heatmap development team).
Statistics
Data were analyzed using the two-tailed Student’s t-test. P < 0.05 (*) and P < 0.01(**) were considered statistically significant. Data are presented as mean ± standard deviation.
RESULTS AND DISCUSSION
TERT accelerates tumorigenesis and gives rise to tumor heterogeneity independent of its enzymatic activity
Accumulating evidence has demonstrated that TERT plays a crucial role in cell survival and proliferation independent of its telomere lengthening enzymatic activity (Cao et al., 2002; Lee et al., 2008; Rahman et al., 2005; Stewart et al., 2002). However, these telomerase activity-independent functions of TERT and their role in tumor initiation and progression are not well characterized. Therefore, to investigate the telomere elongationindependent role of human TERT in tumor progression, we performed in vivo tumor formation assays with U87MG glioma cells that were transduced with a telomerase activity-deficient mutant of TERT (TERT-D712A, hereafter referred to as TERT712) (Beattie et al., 2001) or with TERT-specific shRNA (Fig. 1A). Repression of endogenous TERT expression by TERT-shRNA dramatically reduced the tumor formation ability of U87MG cells. In contrast, ectopic expression of TERT712 in U87MG cells accelerated in vivo tumor formation (Fig. 1B). These results indicate that the telomerase activity-independent function of TERT is associated with tumor progression (Cao et al., 2002; Lee et al., 2008; Rahman et al., 2005; Stewart et al., 2002).
Fig. 1. TERT accelerates tumorigenesis and gives rise to heterogeneous tumors in a telomerase activity-indepen-dent manner. (A) U87MG glioma cells were ectopically transduced with a telomerase activity-deficient TERT mu-tant (TERT712), or with TERTspecific shRNA (shTERT) to repress endogenous TERT expression. Nonspecific Scramble shRNA was also transduced into U87MG glioma cells as a negative control for the shRNA study. TERT mRNA expression was analyzed by real-time RT-PCR. *p < 0.05. (B) Representative photographs of tumors and graph showing tumor volumes at 8 weeks after subcutaneous injection of the cells described in (A) into nude mice (n = 6). *p < 0.05. (C) Immunofluorescence analysis showing the expression of markers specific for glioma stem cells (CD133 and Nestin), astrocytes (GFAP), and neurons (Tuj1) in tumors derived from U87MG-control and U87MGTERT712 cells.
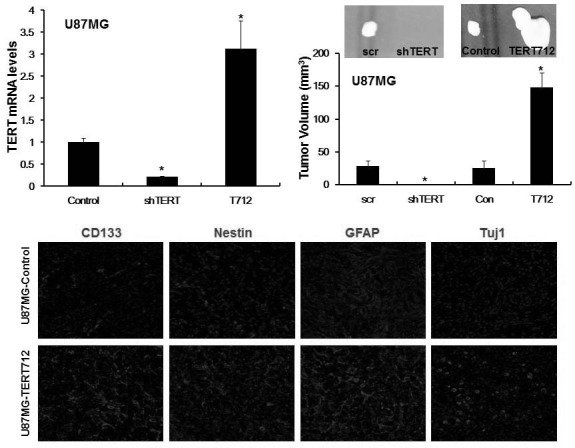
A recent study demonstrated that TERT functions as a potent developmental regulator by modulating the Myc and Wnt signaling pathways that are pivotal drivers of stemness in many types of normal and cancer stem cells (Choi et al., 2008; Park et al., 2009). Cancer stem cells are known to give rise to tumor heterogeneity; therefore, we determined whether tumors derived from U87MG-TERT712 cells possess heterogeneous cell populations containing neural stem cells, astrocytes, and neurons. Notably, U87MG-driven tumors were composed primarily of GFAP+ astrocytic cells with a few Nestin+ and CD133+ glioma stem cells and rare Tuj1+ neuronal cells indicating cellular homogeneity, whereas U87MG-TERT712-driven tumors appeared to consist of a mixture of CD133+, Nestin+, GFAP+, and Tuj1+ cells, indicative of heterogeneous tumors composed of various cell lineages including neural stem cells, astrocytes, and neurons (Fig. 1C) (Singh et al., 2004; Vescovi et al., 2006). These results suggest that ectopic expression of TERT allows cancer cells to acquire properties of stem cells and ultimately become cancer stem cells, resulting in tumor heterogeneity, a common histopathological feature of human GBMs (Singh et al., 2004).
