SUMMARY
Some plant-based diets lower the cardiometabolic risks and prevalence of hypertension. New evidence implies a role for the transient receptor potential vanilloid 1 (TRPV1) cation channel in the pathogenesis of cardiometabolic diseases. Little is known about impact of chronic TRPV1 activation on the regulation of vascular function and blood pressure. Here we report that chronic TRPV1 activation by dietary capsaicin increases the phosphorylation of protein kinase A (PKA) and eNOS and thus production of nitric oxide (NO) in endothelial cells, which is calcium dependent. TRPV1 activation by capsaicin enhances endothelium-dependent relaxation in wild-type mice, an effect absent in TRPV1-deficient mice. Long-term stimulation of TRPV1 can activate PKA, which contributes to increased eNOS phosphorylation, improves vasorelaxation, and lowers blood pressure in genetically hypertensive rats. We conclude that TRPV1 activation by dietary capsaicin improves endothelial function. TRPV1-mediated increase in NO production may represent a promising target for therapeutic intervention of hypertension.
INTRODUCTION
Hypertension is a major risk factor for the increased incidence of stroke, coronary heart disease, and renal insufficiency (Ong et al., 2007). Lowering high blood pressure reduces the risks to develop cardiovascular events (Cushman, 2001). Epidemiological studies show that multiple dietary factors affect blood pressure. Over the past decade, reducing sodium intake, increasing potassium intake, and consuming fruits and vegetables based on the “DASH diet” have emerged as effective antihypertensive strategies (Appel et al., 1997; Lichtenstein et al., 2006). Capsium species, or hot peppers, are consumed worldwide as vegetables and spices. Capsaicin (8-methyl-N-vanillyl-trans-6-nonenamide) is the major pungent ingredient in hot pepper and gives a flavor to food without increasing calories. Capsaicin increases thermogenesis by enhancing catecholamine secretion from adrenal medulla, decreases weight gain, and adipogenesis by enhancing energy metabolism (Hachiya et al., 2007; Kawabata et al., 2006; Ohnuki et al., 2001; Zhang et al., 2007). Capsaicin is a highly selective agonist for the transient receptor potential vanilloid 1 (TRPV1) cation channel (Caterina et al., 1997). TRPV1, a polymodal nonselective cation channel, is expressed in sensory neurons and also present in nonneuronal tissues including blood vessels (Nilius, 2007; Pedersen et al., 2005; Vennekens et al., 2008). Apart from its role as a potent analgesic (Caterina et al., 2000), capsaicin exerts effects in the cardiovascular system (Gupta et al., 2007; Pacher et al., 2004). However, the effects of capsaicin on vascular tone and arterial pressure are a paradox. Acute or short-term administration of capsaicin has mixed effects, either increasing or lowering arterial pressure transiently in human and rodents (del Carmen Garcia et al., 2003; Hachiya et al., 2007; Li and Wang, 2003). Capsaicin produces relaxation in several arterial in vitro preparations, which may be mediated by the releases of calcitonin gene-related peptide (CGRP) and substance P from perivascular sensory nerve terminals (Holzer, 1992; Li and Wang, 2003; Rubino and Burnstock, 1996). Alternatively, capsaicin is reported to increase the nitric oxide (NO) metabolites production by human vein endothelial cells (ECs) (Lo et al., 2003). Although TRPV1 is proposed to be involved in Dahl salt-sensitive hypertension (Wang and Wang, 2006), its exact mechanism is poorly understood. Little is known about the impact of chronic activation of TRPV1 on the regulation of vascular function and blood pressure. We hypothesized that dietary capsaicin chronically activates TRPV1, which contributes to the vascular benefits. We provide in vivo and in vitro experimental evidence demonstrating that long-term activation of TRPV1 can actually increase the phosphorylated levels of protein kinase A (PKA) and endothelial NO synthase (eNOS) in mesenteric arteries and plasma levels of NO metabolites, augment endothelium-dependent relaxation, and lower arterial pressure in hypertensive rats. Our results suggest that endothelial TRPV1 is a potential therapeutic target in the management of hypertension and related vascular diseases.
RESULTS
Location and Functional Characterization of TRPV1 in Arteries
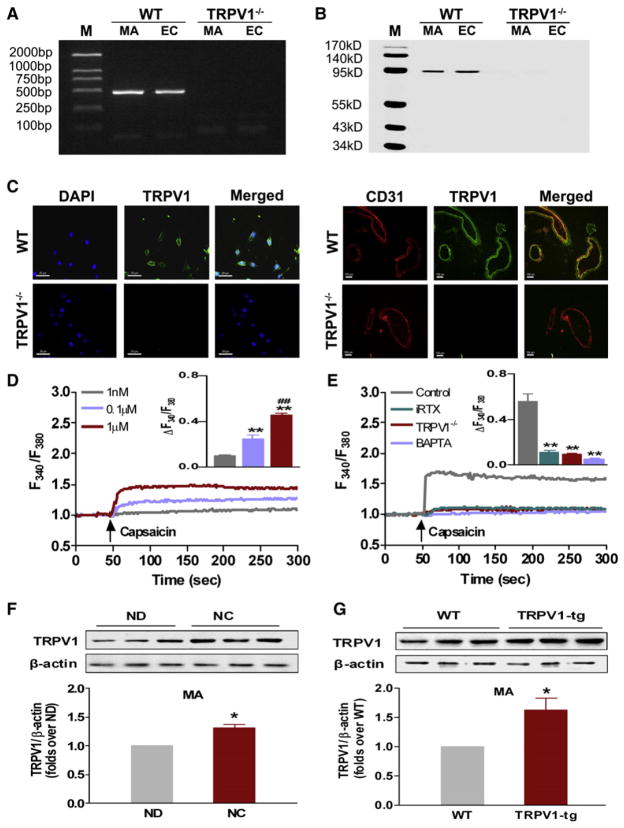
TRPV1 is reportedly present in ECs of arteries (Inoue et al., 2006; Yao and Garland, 2005). To validate the expression of TRPV1 in the endothelium, ECs were isolated from aortas of wild-type (WT) and TRPV1−/− mice and cultured. The expression of TRPV1 mRNA (Figure 1A) and protein (Figure 1B) was clearly detected by RT-PCR and immunoblotting in both cultured ECs and freshly isolated mesenteric arteries (MAs) of WT mice but absent in ECs and MA from TRPV1−/− mice. Further confirmation for the presence of TRPV1 is verified by immunofluorescence staining (Cristino et al., 2006) (Figure 1C). TRPV1 belongs to the family of nonselective cation channels, displaying high Ca2+ permeability (Vennekens et al., 2008). Acute exposure to capsaicin stimulates an increase in the cytosolic free calcium concentration ([Ca2+]i) in cultured ECs (Figure 1D). Specific blockade of TRPV1 by 5′-iodo-resiniferatoxin (iRTX), quenching of [Ca2+]i by the Ca2+ chelator BAPTA-AM, and TRPV1 deficiency abolished the capsaicin-induced [Ca2+]i increase (Figure 1E). Capsaicin treatment for 6 months significantly increased TRPV1 protein expression in mesenteric arteries from WT mice (Figure 1F), and the level of TRPV1 protein was higher in mesenteric arteries from TRPV1 transgenic mice (Figure 1G). Thus, functional TRPV1 is present in ECs and intact arteries, and its expression can be upregulated chronically by capsaicin.
Figure 1. Location and Functional Characterization of TRPV1 Channels in Arteries.
(A and B) TRPV1 mRNA was detected by RT-PCR, and TRPV1 protein level was detected by immunoblotting in mesenteric arteries (MA) and cultured endothelial cells (ECs) from WT but not from TRPV1−/− mice. M, marker.
(C) Representative immunofluorescence images of TRPV1 in ECs (left panel, bar denotes 50 μm) and in mesenteric arteries (MA) (right panel, bar denotes 100 μm) from WT (upper panel) and TRPV1−/− mice (lower panel). DAPI denotes visualizing nuclei. Coexpression of CD31 (red) with TRPV1 (green) is shown.
