Abstract
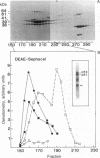
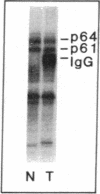
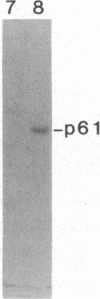
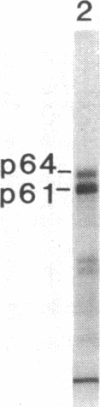
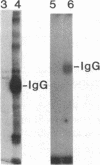
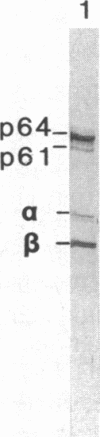
We have identified and substantially purified a tyrosine protein kinase from normal bovine brain that is immunologically related to the product of the Rous sarcoma virus oncogene (pp60v-src). The enzyme, a 61-kDa protein (p61), is solubilized with detergent from bovine cerebral cortical membranes and purified by column chromatography. In the purest preparations, this protein is phosphorylated only on tyrosine, but it can also be a substrate for serine- and threonine-specific protein kinases. The p61 protein phosphorylates the heavy chain of immunoglobulins from rabbits bearing Rous sarcoma virus-induced tumors (TBR IgG) but not normal IgG. TBR IgG precipitates the 61-kDa phosphoprotein and protein kinase activity from purified preparations. The activity of the purified brain tyrosine kinase is 10 times higher in the presence of 7-10 mM Mn2+ and 6 mM Mg2+ than it is with 6 mM Mg2+ alone. With Mn2+, the p61 enzyme has a Km for ATP of 2 microM. All preparations of p61 also contain a 64-kDa protein (p64) that is phosphorylated on tyrosine. Measurement of the Stokes radius of p61 and p64 by gel filtration shows that they are not physically associated in buffer containing the nonionic detergent Lubrol 12A9. The p64 protein is not precipitated by TBR IgG. We do not know whether p64 is only a substrate for the p61 tyrosine kinase or is itself a kinase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Barnekow A., Schartl M., Anders F., Bauer H. Identification of a fish protein associated with a kinase activity and related to the Rous sarcoma virus transforming protein. Cancer Res. 1982 Jun;42(6):2429–2433. [PubMed] [Google Scholar]
- Barnekow A., Schartl M. Cellular src gene product detected in the freshwater sponge Spongilla lacustris. Mol Cell Biol. 1984 Jun;4(6):1179–1181. doi: 10.1128/mcb.4.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blithe D. L., Richert N. D., Pastan I. H. Purification of a tyrosine-specific protein kinase from Rous sarcoma virus-induced rat tumor. J Biol Chem. 1982 Jun 25;257(12):7135–7142. [PubMed] [Google Scholar]
- Blithe D. L., Richert N. D., Pastan I. H. Purification of a tyrosine-specific protein kinase from Rous sarcoma virus-induced rat tumor. J Biol Chem. 1982 Jun 25;257(12):7135–7142. [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Hellstrom K. E., Krebs E. G. A lymphoma cell line expressing elevated levels of tyrosine protein kinase activity. J Biol Chem. 1983 Sep 10;258(17):10738–10742. [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta J. D., Swarup G., Garbers D. L. Tyrosine protein kinase activity in normal rat tissues: brain. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:461–470. [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Gateff E. Cancer, genes, and development: the Drosophila case. Adv Cancer Res. 1982;37:33–74. doi: 10.1016/s0065-230x(08)60881-7. [DOI] [PubMed] [Google Scholar]
- Gateff E. Cancer, genes, and development: the Drosophila case. Adv Cancer Res. 1982;37:33–74. doi: 10.1016/s0065-230x(08)60881-7. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Pike L. J., Kuenzel E. A., Casnellie J. E., Krebs E. G. A comparison of the insulin- and epidermal growth factor-stimulated protein kinases from human placenta. J Biol Chem. 1984 Aug 10;259(15):9913–9921. [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes of spontaneous and chemically induced tumors. Adv Cancer Res. 1982;36:149–163. doi: 10.1016/s0065-230x(08)60424-8. [DOI] [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]