TERT induces expression of EGFR and bFGF in glioma cells in vitro and in vivo and expression of TERT correlates with expression of EGFR and bFGF in patients with GBM
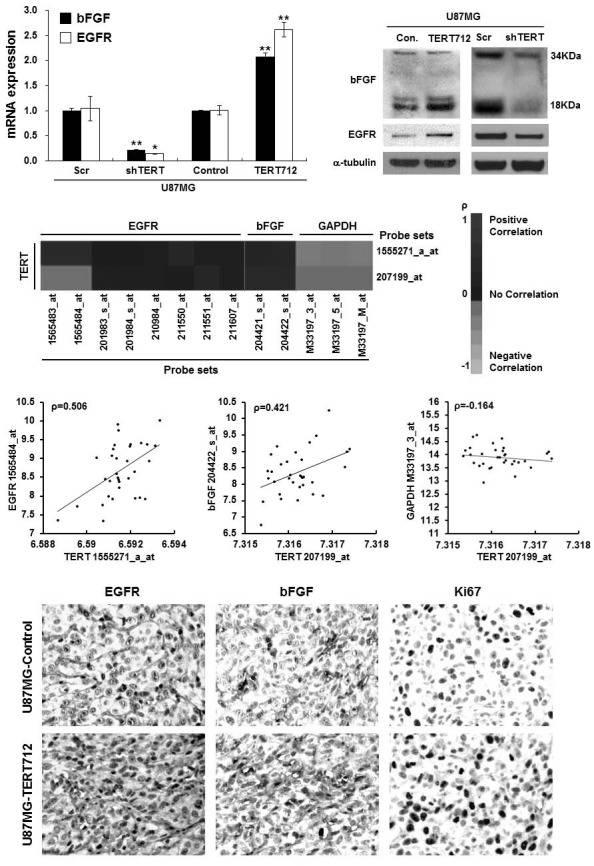
A previous study demonstrated that overexpression of wild-type TERT in human mammary epithelial cells results in a robust increase in expression of EGFR and bFGF (Singh et al., 2004). Moreover, EGF and bFGF signaling is sufficient to enhance self-renewal of glioma stem cells (Jeon et al., 2008; Lee et al., 2006). Therefore, we examined whether expression of EGFR and bFGF is induced in telomerase activity deficient TERToverexpressing U87MG cells to address the potential mechanism underlying the acquisition of stemness characteristics following ectopic TERT712 expression. Indeed, we found that both mRNA and protein levels of EGFR and bFGF (expressed as a 18-kDa secreted form and 34-kDa non-secreted form) were markedly elevated in U87MG-TERT712 cells, and significantly diminished in U87MG-shTERT cells (Figs. 2A and 2B). Next, we analyzed mRNA expression patterns of EGFR, bFGF and TERT in patients with GBM using the REMBRANDT database (National Cancer Institute, 2005) to validate a possible correlation in their expressions in brain malignancies, although this database did not discriminate expressions of wild-type and telomerase activity-deficient mutant TERT. TERT displayed a high correlation with expression of EGFR and bFGF, but not control GAPDH (Figs. 2C and 2D). Consistent with these findings, we also observed that tumors derived from mouse xenografts of U87MG-TERT712 cells showed higher levels of EGFR and bFGF expression, and possessed increased numbers of Ki67+ proliferating cells compared with tumors derived from U87MG-control cells (Fig. 2E). Our findings suggest that, independent of its enzymatic activity, ectopic expression of TERT leads to activation of EGF and bFGF signaling through induction of EGFR and bFGF expression, which might be sufficient to drive the generation of cancer stem cells and give rise to heterogeneous tumors.