(D) Representative tracings, with inset summary data showing capsaicin (CAP) stimulated increases of [Ca2+]i in ECs; **p < 0.01 versus capsaicin 1 nM, ##p < 0.01 versus capsaicin 0.1 μM. Data are means ± SEM (six separate experiments).
(E) Representative tracings, with inset summary data showing the inhibitory effects of specific TRPV1 antagonist 5′-iodo-resiniferatoxin (iRTX), the intracellular Ca2+ chelator, BAPTA-AM and TRPV1 deficiency (TRPV1−/−) on capsaicin-induced Ca2+ entry in cultured ECs. **p < 0.01 versus control. Data are means ± SEM (six experiments).
(F) TRPV1 protein expression in mesenteric arteries (MA) from WT mice fed with normal diet (ND) and normal diet plus capsaicin (NC) for 6 months; *p < 0.05 versus ND. Data are means ± SEM (three to six experiments).
(G) TRPV1 expression in mesenteric arteries from TRPV1 transgenic mice (TRPV1-tg) and WT littermates; *p < 0.05 versus WT. Data are means ± SEM (three to six experiments).
Activation of TRPV1 Increases [Ca2+]i and Phosphorylation of eNOS in Arteries
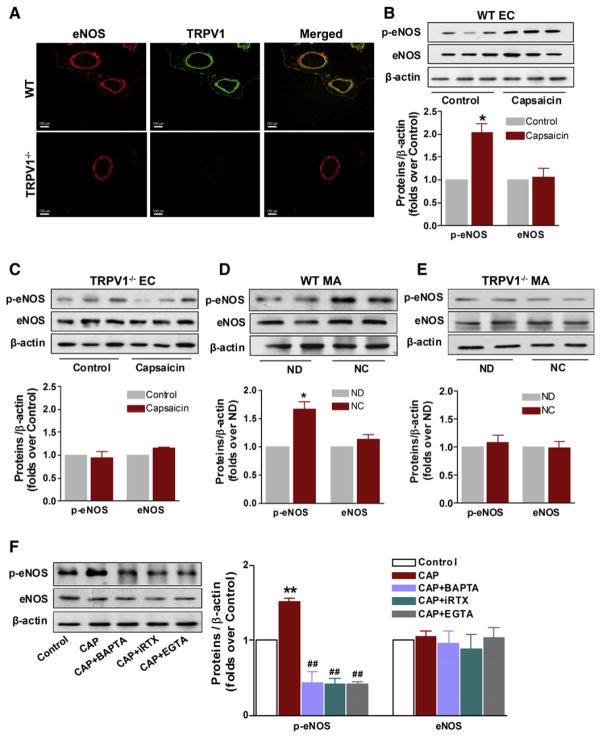
We next examined the physiological relevance of TRPV1 activation. Previous studies showed that capsaicin or its derivative increases the protein level of eNOS in vein ECs (Lo et al., 2003) and NO production in rat mesenteric arteries (Poblete et al., 2005). We tested the hypothesis that TRPV1 activation causes an increase in [Ca2+]i in ECs, which in turn increases the phosphorylation of eNOS and promotes NO formation. First, both eNOS and TRPV1 are colocalized in mesenteric arteries of WT mice, but not in TRPV1−/− mice (Figure 2A). Second, acute capsaicin treatment increased the level of phosphorylated-eNOSser1177 (p-eNOS) in cultured ECs from WT mice (Figure 2B), but not in those from TRPV1−/− mice (Figure 2C). Third, chronic consumption of capsaicin for 6 months significantly increased levels of p-eNOS in mesenteric arteries from WT mice compared with nontreated mice (Figure 2D). Capsaicin failed to produce such effect in mesenteric arteries of TRPV1−/− mice, suggesting specificity of its action (Figure 2E). The increased level of p-eNOS in capsaicin-treated ECs was inhibited by iRTX, by BAPTA-AM, and by removal of extracellular Ca2+ using EGTA (Figure 2F). The present results indicate that endothelial TRPV1 activation increases Ca2+-dependent phosphorylation of eNOS at Ser1177.
Figure 2. Activation of TRPV1 Increases Intracellular Calcium and Enhances eNOS Activity in the Vasculature.
(A) Colocalization of eNOS and TRPV1 in mesenteric arteries from WT (upper panel) and TRPV1-deficient mice (TRPV1−/−, lower panel), which were detected by immunofluorescence staining for eNOS (red) and TRPV1 (green). Scale bar denotes 100 μm. Photomicrographs are representative of three samples performed for each combination.
(B and C) Immunoblottings showing the effect of capsaicin (1 μM) on the levels of p-eNOS and eNOS in cultured ECs from WT and from TRPV1−/− mice. *p < 0.05 versus control. Data are means ± SEM. Each n = 3.
(D and E) Immunoblottings showing the effect of dietary capsaicin (0.01%) to WT and TRPV1−/− mice for 6 months on levels of p-eNOS and eNOS in mesenteric arteries (MA); *p < 0.05 versus ND. ND, normal diet; NC, normal diet plus capsaicin. The pooled data are means ± SEM. Each n = 3.
(F) Levels of p-eNOS and eNOS by immunoblotting and summary data showing inhibition of capsaicin-induced increase of p-eNOS by specific TRPV1 antagonist 5′-iodo-RTX (iRTX), or the intracellular Ca2+ chelator BAPTA-AM, or EGTA in cultured ECs from WT mice; **p < 0.01 versus control, ##p < 0.01 versus capsaicin group. CAP, capsaicin. Data are means ± SEM. Each n = 3.
Activation of TRPV1-Mediated NO Production Is Associated with Increases of [Ca2+]i in ECs
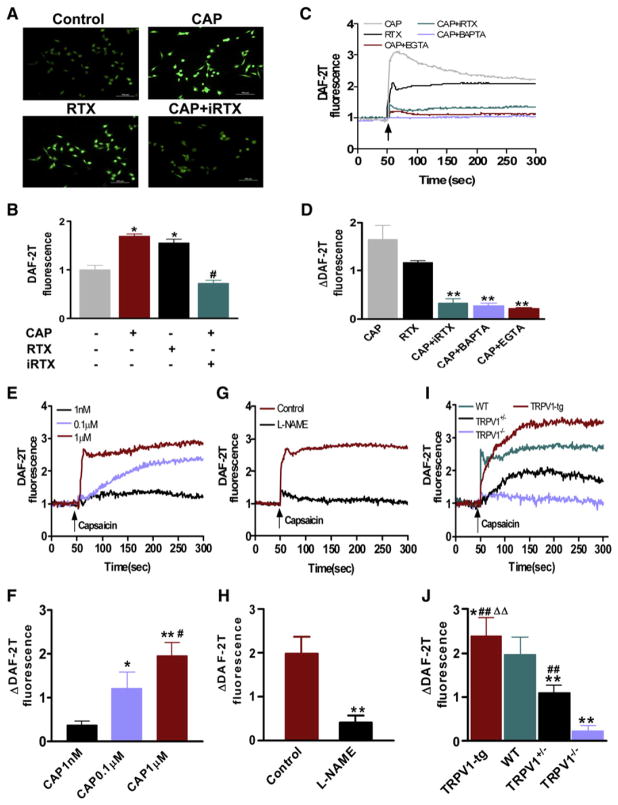
To confirm the positive role of Ca2+ influx via TRPV1 in the increased eNOS activity, NO production was measured using the NO-sensitive DAF-2DA fluorescence dye in cultured ECs and in freshly isolated mouse mesenteric arteries (Mogami et al., 2005). Capsaicin and resiniferatoxin (RTX), a chemically unrelated TRPV1 agonist, triggered a rise of intracellular NO in ECs, a response that was inhibited by iRTX, BAPTA-AM, or the absence of extracellular calcium ions (Figures 3A–3D). In WT mouse mesenteric arteries with intact endothelium, capsaicin stimulated NO production in a concentration-dependent manner (Figures 3E and 3F), which was abolished by NOS inhibition using NG-nitro-L-arginine methyl ester (L-NAME) (Figures 3G and 3H). To more firmly establish the essential role of TRPV1 in mediating NO production, the effect of capsaicin was examined in mesenteric arteries from TRPV1 transgenic (TRPV1-tg), WT, TRPV1 heterozygous (TRPV1+/−), and TRPV1−/− mice. The stimulatory effect of capsaicin was positively correlated with the expression level of TRPV1, being the greatest in TRPV1-tg and the least in TRPV1−/− mice (Figures 3I and 3J). Collectively, the present results support the notion that capsaicin acutely stimulates NO production through stimulation of TRPV1-mediated Ca2+ influx in ECs.