Fig. 2. Induction of EGFR and bFGF expression by TERT in vitro and in vivo, and correlation of TERT expression with EGFR and bFGF expression in GBM patients. (A) bFGF and EGFR mRNA levels in U87MGcontrol, U87MG-Scramble, U87MG-shTERT and U87MG-TERT712 cells determined by real-time RT-PCR. *p < 0. 05; * *p < 0.01. (B) bFGF and EGFR protein levels in the cells described in (A) determined by Western blot analysis. α-tubulin was used for an equal loading control. (C) Heatmap showing correlation of TERT expression with expression of EGFR, bFGF and GAPDH mRNA in GBM patients from the REMBRANDT database. The color of each rectangle indicates the Pearson Correlation Coefficient value (ρ) between probe sets. (D) Representative plots showing correlation of TERT levels with EGFR, bFGF and GAPDH mRNA expression for the data shown in (C). Each axis shows log2 signal value of mRNA expression, and the corresponding Pearson Correlation Coefficient value (ρ) is indicated in each plot. (E) Immunohistochemistry analysis showing EGFR and bFGF protein levels and the Ki67+ proliferating cell population in tumors derived from transplantation of U87MG-control and U87MG-TERT712 cells into nude mice.
Decreased TERT expression in glioma stem cells leads to reduced expression of EGFR and bFGF, and promotes loss of glioma stem cell properties
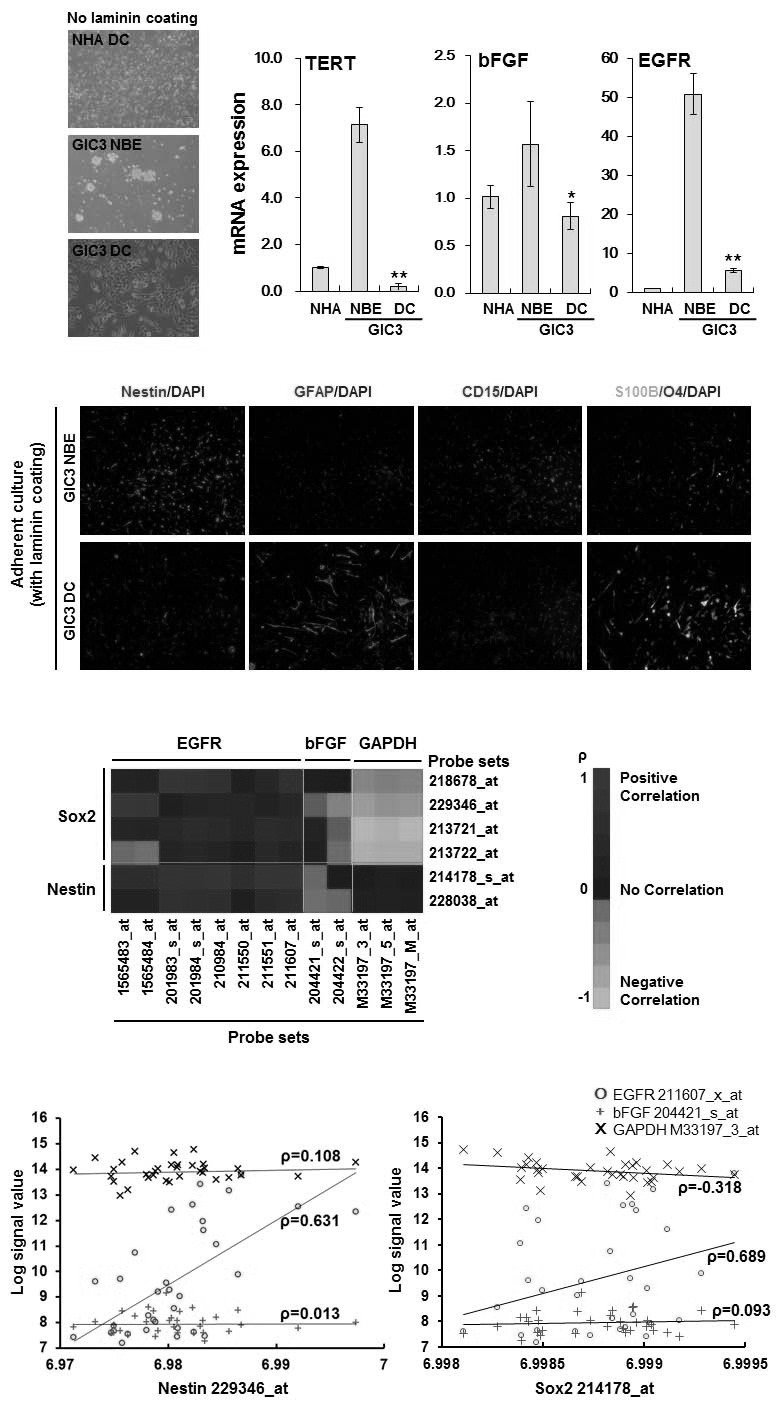
To further validate our findings in a more clinically relevant model, we used GIC3 glioma stem cells generated from a patient with glioma. GIC3 cells grown in a differentiation culture (DC) medium (DMEM + 5% FBS) appea-red to attach to the culture dish substrate without neurosphere formation; however, they were capable of forming neuros-pheres in a suspension NBE culture (not laminin-coated) (Fig. 3A). Expression of TERT, bFGF, a nd E GFR w as l ower i n GIC3 c ells g rown i n D C medium compared with those grown in NBE culture (Fig. 3B). Furthermore, in adherent culture (laminin-coated) (Pollard et al., 2009), most of the cells grown in NBE were positive for the glioma stem cell markers Nestin and CD15 (SSEA-1) (Lee et al., 2006; Pollard et al., 2009), whereas cells grown in DC medium appeared to differentiate into astrocytes (GFAP+ and S100β+) and oligodendrocytes (O4+) (Fig. 3C). Therefore, we wondered whether elevated expression of TERT might directly regulate cancer stemness through induction of EGFR and bFGF. Before testing this hypothesis, we first used the REMBRANDT database to compare expression levels of EGFR and bFGF with those of the two best-characterized glioma stem cell markers, Nestin and Sox2, in patients with GBM. As shown in Figs. 3D and 3E, expression levels of Nestin and Sox2 were highly correlated with expression levels of EGFR, but not bFGF or GAPDH. Although TERT expression showed a high correlation with expression of both EGFR and bFGF, these findings suggest that upregulation of EGFR, rather than bFGF, by TERT might be the critical factor in the regulation of selfrenewal and maintenance of glioma stem cells.
Fig. 3. Reduction of EGFR and bFGF expression by downregu-lation of TERT in glioma stem cells, and correlation of EGFR expression with Nestin and Sox2 expression in GBM patients. (A) Representative photographs showing GIC3 glioma stem cells grown in suspension neurobasal medium with bFGF and EGF (NBE) or in differentiation culture (DC; DMEM + 10% FBS), and normal human astrocytes (NHA) cells grown in DC. (B) Real-time RT-PCR showing reduced expression levels of TERT, bFGF, and EGFR in GIC3 cells grown in DC adherent culture, compared with NBE culture. Gene expression in GIC3 cells was normalized to expression in NHA cells. *p < 0.05; **p < 0.01. (C) Immunofluorescence analysis of cells positive for lineage-specific markers in GIC3 grown in suspension in NBE or adherent in DC cultures. Nestin and CD15, glioma stem cell markers; GFAP and S100β, astrocyte markers; O4, oligodendrocyte marker. Nuclei were stained with DAPI. (D) Heatmap showing correlation of EGFR, bFGF, and GAPDH levels with Sox2 and Nestin mRNA expression in GBM patients from the REMBRANDT database. The color of each rectangle indicates the Pearson Correlation Coefficient value between probe sets. (E) Representative plots showing correlation of EGFR, bFGF, and GAPDH with Nestin (left graph) and Sox2 (right graph) for the data shown in (D). Each axis shows log2 signal value of mRNA expression, and the corresponding Pearson Correlation Coefficient value is indicated in each plot.