Figure 3. TRPV1-Mediated NO Production Is Associated with Calcium Influx.
(A and B) Representative pictures and summary data showing quantification of capsaicin-induced NO production in ECs using a dye 4,5-diaminofluorescein diacetate (DAF-2DA), which is converted into the fluorescent DAF-2T in the presence of NO. RTX, resiniferatoxin; iRTX, 5′-iodo-resiniferatoxin. *p < 0.05 versus control, #p < 0.05 versus capsaicin group. Scale bar denotes 100 μm.
(C and D) Representative tracings and summary data showing the effects of iRTX, BAPTA-AM, and EGTA on capsaicin-induced NO production in cultured ECs. **p < 0.01 versus capsaicin group (CAP).
(E and F) Representative tracings and summary data showing concentration-dependent effect of capsaicin-induced increase of NO production in freshly isolated mesenteric arteries from WT mice. *p < 0.05, **p < 0.01 versus capsaicin (CAP) 1 nM, #p < 0.05 versus CAP 0.1 μM.
(G and H) Representative tracings and summary data showing the effects of L-NAME on capsaicin-induced NO production in mesenteric arteries from WT mice; **p < 0.01 compared with control group.
(I and J) Representative tracings and summary data showing capsaicin-induced NO production in mesenteric arteries from mice with different TRPV1 genotypes. *p < 0.05, **p < 0.01 versus WT; ##p < 0.01 versus TRPV1−/− mice; ΔΔp < 0.01 versus TRPV1 heterozygote mice (TRPV1+/−). Data are means ± SEM (three to six experiments).
Chronic Activation of TRPV1 Activates PKA in Arteries
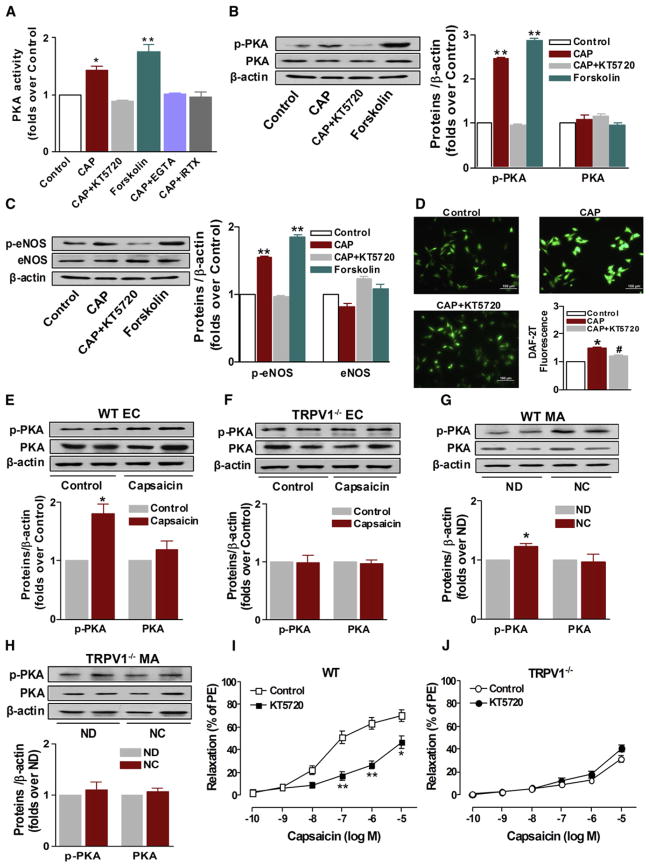
In addition to Ca2+-dependent eNOS activation, the level of eNOS phosphorylation can be regulated by several kinases, perhaps as long-term modulators of eNOS activity (Boo et al., 2002; Michell et al., 2001). Thus, we examined changes in the levels of Akt, AMPK, ERK in mesenteric arteries from WT mice. Chronic dietary capsaicin for 6 months did not influence the protein expression of these kinases (see Figure S1 available online). In addition, inhibition of PI3K (Wortmannin and LY294002) or Akt (Akt inhibitor II) had no effect on capsaicin-induced vaso-relaxation (Figures S2A and S2B). Previous studies showed that PKA is involved in the regulation of TRPV1 function (Mohapatra and Nau, 2005; Varga et al., 2006). We next tested whether TRPV1-mediated eNOS phosphorylation requires PKA activation. First, we showed that both capsaicin and forskolin, a PKA activator, increased PKA activity. However, removal of extracellular Ca2+ by EGTA or inhibition of TRPV1 using iRTX blocked capsaicin-induced increase of PKA activity in cultured ECs (Figure 4A). Second, both capsaicin and forskolin increased phosphorylated-PKA (p-PKA) and p-eNOS levels. However, inhibition of PKA using KT5720 reduced the capsaicin-induced elevation of p-PKA and p-eNOS in cultured ECs (Figures 4B and 4C). Third, inhibition of PKA using KT5720 significantly reduced capsaicin-induced NO production in cultured ECs (Figure 4D). Fourth, capsaicin increased the p-PKA level in cultured ECs of WT mice (Figure 4E), an effect absent in TRPV1−/− mice (Figure 4F). Fifth, chronic dietary capsaicin increased the p-PKA level in mesenteric arteries from WT mice (Figure 4G), but this effect was absent in TRPV1−/− mice (Figure 4H). Sixth, inhibition of PKA by KT5720 prevented capsaicin-induced relaxation in WT mice (Figure 4I), but not in TRPV1−/− mice (Figure 4J). Collectively, these results suggest that Ca2+-dependent PKA activation participates in ability of chronic TRPV1 activation to phosphorylate eNOS.
Figure 4. Effects of TRPV1 Activation on PKA Phosphorylation and Activity.
(A) The effect of TRPV1 activation on PKA activity in cultured ECs. CAP, capsaicin; EGTA, ethylene glycol tetraacetic acid; iRTX, 5′-iodo-RTX; *p < 0.05, **p < 0.01 versus control. Data are means ± SEM. Each n = 6.
(B) The effect of TRPV1 activation on PKA phosphorylation in cultured ECs. CAP, capsaicin; **p < 0.01 versus control. Data are means ± SEM. Each n = 3.
(C) The effect of TRPV1 activation on eNOS phosphorylation in cultured ECs. CAP, capsaicin; **p < 0.01 versus control. Data are means ± SEM. Each n = 3.
(D) The effect of PKA inhibitor KT5720 on capsaicin-induced increase of NO production in cultured ECs. CAP, capsaicin; *p < 0.05 versus control; #p < 0.05 versus CAP. Data are means ± SEM. Each n = 3–6. Scale bar denotes 100 μm.
(E) The effect of TRPV1 activation on PKA phosphorylation in primary cultured aortic ECs from WT mice. *p < 0.05 versus control. Data are means ± SEM. Each n = 3.
(F) Lack of effect of TRPV1 activation on PKA phosphorylation in primary cultured aortic ECs from TRPV1−/− mice. Data are means ± SEM. Each n = 3.
(G) The effect of chronic TRPV1 activation on PKA phosphorylation in mesenteric arteries (MA) from WT mice. ND, normal diet; NC, capsaicin diet; *p < 0.05 versus ND. Data are means ± SEM. Each n = 3.
(H) Lack of effect of chronic TRPV1 activation on PKA phosphorylation in mesenteric arteries (MA) from TRPV1-deficient mice. ND, normal diet; NC, capsaicin diet. Data are means ± SEM. Each n = 3.
(I) Effect of PKA inhibitor KT5720 (2 μM) on capsaicin-induced relaxation in freshly isolated mesenteric arteries from WT mice; values are expressed as means ± SEM (four to eight arterial rings); *p < 0.05, **p < 0.01 versus control.
(J) Effect of PKA inhibitor KT5720 (2 μM) on capsaicin-induced relaxation in freshly isolated mesenteric arteries from TRPV1−/− mice. Data are means ± SEM (four to eight arterial rings).
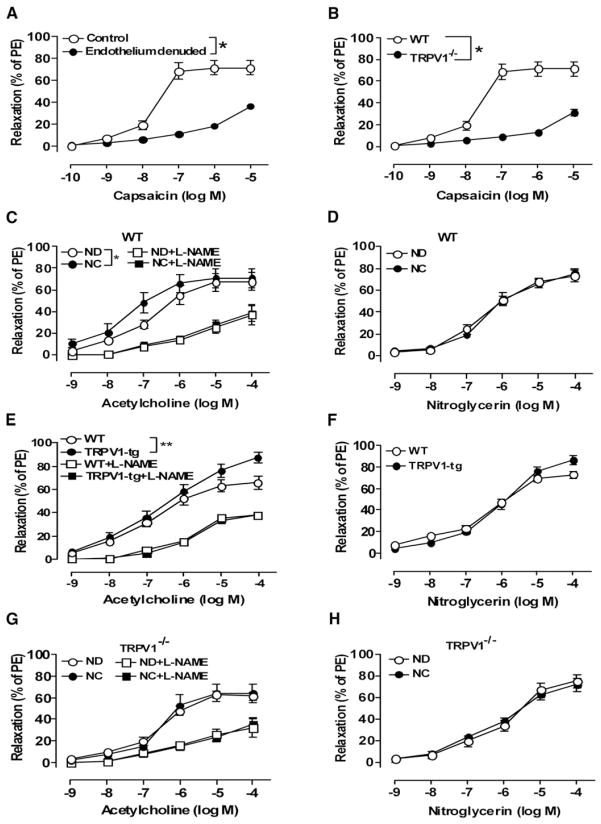
Chronic Activation of TRPV1 Enhances Endothelium-Dependent Relaxation
Capsaicin concentration-dependently relaxed mesenteric arteries of WT mice, with less of an effect in arteries lacking endothelium (Figure 5A). Marked attenuation was observed in TRPV1−/− mice arteries with endothelium (Figure 5B). Chronic activation of TRPV1 by 6 month consumption of dietary capsaicin (0.01%) in WT mice moderately increased acetylcholine-induced endothelium-dependent relaxation (pD2, 6.88 ± 0.16 in control and 7.52 ± 0.17 in capsaicin, p < 0.05, Figure 5C, Table S1). Furthermore, the maximum relaxation to acetylcholine was significantly enhanced in TRPV1-tg mice (Figure 5E, Table S2). Such an effect was absent in capsaicin-treated TRPV1−/− mice (Figure 5G, Table S3). Acetylcholine-induced relaxation was inhibited by L-NAME in arteries from all groups (Figures 5C, 5E, and 5G, Tables S1–S3). By contrast, endothelium-independent relaxation to nitroglycerin was similar in different groups (Figures 5D, 5F, and 5H, Tables S1–S3). These data demonstrate that TRPV1-mediated relaxation is endothelium dependent and that dietary capsaicin improves relaxations in WT mice, likely through TRPV1 activation.
Figure 5. Chronic Activation of TRPV1 Enhances Endothelium-Dependent Relaxation.
(A) Effect of endothelium denudation on capsaicin-induced relaxation in freshly isolated mesenteric arteries from WT mice; *p < 0.05 versus control. PE, phenylephrine.
(B) Effect of TRPV1 deficiency (TRPV1−/−) on capsaicin-induced relaxation in mesenteric arteries; *p < 0.05 versus WT mice.
(C and D) Acetylcholine- and nitroglycerin-induced relaxation in the presence or absence of L-NAME (100 μM) in isolated mesenteric arteries from WT mice on normal diet (ND) or capsaicin diet (NC); *p < 0.05.
(E and F) Acetylcholine- and nitroglycerin-induced relaxation in the presence or absence of L-NAME (100 μM) in isolated mesenteric arteries from TRPV1 transgenic mice (TRPV1-tg) and their WT littermates; **p < 0.01.
(G and H) Acetylcholine- and nitroglycerin-induced relaxation in the presence or absence of L-NAME (100 μM) in isolated mesenteric arteries from TRPV1-deficient mice (TRPV1−/−) on normal diet (ND) or capsaicin diet (NC).
Data are means ± SEM (four to eight arterial rings).
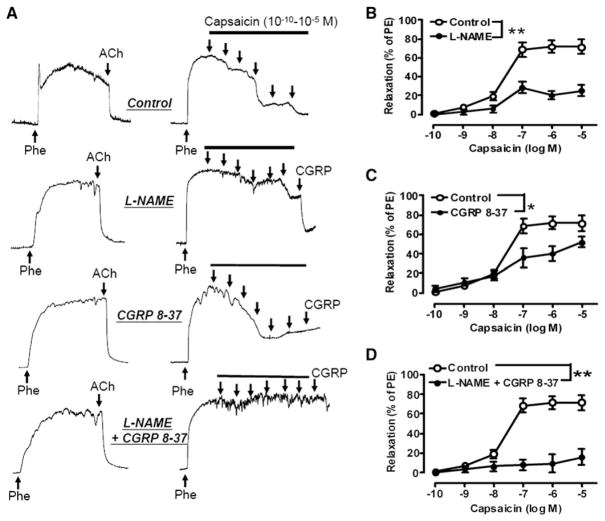
Role of Perivascular Sensory Nerves in TRPV1-Mediated Relaxations
Capsaicin is known to stimulate the release of CGRP and substance P from perivascular sensory nerve terminals in several vascular beds (Holzer, 1992; Rubino and Burnstock, 1996). We found that capsaicin-induced relaxation was partially inhibited by the CGRP receptor antagonist CGRP8-37 or NOS inhibitor L-NAME, and was abolished by cotreatment with CGRP8-37 plus L-NAME in the mesenteric arteries from WT mice (Figures 6A–6D). Exogenous CGRP-induced relaxation was unaffected by L-NAME or ODQ, a guanylate cyclase inhibitor (Figure S3A). CGRP-induced relaxation was partially dependent on the presence of endothelium, and CGRP caused less relaxation of arteries precontracted by 60 mM KCl (Figure S3B). Substance P did not relax the same preparations (data not shown). Together, the present study underscores that capsaicin acutely relaxes mesenteric arteries through at least two mechanisms: a direct action on ECs which is NO dependent and L-NAME sensitive and also the release of CGRP, which activates an L-NAME-insensitive pathway but to a lesser degree.
Figure 6. Role of Perivascular Sensory Nerves in TRPV1-Mediated Relaxation.
(A) Representative tracings showing inhibitory effect of L-NAME and CGRP receptor antagonist CGRP 8-37 on capsaicin-induced relaxation in mouse mesenteric arteries.
(B) Effect of L-NAME (100 μM) on capsaicin-induced relaxation in freshly isolated mesenteric arteries from C57BL/6J mice; **p < 0.01.
(C) Effect of CGRP 8-37 (3 μM) on capsaicin-induced relaxation in freshly isolated mesenteric arteries from C57BL/6J mice; *p < 0.05.
(D) Effects of L-NAME (100 μM) and CGRP 8-37 (3 μM) on capsaicin-induced relaxation in freshly isolated mesenteric arteries from C57BL/6J mice; **p < 0.01.