Upregulation of EGFR by TERT plays a critical role in promoting stem cell-like features in glioma cells and maintaining glioma stem cell properties
To directly address the importance of the TERT-EGFR axis in controlling cancer stemness properties, we used U87MG cells that are known to retain a subpopulation of glioma stem-like cells when grown in a defined culture medium supplemented with EGF and bFGF (Qiang et al., 2009). We first established several cell lines: control U87MG cells transduced with a nonspecific scramble shRNA (U87MG-Scr); TERT-depleted U87MG cells transduced with TERT-specific shRNA (U87MG-shTERT); and U87MG-shTERT cells transduced with EGFR, a secreted form of bFGF (SF), or full length bFGF (FL). Next, we conducted a neurosphere formation assay using these cell lines. As shown in Fig. 4A, the neurosphere formation ability of U87MG cells was markedly diminished by depletion of TERT, but was significantly recovered by ectopic expression of EGFR in U87MG-shTERT cells. However, ectopic expression of bFGF-SF and bFGF-FL in U87MG-shTERT failed to rescue the neurosphere forming ability. These results indicate that TERT is capable of regulating self-renewal of glioma stem-like cells through induction of EGFR expression. To further understand the role of the TERT-EGFR axis in self-renewal of glioma stem-like cells, we inhibited EGFR signaling in control U87MG cells and in U87MG cells that overexpress wild-type TERT or telomerase activitydeficient TERT712 by treatment with an EGFR inhibitor, AG1478. As shown in Fig. 4B, ectopic expression of TERT and TERT712 in U87MG cells significantly promoted neurosphere formation compared with control U87MG cells. Inhibition of EGFR signaling by AG1478 markedly reduced neurosphere formation in U87MG-TERT and U87MG-TERT712 cells to a level similar to that of control U87MG cells (Fig. 4B).
Fig. 4. Loss of TERT expression in glioma stem cells leads to reduced expression of EGFR and bFGF and diminished glioma stem cell properties. (A) Neurosphere formation of U87MG-control-Scramble, U87MGshTERT- control, U87MG-shTERTEGFR, U87MG-shTERT-bFGF-SF, and U87MG-shTERT-bFGF-FL cells grown in NBE culture for 10 days. **p < 0.01. (B) Neurosphere formation of U87MG-control, U87MG-TERT, and U87MG-TERT712 cells grown in NBE culture medium with or without the EGFR inhibitor AG1478. *p < 0.05; **p < 0.01. (C) Relative gene expression levels in GIC3-shTERT and GIC3- Scramble cells determined by realtime RT-PCR analysis. *p < 0.05. (D) Cell viability of GIC3-shTERT and GIC3-scramble cells grown in the presence of doxorubicin (1 μM) for 24 h determined by a trypan blue exclusion assay. **p < 0 .01. ( E) Neurosphere formation of glioma stem cells (GIC1, GIC2, and GIC3) grown in a defined culture medium supplemented with EGF or bFGF alone, or with both EGF and bFGF. *p < 0.05; **p < 0.01.
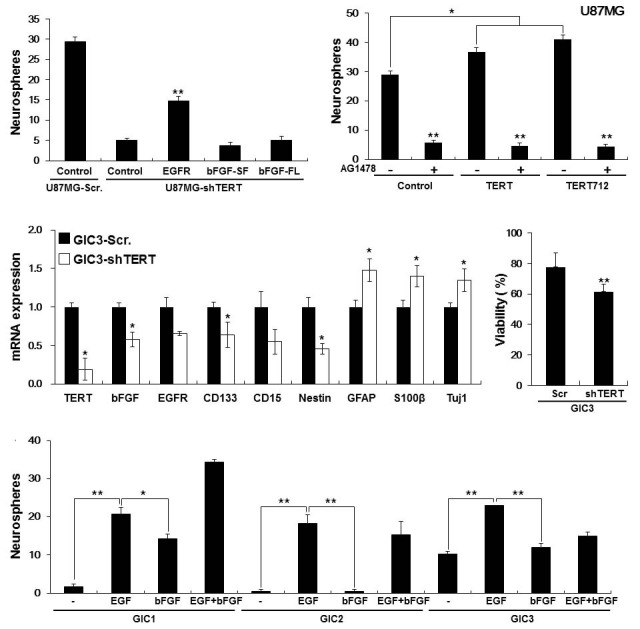
To determine the direct effects of TERT on the expression of EGFR, bFGF, and lineage-specific markers specific for glioma stem cells (CD133, CD15, and Nestin) and differentiation (GFAP, S100β, and Tuj1), as well as on anticancer drug resistance (one of the hallmarks of cancer stem cells) in glioma stem cells derived from GBM patient (Hirschmann-Jax et al., 2004; Vescovi et al., 2006), we transduced TERT-specific shRNA into GIC3 cells. Depletion of TERT in these cells resulted in a significant decrease in expression of TERT, EGFR, bFGF, and glioma stem cell marker mRNAs, but a relative increase in GFAP, S100β, and Tuj1 mRNA levels (Fig. 4C). In addition, these cells displayed significantly reduced cell viability when treated with doxorubicin (Fig. 4D). These results indicate that persistent TERT expression in glioma stem cells is required to maintain their undifferentiated status and resistance to certain anticancer drugs.