Data are means ± SEM (four to six experiments).
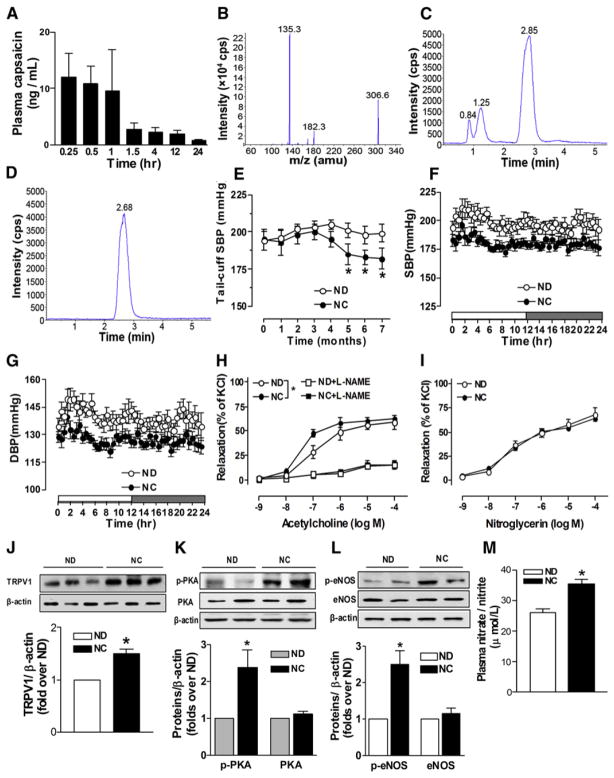
Activation of TRPV1 by Dietary Capsaicin Attenuates Hypertension
To examine whether chronic activation of TRPV1 could favorably modulate vascular function and hence reduce arterial pressure in hypertension, adult spontaneously hypertensive rats (SHRs) were fed with capsaicin diet for 7 months. We observed that both SHR and WT mice had an aversive response in that food intake transiently declined during the first days after initiating capsaicin administration, changes that were absent in TRPV1−/− mice (Figures S4A and S4B). Body weight, heart rate, and biochemical parameters in both mice and rats were not different between treated and untreated groups (Figures S4C and S4D, Tables S4 and S5).
In Wistar rats we assessed the time-dependent response to intragastric administration of capsaicin (15 mg/kg body weight) (Appendino et al., 2002; Melck et al., 1999). The plasma concentration of capsaicin reached a peak of ~10 ng/ml at the first hour and then declined rapidly (Figures 7A–7D). The absolute bioavailability of oral capsaicin was approximately 0.106%. The small bioavailability may be caused by rapid metabolism of capsaicin in the liver (Beaudry and Vachon, 2009; Chanda et al., 2008). Dietary capsaicin for 3 weeks did not affect endothelium-dependent relaxation, blood pressure, or levels of p-PKA or p-eNOS in mesenteric arteries from SHR (Figures S5A–S5D). During chronic capsaicin treatment, systolic blood pressure (SBP) fell slowly starting at fourth month, reaching a significant reduction at fifth month and onward (Figure 7E). Ambulatory arterial pressure in conscious, unrestrained SHR was monitored by radiotelemetry. The average 24 hr blood pressure was significantly lower in capsaicin-treated compared with untreated SHR (SBP, 181 ± 3 mmHg versus 198 ± 5 mmHg; and diastolic BP, 127 ± 2 mmHg versus 139 ± 3 mmHg, p < 0.01, n = 7–8) (Figures 7F and 7G). Chronic capsaicin treatment improved endothelium-dependent relaxation of SHR mesenteric arteries (pD2, 6.97 ± 0.05 in control and 7.41 ± 0.04 in capsaicin, p < 0.05). This difference was eliminated by L-NAME (pD2, 6.13 ± 0.29 in control and 6.31 ± 0.20 in capsaicin, p > 0.05, Figure 7H and Table S6). Endothelium-independent relaxation by nitroglycerin was comparable in both groups (pD2, 7.16 ± 0.15 in control and 7.21 ± 0.14 in capsaicin, p > 0.05, Figure 7I and Table S6). The levels of TRPV1, p-PKA, and p-eNOSSer1177 in mesenteric arteries and the plasma nitrate/nitrite (NO metabolites) concentration were higher in capsaicin-treated SHR (Figures 7J–7M). Previous studies reported that capsaicin increases plasma levels of CGRP and substance P (Holzer, 1992; Li and Wang, 2003; Rubino and Burnstock, 1996). However, we detected no difference in the plasma concentration of CGRP (47.8 ± 6.1 pg/ml in control and 46.1 ± 11.2 pg/ml in capsaicin, p > 0.05) and substance P (136.6 ± 16.3 pg/ml in control and 140.8 ± 24.2 pg/ml in capsaicin, p > 0.05) in SHR. Thus, we conclude that long-term consumption of capsaicin reduced arterial pressure in SHR primarily due to activation of TRPV1 and subsequently promotes phosphorylation of PKA and eNOS, and increases the production of NO that accounts for the improved endothelium-dependent relaxation in SHR.
Figure 7. Effects of TRPV1 Activation on Blood Pressure and Vascular Relaxation in Spontaneous Hypertensive Rats.
(A) The plasma concentration of capsaicin in rats administered intragastrically with a single dose of capsaicin (15 mg/kg body weight).
(B–D) Identification of capsaicin in plasma: mass spectrometry of capsaicin, chromatogram of extracted plasma sample from capsaicin-treated rats, and chromatogram of authentic capsaicin.
(E) The time course of capsaicin-induced reduction in SBP in SHR fed with normal diet (ND) or normal diet plus capsaicin (NC) for 7 months. SBP was determined using tail cuff method. *p < 0.05 versus ND.
(F and G) Capsaicin-induced reduction of SBP and diastolic blood pressure (DBP) in SHR. Blood pressure was determined for 24 hr using radiotelemetry in SHR fed with normal diet (ND) or normal diet plus capsaicin (NC) for 7 months.
(H) Acetylcholine-induced relaxation in the presence or absence of L-NAME (100 μM) in isolated mesenteric arteries from SHR fed without (ND) or with capsaicin (NC) for 7 months. *p < 0.05 versus ND group.
(I) Nitroglycerin-induced relaxation in isolated mesenteric arteries from SHR fed without (ND) or with capsaicin (NC) for 7 months.
(J) TRPV1 protein expression in mesenteric artery from SHR fed without (ND) or with capsaicin (NC) for 7 months. *p < 0.05 compared with ND group.
(K) Levels of p-PKA and PKA protein in mesenteric arteries from SHR fed without (ND) or with capsaicin (NC) for 7 months. *p < 0.05 versus ND group.
(L) Levels of p-eNOS and eNOS protein in mesenteric arteries from SHR fed without (ND) or with capsaicin (NC) for 7 months. *p < 0.05 compared with ND group.
(M) Increased plasma nitrate/nitrite levels in SHR treated without (ND) or with capsaicin (NC) for 7 months.*p < 0.05 versus ND group.
Data are means ± SEM.
DISCUSSION
The present study provides experimental evidences for the beneficial effect of dietary capsaicin in reducing high blood pressure. Such benefit is related to a direct stimulatory action of capsaicin on endothelial TRPV1 channels, a conclusion supported by both in vitro and in vivo data obtained in TRPV1−/− mice and TRPV1 transgenic mice. We showed that TRPV1 activation elevates the phosphorylation of PKA and eNOS in ECs and plasma NO concentration, improves endothelium-dependent relaxation in mesenteric arteries, and lowers arterial pressure in genetically hypertensive rats. Thus, endothelial TRPV1 activation can be considered as a potential strategy for the management of hypertension.
TRPV1 activation in sensory neurons causes nociception (Julius and Basbaum, 2001). Capsaicin, a specific TRPV1 agonist, is used as a potent analgesic and the modulator of sensory function (Nilius, 2007; Tominaga and Tominaga, 2005). However, the effect of capsaicin on blood pressure is more controversial. Few studies are available concerning the impact of chronic activation of TRPV1 on the regulation of vascular function and blood pressure.
Capsaicin can affect cardiovascular function through stimulating the release of CGRP and substance P from perivascular sensory nerve endings (Li et al., 2003). Capsaicin may exert direct effects on the vasculature, relaxing coronary, mesenteric, hepatic, basilar, and meningeal arteries of pigs and rats (Bratz et al., 2008; Lo et al., 2003). However, it has also been reported that activation of TRPV1 can cause vasoconstriction in mesenteric (Scotland et al., 2004), coronary (Szolcsanyi et al., 2001), and skeletal muscle arteries (Kark et al., 2008) in rodents and canines. Furthermore, capsaicin-induced relaxation of human and porcine coronary arteries is likely to be attributed to a CGRP-independent mechanism (Gupta et al., 2007). In addition, neonatal denervation of sensory nerves does not modify the NO release elicited by capsaicin, indicating that perivascular nerve fibers probably do not participate in capsaicin-induced relaxation (Rocha and Bendhack, 2009). The present study shows that CGRP-induced relaxations of rat mesenteric arteries were unaffected by inhibition of NO synthase. On the other hand, capsaicin-induced endothelium-dependent vasorelaxation was NO dependent. However, the possibility that long-term administration of TRPV1 agonists may affect sensory neurons in vivo to contribute to the observed hypotensive effect by acting partially through CGRP cannot be ruled out.
Our collective results from ECs and mesenteric arteries in vitro indicate that TRPV1 activation increases [Ca2+]i, the activity of eNOS in intact arteries, and the NO production in ECs. First, capsaicin selectively increases the level of p-eNOS in ECs from WT mice, but not those of TRPV1−/− mice. Second, the level of p-eNOS is specifically upregulated in mesenteric arteries from WT mice chronically fed capsaicin diet, but not in arteries from TRPV1−/− mice receiving the same diet. Third, the increased [Ca2+]i is required for the phosphorylation of eNOS and subsequent NO production in ECs upon TRPV1 activation by capsaicin because the stimulatory effect is inhibited by a Ca2+ chelator, by removal of extracellular calcium ions, and by antagonism by a selective TRPV1 blocker. Fourth, capsaicin-induced NO production appears to be related to the expression level of TRPV1 in arteries from mice with different TRPV1 genotypes. Although elevated [Ca2+]i is known to enhance the NO formation in ECs (da Silva et al., 2009; Michel et al., 1997), other factors may regulate the eNOS activity. However, the present acute studies in vitro show that tetrahydrobiopterin (BH4) does not modulate capsaicin-induced relaxations (Figure S6). Moreover, capsaicin does not affect the level of caveolin-1(cav-1) (Figure S7A), heat shock protein 90 (hsp90) (Figure S7B), and VEGF (Figure S7C) in ECs.
The present study suggests that TRPV1 activation and subsequent Ca2+ influx are required to enhance eNOS activity. However, we cannot exclude the possibility that other mechanisms may also contribute to the upregulation of eNOS activity in vivo during chronic capsaicin consumption. It is well known that the eNOS activity can be regulated via its phosphorylation by various kinases, including Akt, AMP-activated protein kinase (AMPK), extracellular signal-regulated kinase 1/2 (ERK), and PKA (Michell et al., 2001; Boo et al., 2002). Such kinases may mediate long-term modulation of eNOS activity. However, we find that chronic dietary capsaicin did not alter the expression of Akt, AMPK, and ERK in WT mouse mesenteric arteries. Previous studies report that PKA modulates TRPV1 function (Mohapatra and Nau, 2005; Varga et al., 2006). Our data show that TRPV1 activation can enhance the activity and expression of PKA in ECs. Chronic dietary capsaicin increased p-PKA level in mesenteric arteries from WT mice, but this effect was absent in TRPV1−/− mice. PKA inhibition reduced capsaicin-induced relaxation of mesenteric arteries and NO production in ECs. Our present results clearly show that TRPV1-activated Ca2+ influx is involved in the PKA-mediated eNOS phosphorylation following long-term administration of capsaicin.
Although our present results agree with other reports showing that TRPV1 activation causes relaxation of isolated arteries, the effect of acute or short term administration of capsaicin on arterial pressure in vivo is less clear. Oral intake of hot pepper is reported to transiently increase blood pressure and heart rate in humans (Hachiya et al., 2007), while 2 week administration of CH-19, a nonpungent cultivar of red pepper, does not produce a pressor effect (Kawabata et al., 2006). Acute intravenous administration of capsaicin also causes a transient elevation in arterial pressure and heart rate in dogs and rats (Chanda et al., 2005; Giles and Sander, 1986). This pressor effect is in part associated with an increased sympathetic nerve activity (Hachiya et al., 2007), and TRPV1-mediated myogenic constriction involves sensory nerve-derived substance P (Scotland et al., 2004). By contrast, intravenous administration of capsaicin transiently lowers arterial pressure in rats, while the TRPV1 antagonist capsazepine elicits an acute pressor effect (Wang and Wang, 2006). However, these studies did not distinguish between a direct action of capsaicin on endothelial TRPV1 as opposed to vascular effects secondary to nerve activation. Transdermal application of capsaicin improves the ischemic threshold in patients with coronary artery disease, and this benefit is related to the augmented plasma levels of NO and thus improved arteriolar vasodilatation, events unrelated to a change in the plasma concentration of CGRP (Fragasso et al., 2004). The present study shows that short-term dietary capsaicin did not affect blood pressure, the endothelium-dependent relaxation, and PKA/eNOS phosphorylation in mesenteric arteries of SHR. We also detected no significant changes in the plasma levels of CGRP and substance P after capsaicin treatment in hypertensive rats. Acetylcholine-induced relaxation was augmented in mice receiving chronic dietary capsaicin and in TRPV1 transgenic mice. The present study provides evidence that chronic activation of TRPV1 channels improves endothelium-dependent relaxation, because such a benefit is absent in TRPV1−/− mice. Acute administration of capsaicin or its derivative lowers mean arterial pressure in SHR accompanied with increased plasma levels of CGRP (Li and Wang, 2003; Lo et al., 2003). Most published studies only show the acute effect (within 10 min to hours) of capsaicin on blood pressure. However, prior to our study, it was unclear whether chronic activation of TRPV1 by capsaicin for a longer duration would affect vascular function and blood pressure. Thus, a major finding of our study is a clear demonstration that chronic capsaicin treatment lowered arterial pressure in conscious adult SHR. Radiotelemetry measurements show that both systolic and diastolic blood pressure were reduced in SHR receiving dietary capsaicin for long term. Notably, at the time, endothelium-dependent relaxation was found to be improved. The antihypertensive effect of chronic capsaicin consumption was accompanied with elevated plasma levels of nitrate/nitrite, an index of NO production, and increased phosphorylation of PKA and eNOS in mesenteric arteries.
Clinical investigations indicate that restoration of endothelial function in hypertensive patients normally takes more than 6 months or even longer (Ishibashi et al., 2008; Schiffrin et al., 2002). Oral administration of molsidomin, a NO donor, produces a measurable reduction of blood pressure only several weeks after treatment (Koeners et al., 2008; Racasan et al., 2005). Therefore, an antihypertensive effect of TRPV1 activation and stimulation on PKA/eNOS phosphorylation may take longer.
However, long-term treatment with TRPV1 agonists may also exert an endothelium-independent vascular action, jointly contributing to the persistent reduction of blood pressure observed in vivo. Collectively, the present results demonstrate that long-term dietary capsaicin leads to a reduced arterial pressure and improved endothelial function in hypertensive rats, most likely through activation of endothelial TRPV1 and a NO-dependent mechanism.