Many types of cancer stem cells, including glioma stem cells, preferentially retain their stemness when grown in a defined culture medium supplemented with EGF and bFGF (Lee et al., 2006), indicating that EGF and bFGF signaling are crucial driving forces for maintaining stem cell character in vitro. Therefore, we compared neurosphere formation capacity of three glioma stem cells derived from patients with GBM (GIC1, GIC2, and GIC3; Joo et al., 2008) by growing the cells in a defined culture medium supplemented with EGF or bFGF alone, or with both EGF and bFGF. As shown in Fig. 4E, EGF promoted neuros-phere formation in all three glioma stem cells more effectively than bFGF, further suggesting that upregulation of EGFR by TERT plays a crucial role in maintaining selfrenewing growth of glioma stem-like cells.
In conclusion, we have delineated a functional link between telomere elongation-independent function of TERT and the acquisition of cancer stem cell properties and anticancer drug resistance. These findings are supported by recent data indicating that most cancer stem cells require EGFR signaling to maintain their stemness (Lee et al., 2006; Singh et al., 2004; Vescovi et al., 2006), and are much more resistant to chemotherapy and radiotherapy than other cancer cells (Bao et al., 2006). Further investigation of the mechanisms underlying TERT-driven EGFR expression will be particularly valuable in the development of new therapeutic agents and modalities that target cancer stem cells.
Acknowledgments
This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea. (A080098). X.J. was supported by a BK21 Research Fellowship from the Ministry of Education and Human Resources Development. S.H.K was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-351-C00137).
References
- 1.Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Bigner D.D., Rich J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. (2006);444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 2.Beattie T.L., Zhou W., Robinson M.O., Harrington L. Functional multimerization of the human telomerase reverse transcriptase. Mol. Cell. Biol. (2001);21:6151–6160. doi: 10.1128/MCB.21.18.6151-6160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. (2008);455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y., Li H., Deb S., Liu J.P. TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene. (2002);21:3130–3138. doi: 10.1038/sj.onc.1205419. [DOI] [PubMed] [Google Scholar]
- 5.Choi J., Southworth L.K., Sarin K.Y., Venteicher A.S., Ma W., Chang W., Cheung P., Jun S., Artandi M.K., Shah N., et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. (2008);4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhardt M., Drullinsky P., Guillem J., Moore M.A. Telomerase and telomere length in the development and progression of premalignant lesions to colorectal cancer. Clin. Cancer Res. (1997);3:1931–1941. [PubMed] [Google Scholar]
- 7.Furugori E., Hirayama R., Nakamura K.I., Kammori M., Esaki Y., Takubo K. Telomere shortening in gastric carcinoma with aging despite telomerase activation. J. Cancer Res. Clin. Oncol. (2000);126:481–485. doi: 10.1007/s004320000137. [DOI] [PubMed] [Google Scholar]
- 8.Hirschmann-Jax C., Foster A.E., Wulf G.G., Nuchtern J.G., Jax T.W., Gobel U., Goodell M.A., Brenner M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA. (2004);101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland E.C. Gliomagenesis: genetic alterations and mouse models. Nat. Rev. Genet. (2001);2:120–129. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 10.Jeon H.M., Jin X., Lee J.S., Oh S.Y., Sohn Y.W., Park H.J., Joo K.M., Park W.Y., Nam D.H., DePinho R.A., et al. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes. Dev. (2008);22:2028–2033. doi: 10.1101/gad.1668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin X., Beck S., Sohn Y.W., Kim J.K., Kim S.H., Yin J., Pian X., Kim S.C., Choi Y.J., Kim H. hTERT suppresses p53-mediated anti-apoptotic response via induction of bFGF. Exp. Mol. Med. (2010);42:574–582. doi: 10.3858/emm.2010.42.8.