In conclusion, we demonstrate that dietary capsaicin consumption reduces blood pressure in SHRs. Our mechanistic evidence suggests that this vascular benefit is likely to be caused by chronic activation of endothelial TRPV1 channel, which mediates an increased Ca2+ influx and subsequent phosphorylation of PKA and eNOS. As a result, NO production accounts for the potentiality of the endothelium-dependent relaxation in capsaicin-treated mice and SHR. Our findings provide insights into the physiological role of endothelial TRPV1 channel in a long-term regulation of blood pressure. TRPV1 activation through chronic dietary capsaicin may represent a promising intervention of lifestyle in high-risk populations with hypertension and related vascular disorders.
EXPERIMENTAL PROCEDURES
Animal Treatment
C57BL/6J WT and TRPV1−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The transgenic plasmid pcDNA3.1-rTRPV1 was a kind gift from Ramon Latorre (Laboratory of Biophysics and Molecular Physiology, Chile). Transgenic TRPV1 mice were produced by Shanghai Research Center for Biomodel Organism (Supplemental Information). Eight-week-old male SHR were obtained from Charles Rivers Laboratories. Rats and mice were housed under a 12 hr/12 hr day/night cycle with free access to food and water. Animals were given the normal standard chow (control group) or normal chow plus with 0.01% capsaicin for mice and 0.02% capsaicin for rats (capsaicin group). The institute Animal Care and Use Committee approved all animal protocols.
Endothelial Cell Culture and Measurements of [Ca2+]i
ECs were isolated as described (Kobayashi et al., 2005), and cytosolic free calcium concentration ([Ca2+]i) was measured using the fluorescent dye fura2/AM (Liu et al., 2009; Zhu et al., 1998) (Supplemental Information).
Measurement of NO Production
The ECs or arterial segments from mice were washed twice in Hank’s balanced salt solution (HBSS) before adding HBSS containing DAF-2DA (5 μM, Calbiochem, USA). After further incubation for 30 min for cells and 2 hr for arteries in dark room with or without 15 min treatment with iRTX (1 μM, Alomone, Israel) or BAPTA-AM (10 μM, Sigma-Aldrich), samples were rinsed in HBSS and transferred to black microplates. The DAF-2T fluorescence for ECs was monitored using a Nikon fluorescence microscope (excitation 488 nm, emission 515 nm) equipped with an FITC filter (Thomas et al., 2007), while the fluorescence for mesenteric arteries was measured with PTI (Photon Technology International, USA) (Mogami et al., 2005).
RNA Isolation and PCR Analysis
Total RNA isolation and reverse transcription polymerase chain reaction were performed following standard protocols. Primer sequences are shown in the Supplemental Information.
Immunoblot Analysis and Immunohistochemistry
Immunoblots of TRPV1, β-actin, eNOS, PKA, Akt, AMPK, ERK, and their phosphorylated forms were performed as reported (Liu et al., 2009). Immunohistochemistry was routinely performed using antibodies against TRPV1 (Alomone, Israel), CD31, and eNOS (Santa Cruz Biotechnology, USA) (Supplemental Information).
Blood Pressure Measurement
SBP, measured by tail-cuff plethysmography, was routinely obtained every month. After normal diet or normal diet plus capsaicin for 7 months, SHRs were surgically implanted with telemetric transmitters (TL11M2-C50-PXT, Data Sciences International, MN, USA). The catheter of the implant was placed into the distal portion of the descending aorta. Rats were allowed to recover from surgery for 10 days, and then 24 hr ambulatory systolic and diastolic pressures were measured by telemetry in conscious, unrestrained rats. We collected data for 10 s every 30 min and used the 24 hr mean values for analysis.
Measurement of Vascular Reactivity
Vascular reactivity of freshly isolated mesenteric arteries was studied in myograph (Danish Myo Technology, Denmark) as described (Huang et al., 2003). After mice or rats were anesthetized with pentobarbital sodium, the mesenteric vascular bed was removed and placed in a cold Krebs solution containing (mM) 118 NaCl, 25 NaHCO3, 11 D-glucose, 4.7 KCl, 1.2 KH2PO4, 1.17 MgSO4, and 2.5 CaCl2. The first (of mouse) or second (of rat) branches of the mesenteric artery were dissected out and cleaned of connective tissue. In some arteries, the endothelium of the rings was removed by gently rubbing the lumen with human hair. Arterial segments (100–200 μm in luminal diameter, 2–2.5 mm in length) were mounted in myograph. Each ring was bathed in Krebs solution aerated with 95%O2 and 5%CO2 at 37°C (pH 7.4). After measurement of passive-tension internal circumference characteristics, tension was set to the estimated in vivo internal circumference. After 60 min stabilization, functional integrity of rings was confirmed by contraction to KCl (60 mM). The presence of endothelium was confirmed by a relaxant response to acetylcholine (ACh, 1 μM) in rings contracted with phenylephrine (1 μM) in the presence of propranolol (100 nM) to block β-adrenoceptors. Segments relaxing >80% were considered being endothelium intact, while those relaxing <5% were defined as being endothelium-denuded. Capsaicin, RTX, phenylephrine (PE), propranolol, ACh, nitroglycerin (NTG) and NG-nitro-L-arginine methyl ester (L-NAME) were purchased from Sigma-Aldrich.
Biochemical Assays
Blood was withdrawn from jugular vein, and the plasma was separated and immediately frozen at −70°C until assayed. Levels of plasma CGRP, nitrate/ nitrite, substance P, insulin, lipids, and glucose were measured using commercially available assay kits. PKA activity was assessed using a commercially available kit (Westtang Inc., Shanghai).
Mass Spectrometric Quantification of Capsaicin
Mass spectrometric quantification of capsaicin in blood was performed using Agilent 1100 Series, and an Ultra C18 (5 μm, 2.1 mm × 50 mm) column (Supplemental Information).
Statistics
Results represent means ± SEM of n experiments. Statistical differences between groups were assessed by Student’s t test or one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison post hoc tests, as appropriate. Two-sided p values less than 0.05 were regarded to be statistically significant.
Acknowledgments
We thank Dr. Bernd Nilius (Katholieke Univ. Leuven, Belgium) for critical review of the manuscript. We acknowledge Dr. R. Latorre (Laboratory of Biophysics and Molecular Physiology, Chile) for the gift of plasmid pcDNA3.1-rTRPV1. We thank Drs. Bo Yang and Rongfeng Xiang for help with the identification of plasma capsaicin and Lijuan Wang for technical assistance. This research was supported by grants from National Natural Science Foundation of China (30890042), the National Basic Research Program of China (2006CB503905 and 2006CB503804), HKGRF (4653/08M), and CUHK Focused Investment Scheme. This work was also supported by the Program for Changjiang Scholars from the Ministry of Education in China.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures, six tables, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.cmet.2010.05.015.
References
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Appendino G, Minassi A, Morello AS, De Petrocellis L, Di Marzo V. N-Acylvanillamides: development of an expeditious synthesis and discovery of new acyl templates for powerful activation of the vanilloid receptor. J Med Chem. 2002;45:3739–3745. doi: 10.1021/jm020844o. [DOI] [PubMed] [Google Scholar]
- Beaudry F, Vachon P. Quantitative determination of capsaicin, a transient receptor potential channel vanilloid 1 agonist, by liquid chromatography quadrupole ion trap mass spectrometry: evaluation of in vitro metabolic stability. Biomed Chromatogr. 2009;23:204–211. doi: 10.1002/bmc.1107. [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;294:H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired no-ciception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chanda S, Mould A, Esmail A, Bley K. Toxicity studies with pure trans-capsaicin delivered to dogs via intravenous administration. Regul Toxicol Pharmacol. 2005;43:66–75. doi: 10.1016/j.yrtph.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Chanda S, Bashir M, Babbar S, Koganti A, Bley K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab Dispos. 2008;36:670–675. doi: 10.1124/dmd.107.019240. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Cushman WC. Systolic hypertension and cardiovascular risk reduction: a clinical review. Curr Hypertens Rep. 2001;3(Suppl 1):S11–S15. doi: 10.1007/s11906-001-0066-y. [DOI] [PubMed] [Google Scholar]
- da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009;119:871–879. doi: 10.1161/CIRCULATIONAHA.108.764571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Carmen Garcia M, Adler Graschinsky E, Celuch SM. Hypotensive effect of anandamide through the activation of CB1 and VR1 spinal receptors in urethane-anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:270–276. doi: 10.1007/s00210-003-0800-x. [DOI] [PubMed] [Google Scholar]
- Fragasso G, Palloshi A, Piatti PM, Monti L, Rossetti E, Setola E, Montano C, Bassanelli G, Calori G, Margonato A. Nitric-oxide mediated effects of transdermal capsaicin patches on the ischemic threshold in patients with stable coronary disease. J Cardiovasc Pharmacol. 2004;44:340–347. doi: 10.1097/01.fjc.0000137161.76616.85. [DOI] [PubMed] [Google Scholar]
- Giles TD, Sander GE. Comparative cardiovascular responses to intravenous capsaicin, phenyldiguanide, veratrum alkaloids and enkephalins in the conscious dog. J Auton Pharmacol. 1986;6:1–7. doi: 10.1111/j.1474-8673.1986.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Lozano Cuenca J, Villalon CM, de Vries R, Garrelds IM, Avezaat CJ, van Kats JP, Saxena PR, MaassenVanDenBrink A. Pharmacological characterisation of capsaicin-induced relaxations in human and porcine isolated arteries. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:29–38. doi: 10.1007/s00210-007-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya S, Kawabata F, Ohnuki K, Inoue N, Yoneda H, Yazawa S, Fushiki T. Effects of CH-19 Sweet, a non-pungent cultivar of red pepper, on sympathetic nervous activity, body temperature, heart rate, and blood pressure in humans. Biosci Biotechnol Biochem. 2007;71:671–676. doi: 10.1271/bbb.60359. [DOI] [PubMed] [Google Scholar]
- Holzer P. Peptidergic sensory neurons in the control of vascular functions: mechanisms and significance in the cutaneous and splanchnic vascular beds. Rev Physiol Biochem Pharmacol. 1992;121:49–146. doi: 10.1007/BFb0033194. [DOI] [PubMed] [Google Scholar]
- Huang Y, Chan FL, Lau CW, Tsang SY, Chen ZY, He GW, Yao X. Roles of cyclic AMP and Ca2+-activated K+ channels in endothelium-independent relaxation by urocortin in the rat coronary artery. Cardiovasc Res. 2003;57:824–833. doi: 10.1016/s0008-6363(02)00773-3. [DOI] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Takahashi N, Tokumaru A, Karino K, Sugamori T, Sakane T, Yoshitomi H, Sato H, Oyake N, Murakami Y, et al. Effects of long-term nicorandil administration on endothelial function, inflammation, and oxidative stress in patients without coronary artery disease. J Cardiovasc Pharmacol. 2008;51:311–316. doi: 10.1097/FJC.0b013e318163a95f. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- Kawabata F, Inoue N, Yazawa S, Kawada T, Inoue K, Fushiki T. Effects of CH-19 sweet, a non-pungent cultivar of red pepper, in decreasing the body weight and suppressing body fat accumulation by sympathetic nerve activation in humans. Biosci Biotechnol Biochem. 2006;70:2824–2835. doi: 10.1271/bbb.60206. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- Koeners MP, Braam B, van der Giezen DM, Goldschmeding R, Joles JA. A perinatal nitric oxide donor increases renal vascular resistance and ameliorates hypertension and glomerular injury in adult fawn-hooded hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1847–R1855. doi: 10.1152/ajpregu.00073.2008. [DOI] [PubMed] [Google Scholar]
- Li J, Wang DH. High-salt-induced increase in blood pressure: role of capsaicin-sensitive sensory nerves. J Hypertens. 2003;21:577–582. doi: 10.1097/00004872-200303000-00024. [DOI] [PubMed] [Google Scholar]
- Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- Liu D, Yang D, He H, Chen X, Cao T, Feng X, Ma L, Luo Z, Wang L, Yan Z, et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53:70–76. doi: 10.1161/HYPERTENSIONAHA.108.116947. [DOI] [PubMed] [Google Scholar]
- Lo YC, Hsiao HC, Wu DC, Lin RJ, Liang JC, Yeh JL, Chen IJ. A novel capsaicin derivative VOA induced relaxation in rat mesenteric and aortic arteries: involvement of CGRP, NO, cGMP, and endothelium-dependent activities. J Cardiovasc Pharmacol. 2003;42:511–520. doi: 10.1097/00005344-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, Di Marzo V. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem Biophys Res Commun. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- Mogami K, Kishi H, Kobayashi S. Sphingomyelinase causes endothelium-dependent vasorelaxation through endothelial nitric oxide production without cytosolic Ca2+ elevation. FEBS Lett. 2005;579:393–397. doi: 10.1016/j.febslet.2004.11.100. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Ohnuki K, Moritani T, Ishihara K, Fushiki T. Capsaicin increases modulation of sympathetic nerve activity in rats: measurement using power spectral analysis of heart rate fluctuations. Biosci Biotechnol Biochem. 2001;65:638–643. doi: 10.1271/bbb.65.638. [DOI] [PubMed] [Google Scholar]
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Poblete IM, Orliac ML, Briones R, Adler Graschinsky E, Huidobro Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol. 2005;568:539–551. doi: 10.1113/jphysiol.2005.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racasan S, Braam B, Koomans HA, Joles JA. Programming blood pressure in adult SHR by shifting perinatal balance of NO and reactive oxygen species toward NO: the inverted Barker phenomenon. Am J Physiol Renal Physiol. 2005;288:F626–F636. doi: 10.1152/ajprenal.00314.2004. [DOI] [PubMed] [Google Scholar]
- Rocha ML, Bendhack LM. Relaxation evoked by extracellular Ca2+ in rat aorta is nerve-independent and involves sarcoplasmic reticulum and L-type Ca2+ channel. Vascul Pharmacol. 2009;50:98–103. doi: 10.1016/j.vph.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Rubino A, Burnstock G. Capsaicin-sensitive sensory-motor neurotransmission in the peripheral control of cardiovascular function. Cardiovasc Res. 1996;31:467–479. [PubMed] [Google Scholar]
- Schiffrin EL, Park JB, Pu Q. Effect of crossing over hypertensive patients from a beta-blocker to an angiotensin receptor antagonist on resistance artery structure and on endothelial function. J Hypertens. 2002;20:71–78. doi: 10.1097/00004872-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ Res. 2004;95:1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J, Oroszi G, Nemeth J, Szilvassy Z, Blasig IE, Tosaki A. Functional and biochemical evidence for capsaicin-induced neural endothelin release in isolated working rat heart. Eur J Pharmacol. 2001;419:215–221. doi: 10.1016/s0014-2999(01)00973-6. [DOI] [PubMed] [Google Scholar]
- Thomas S, Kotamraju S, Zielonka J, Harder DR, Kalyanaraman B. Hydrogen peroxide induces nitric oxide and proteosome activity in endothelial cells: a bell-shaped signaling response. Free Radic Biol Med. 2007;42:1049–1061. doi: 10.1016/j.freeradbiomed.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- Varga A, Bolcskei K, Szoke E, Almasi R, Czeh G, Szolcsanyi J, Petho G. Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo. Neuroscience. 2006;140:645–657. doi: 10.1016/j.neuroscience.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des. 2008;14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Liu DY, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zhang SH, Wagner C, Kurtz A, Maeda N, Coffman T, Arendshorst WJ. Angiotensin AT1B receptor mediates calcium signaling in vascular smooth muscle cells of AT1A receptor-deficient mice. Hypertension. 1998;31:1171–1177. doi: 10.1161/01.hyp.31.5.1171. [DOI] [PubMed] [Google Scholar]