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo K.M., Kim S.Y., Jin X., Song S.Y., Kong D.S., Lee J.I., Jeon J.W., Kim M.H., Kang B.G., Jung Y., et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab. Invest. (2008);88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 13.Lee J., Kotliarova S., Kotliarov Y., Li A., Su Q., Donin N.M., Pastorino S., Purow B.W., Christopher N., Zhang W., et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cells. (2006);9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Lee J., Sung Y.H., Cheong C., Choi Y.S., Jeon H.K., Sun W., Hahn W.C., Ishikawa F., Lee H.W. TERT promotes cellular and organismal survival independently of telomerase activity. Oncogene. (2008);27:3757–3760. doi: 10.1038/sj.onc.1211037. [DOI] [PubMed] [Google Scholar]
- 15.Lin X., Gu J., Lu C., Spitz M.R., Wu X. Expression of telomere-associated genes as prognostic markers for overall survival in patients with non-small cell lung cancer. Clin. Cancer Res. (2006);12:5720–5725. doi: 10.1158/1078-0432.CCR-05-2809. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. http://rembrandt.nci.nih.gov. [Accessed 2009 September 1];REMBRANDT home page. (2005)
- 17.Ohashi K., Tsutsumi M., Nakajima Y., Kobitsu K., Nakano H., Konishi Y. Telomere changes in human hepatocellular carcinomas and hepatitis virus infected noncancerous livers. CA. Cancer J. Clin. (1998);77:1747–1751. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1747::AID-CNCR50>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Park D.M., Rich J.N. Biology of glioma cancer stem cells. Mol. Cells. (2009);28:7–12. doi: 10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 19.Park J.I., Venteicher A.S., Hong J.Y., Choi J., Jun S., Shkreli M., Chang W., Meng Z., Cheung P., Ji H., et al. Telomerase modulates Wnt signaling by association with target gene chromatin. Nature. (2009);460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard S.M., Yoshikawa K., Clarke I.D., Danovi D., Stricker S., Russell R., Bayani J., Head R., Lee M., Bernstein M., et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. (2009);4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Qiang L., Yang Y., Ma Y.J., Chen F.H., Zhang L.B., Liu W., Qi Q., Lu N., Tao L., Wang X.T., et al. Isolation and characterization of cancer stem like cells in human glioblastoma cell lines. Cancer Lett. (2009);279:13–21. doi: 10.1016/j.canlet.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Rahman R., Latonen L., Wiman K.G. hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene. (2005);24:1320–1327. doi: 10.1038/sj.onc.1208232. [DOI] [PubMed] [Google Scholar]
- 23.Shay J.W., Wright W.E. Telomerase therapeutics for cancer: challenges and new directions. Nat. Rev. Drug. Discov. (2006);5:577–584. doi: 10.1038/nrd2081. [DOI] [PubMed] [Google Scholar]
- 24.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. (2004);432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 25.Smith L.L., Coller H.A., Roberts J.M. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat. Cell. Biol. (2003);5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S.A., Hahn W.C., O’Connor B.F., Banner E.N., Lundberg A.S., Modha P., Mizuno H., Brooks M.W., Fleming M., Zimonjic D.B., et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl. Acad. Sci. USA. (2002);99:12606–12611. doi: 10.1073/pnas.182407599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabori U., Ma J., Carter M., Zielenska M., Rutka J., Bouffet E., Bartels U., Malkin D., Hawkins C. Human telomere reverse transcriptase expression predicts progression and survival in pediatric intracranial ependymoma. J. Clin. Oncol. (2006);24:1522–1528. doi: 10.1200/JCO.2005.04.2127. [DOI] [PubMed] [Google Scholar]
- 28.Vescovi A.L., Galli R., Reynolds B.A. Brain tumour stem cells. Nat. Rev. Cancer. (2006);6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]


